A Contact-Free Optical Device for the Detection of Pulmonary Congestion—A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Ethics and Study Population
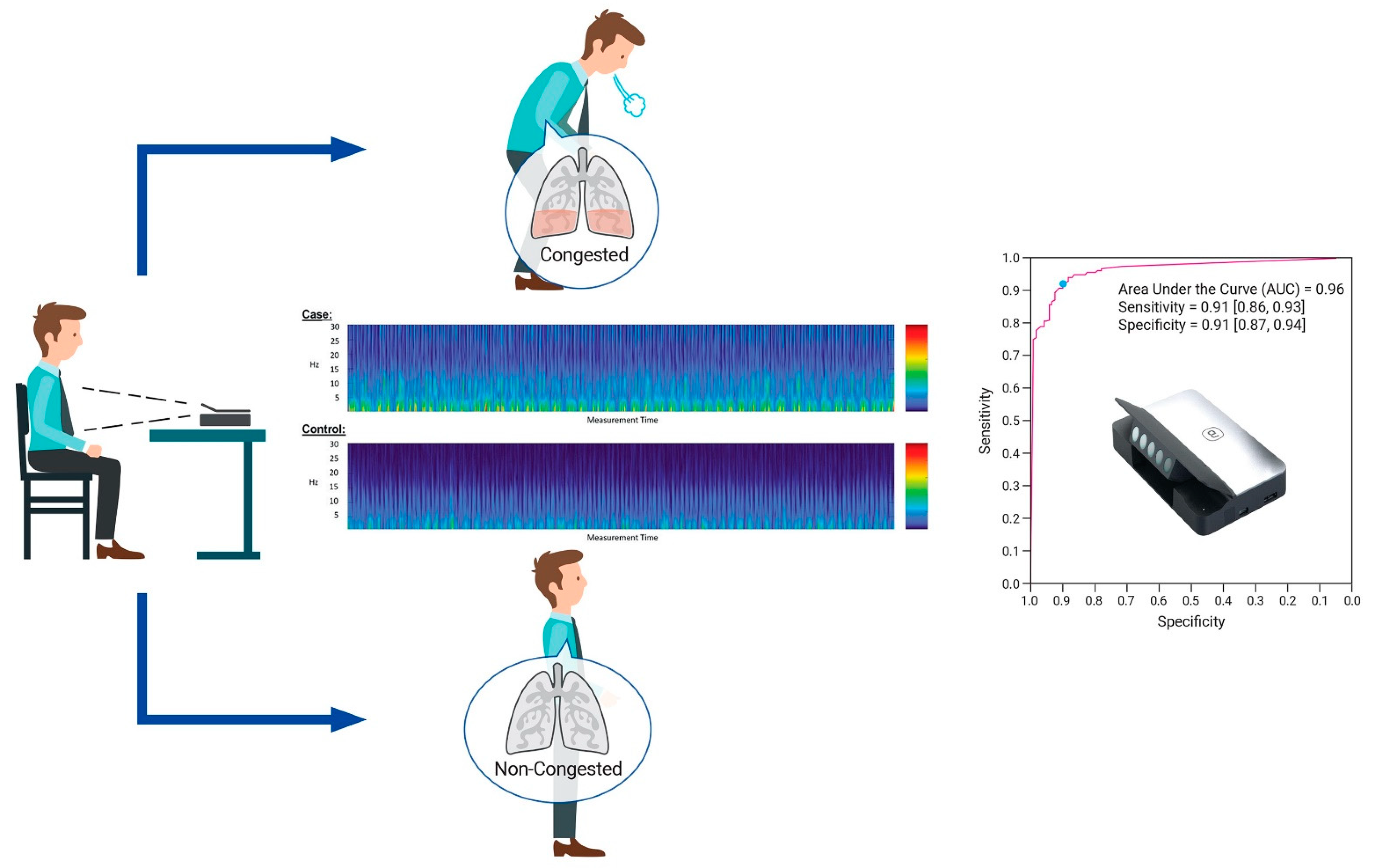
2.2. Study Devices
2.3. Procedure and Test Methods
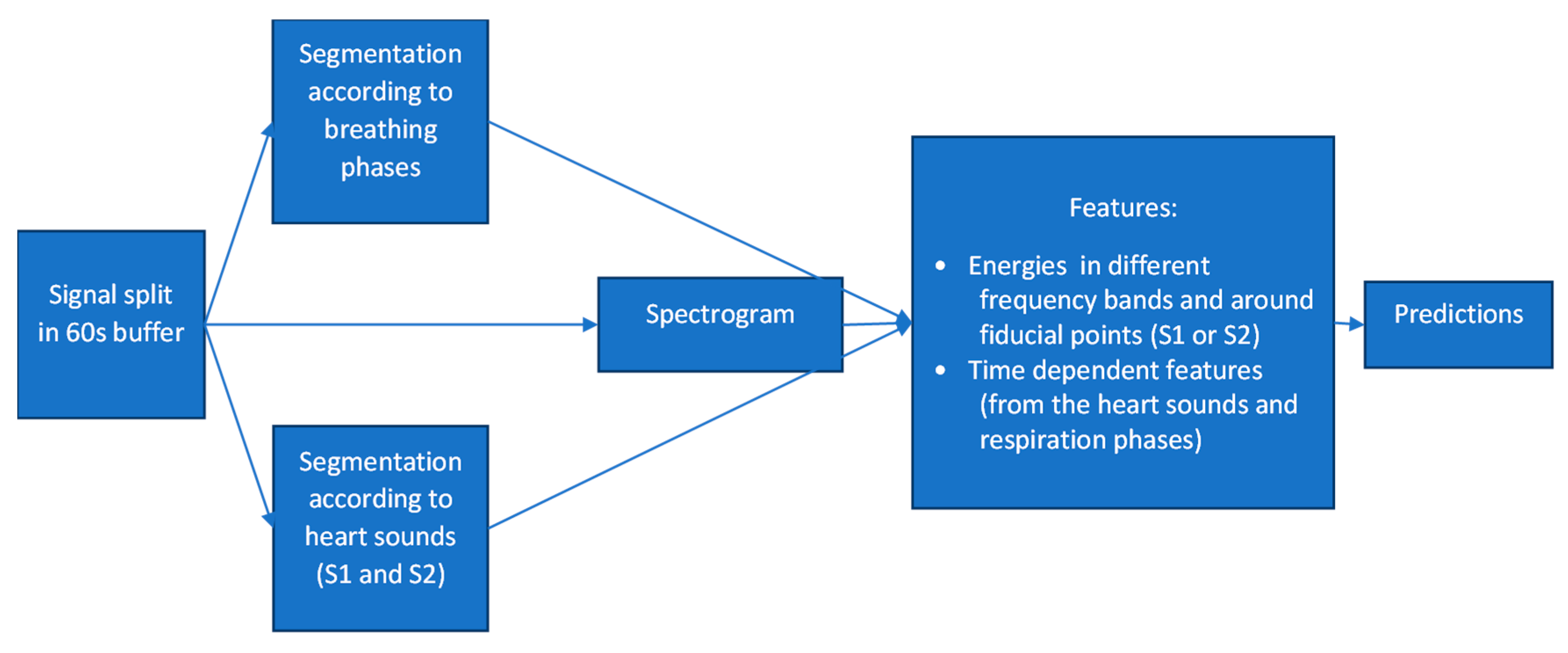
3. The Algorithm’s Diagnostic Accuracy: Development and Evaluation
4. Covariate Effects and Additional Statistical Considerations
5. Results
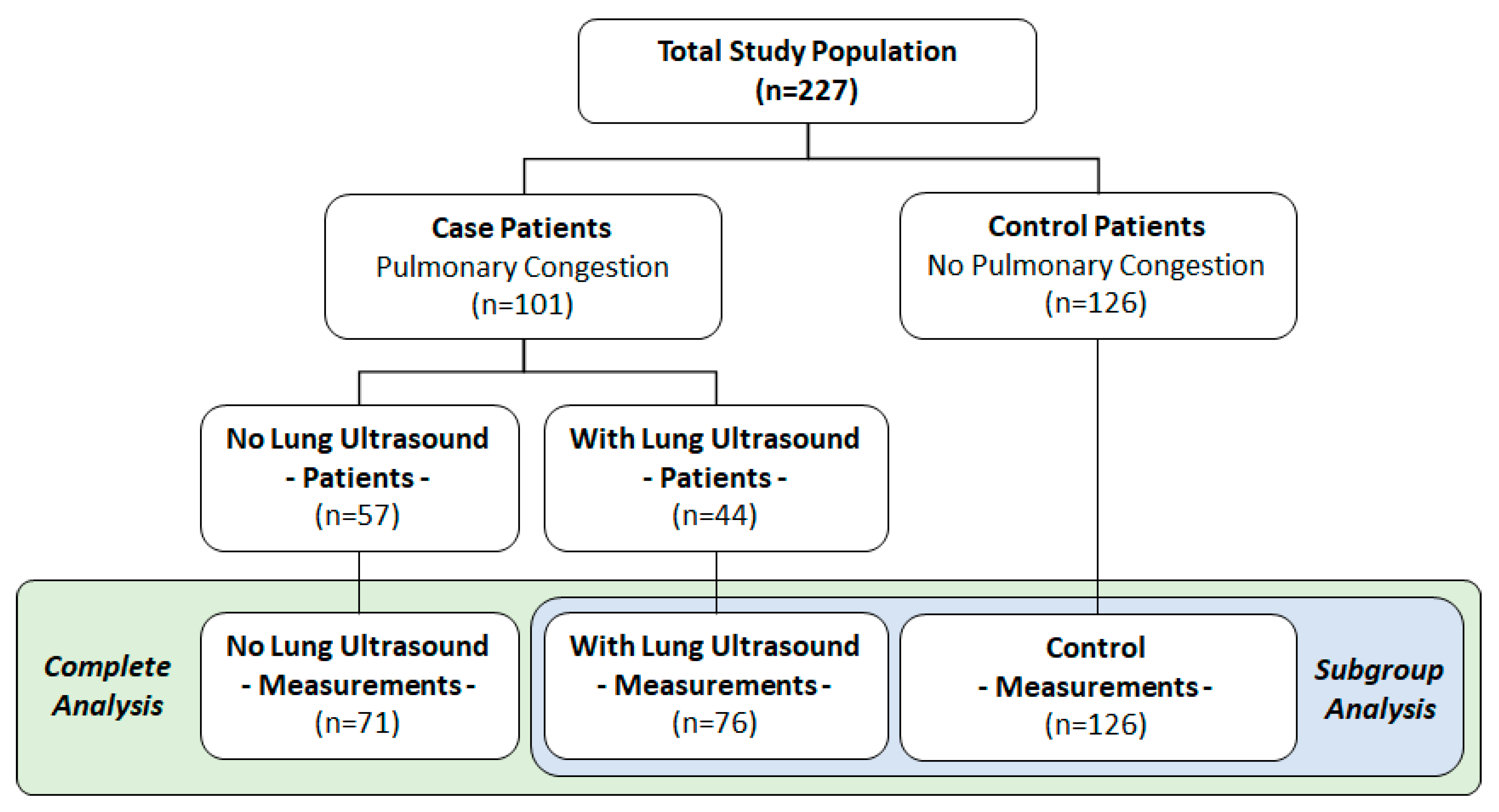
5.1. Study Population
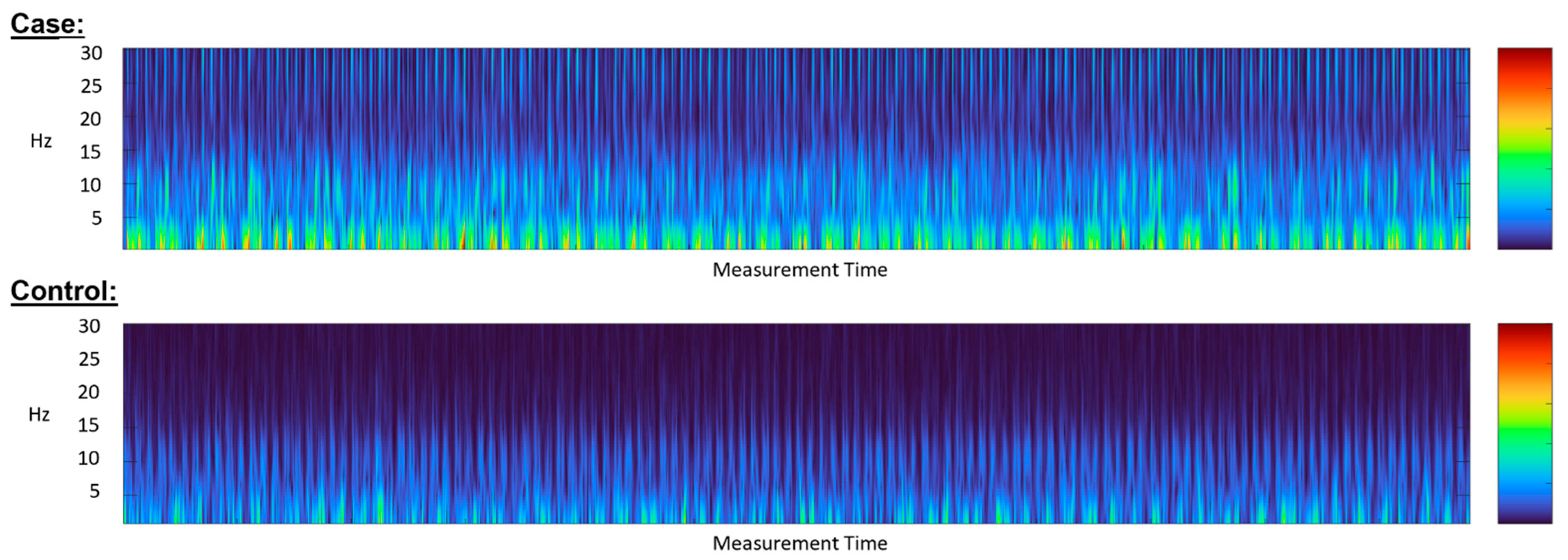
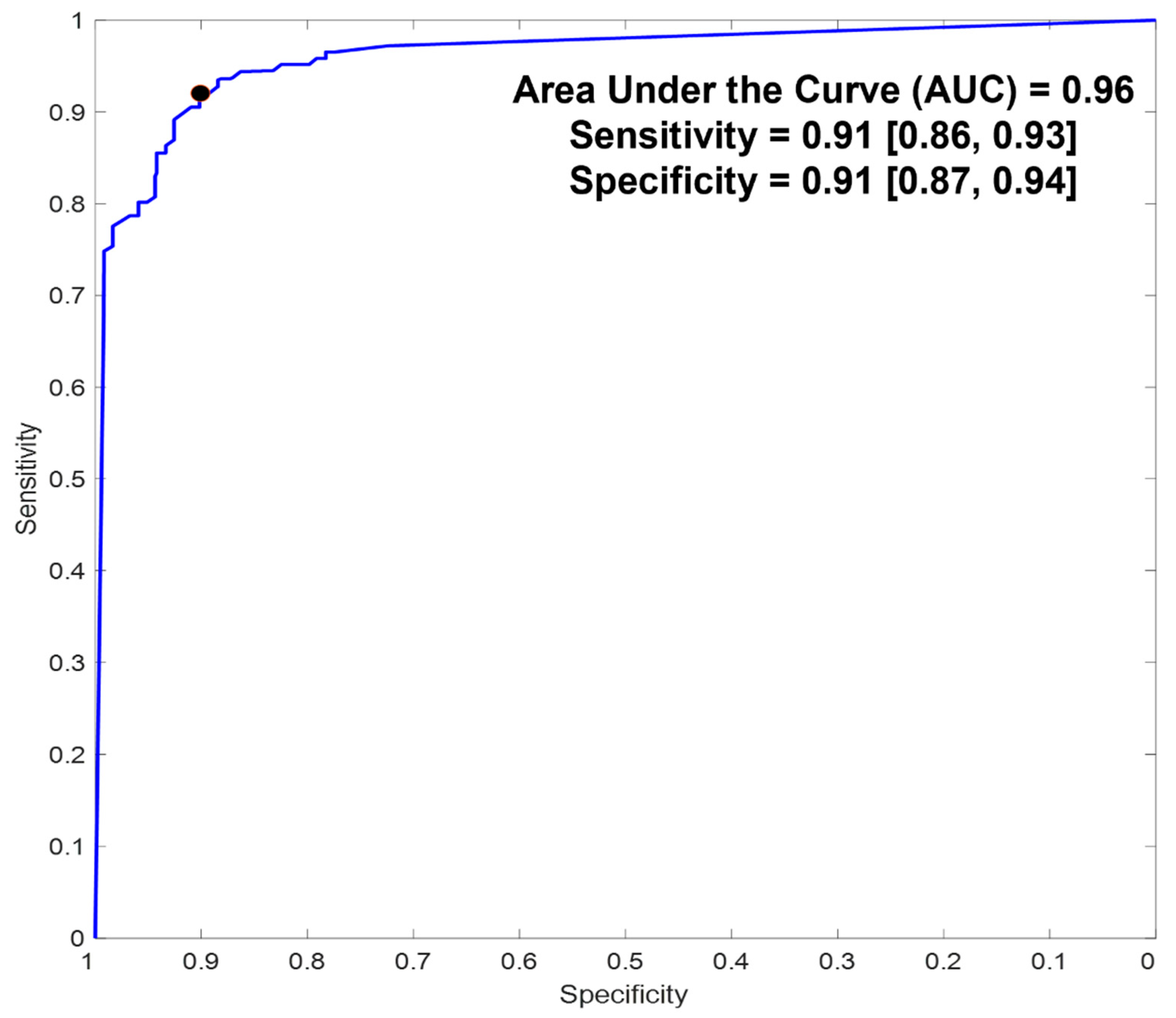
5.2. Diagnostic Accuracy
5.3. Effect of Measurement Duration on Algorithm’s Performance
5.4. Covariate Effects
5.5. Subgroup Analysis
5.6. The Potential Effects of Inconclusive Measurements: Analysis
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Tong, X.; King, R.J.; Loustalot, F.; Hong, Y.; Ritchey, M.D. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ. Heart Fail. 2018, 11, e004873. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.S.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.J.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Fida, N.; Piña, I.L. Trends in heart failure hospitalizations. Curr. Heart Fail. Rep. 2012, 9, 346–353. [Google Scholar] [CrossRef]
- Alghamdi, F.; Chan, M. Management of heart failure in the elderly. Curr. Opin. Cardiol. 2017, 32, 217–223. [Google Scholar] [CrossRef]
- Akintoye, E.; Briasoulis, A.; Egbe, A.; Dunlay, S.M.; Kushwaha, S.; Levine, D.; Afonso, L.; Mozaffarian, D.; Weinberger, J. National Trends in Admission and In-Hospital Mortality of Patients With Heart Failure in the United States (2001–2014). JAHA 2017, 6, e006955. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Sreenivasan, J.; Lateef, N.; Abougergi, M.S.; Greene, S.J.; Ahmad, T.; Anker, S.D.; Fonarow, G.C.; Butler, J. Trends in 30- and 90-Day Readmission Rates for Heart Failure. Circ. Heart Fail. 2021, 14, e008335. [Google Scholar] [CrossRef]
- Platz, E.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.F.; Lee, M.M.Y.; Merz, A.A.; Silverman, M.; Swamy, V.; et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC Heart Fail. 2019, 7, 849–858. [Google Scholar] [CrossRef]
- Givertz, M.M.; Stevenson, L.W.; Costanzo, M.R.; Bourge, R.C.; Bauman, J.G.; Ginn, G.; Abraham, W.T.; CHAMPION Trial Investigators. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 1875–1886. [Google Scholar] [CrossRef]
- Schmier, J.K.; Ong, K.L.; Fonarow, G.C. Cost-Effectiveness of Remote Cardiac Monitoring with the CardioMEMS Heart Failure System. Clin. Cardiol. 2017, 40, 430–436. [Google Scholar] [CrossRef]
- Walton, A.S.; Krum, H. The Heartpod implantable heart failure therapy system. Heart Lung Circ. 2005, 14 (Suppl. 2), S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Veenis, J.F.; Radhoe, S.P.; Hooijmans, P.; Brugts, J.J. Remote Monitoring in Chronic Heart Failure Patients: Is Non-Invasive Remote Monitoring the Way to Go? Sensors 2021, 21, 887. [Google Scholar] [CrossRef] [PubMed]
- Faragli, A.; Abawi, D.; Quinn, C.; Cvetkovic, M.; Schlabs, T.; Tahirovic, E.; Düngen, H.-D.; Pieske, B.; Kelle, S.; Edelmann, F.; et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail. Rev. 2021, 26, 1063–1080. [Google Scholar] [CrossRef]
- Mohebali, D.; Kittleson, M.M. Remote monitoring in heart failure: Current and emerging technologies in the context of the pandemic. Heart 2021, 107, 366–372. [Google Scholar] [CrossRef]
- Gensini, G.F.; Alderighi, C.; Rasoini, R.; Mazzanti, M.; Casolo, G. Value of Telemonitoring and Telemedicine in Heart Failure Management. Card. Fail. Rev. 2017, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Sadeh, B.; Merdler, I.; Sadon, S.; Lupu, L.; Borohovitz, A.; Ghantous, E.; Taieb, P.; Granot, Y.; Goldstein, O.; Soriano, J.C.; et al. A Novel Contact-Free Atrial Fibrillation Monitor- A Pilot Study. Eur. Heart J. Digit. Health 2021, 3, 105–113. [Google Scholar] [CrossRef]
- Havakuk, O.; Sadeh, B.; Merdler, I.; Zalevsky, Z.; Garcia-Monreal, J.; Polani, S.; Arbel, Y. Validation of a novel contact-free heart and respiratory rate monitor. J. Med. Eng. Technol. 2021, 45, 344–350. [Google Scholar] [CrossRef]
- Zalevsky, Z.; Beiderman, Y.; Margalit, I.; Gingold, S.; Teicher, M.; Mico, V.; Garcia, J. Simultaneous remote extraction of multiple speech sources and heart beats from secondary speckles pattern. Opt. Express 2009, 17, 21566. [Google Scholar] [CrossRef] [Green Version]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Schapire, R.E. A decision-theoretic generalization of on-line learning and an application to boosting. In Computational Learning Theory; Vitányi, P., Ed.; EuroCOLT 1995. Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 904. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Angraal, S.; Mortazavi, B.J.; Gupta, A.; Khera, R.; Ahmad, T.; Desai, N.R.; Jacoby, D.L.; Masoudi, F.A.; Spertus, J.A.; Krumholz, H.M. Machine Learning Prediction of Mortality and Hospitalization in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 12–21. [Google Scholar] [CrossRef]
- Brasier, N.; Raichle, C.J.; Dörr, M.; Becke, A.; Nohturfft, V.; Weber, S.; Bulacher, F.; Salomon, L.; Noah, T.; Birkemeyer, R.; et al. Detection of atrial fibrillation with a smartphone camera: First prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Proesmans, T.; Mortelmans, C.; Van Haelst, R.; Verbrugge, F.; Vandervoort, P.; Vaes, B. Mobile Phone-Based Use of the Photoplethysmography Technique to Detect Atrial Fibrillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR mHealth uHealth 2019, 7, e12284. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Lewis, E.F.; Uno, H.; Peck, J.; Pivetta, E.; Merz, A.A.; Hempel, D.; Wilson, C.; Frasure, S.E.; Jhund, P.S.; et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur. Heart J. 2016, 37, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jácome, C.; Aviles-Solis, J.C.; Uhre, Å.M.; Pasterkamp, H.; Melbye, H. Adventitious and Normal Lung Sounds in the General Population: Comparison of Standardized and Spontaneous Breathing. Respir. Care 2018, 63, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Inglis, A.J.; Nalos, M.; Sue, K.-H.; Hruby, J.; Campbell, D.M.; Braham, R.M.; Orde, S.R. Bedside lung ultrasound, mobile radiography and physical examination: A comparative analysis of diagnostic tools in the critically ill. Crit. Care Resusc. 2016, 18, 124. [Google Scholar] [PubMed]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C.; et al. On behalf of the EVEREST trial investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, D.; McCall, J.D.; Primack, B.A. Telehealth Protocol to Prevent Readmission Among High-Risk Patients with Congestive Heart Failure. Am. J. Med. 2017, 130, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Krzesiński, P.; Jankowska, E.A.; Siebert, J.; Galas, A.; Piotrowicz, K.; Stańczyk, A.; Siwołowski, P.; Gutknecht, P.; Chrom, P.; Murawski, P.; et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): A randomised controlled trial. Eur. J. Heart Fail. 2021, 24, 565–577. [Google Scholar] [CrossRef]
- Nachman, D.; Rahamim, E.; Kolben, Y.; Mengesha, B.; Elbaz-Greener, G.; Amir, O.; Asleh, R. In Search of Clinical Impact: Advanced Monitoring Technologies in Daily Heart Failure Care. J. Clin. Med. 2021, 10, 4692. [Google Scholar] [CrossRef]
- Wang, J.T.; Frishman, W.H. Pulmonary Pressure Monitoring for Patients with Heart Failure. Cardiol. Rev. 2017, 25, 53–58. [Google Scholar] [CrossRef]
- Gardner, R.S.; Singh, J.P.; Stancak, B.; Nair, D.G.; Cao, M.; Schulze, C.; Thakur, P.H.; An, Q.; Wehrenberg, S.; Hammill, E.F.; et al. HeartLogic Multisensor Algorithm Identifies Patients during Periods of Significantly Increased Risk of Heart Failure Events: Results From the MultiSENSE Study. Circ. Heart Fail. 2018, 11, e004669. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.-C.; Palot, A.; Patout, M. Technological advances in home non-invasive ventilation monitoring: Reliability of data and effect on patient outcomes. Respirology 2019, 24, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, E130. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Azzam, Z.S.; Gaspar, T.; Faranesh-Abboud, S.; Andria, N.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Validation of remote dielectric sensing (ReDSTM) technology for quantification of lung fluid status: Comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int. J. Cardiol. 2016, 221, 841–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drews, T.E.I.; Laukkanen, J.; Nieminen, T. Non-invasive home telemonitoring in patients with decompensated heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 3696–3708. [Google Scholar] [CrossRef]
- Brahmbhatt, D.H.; Ross, H.J.; Moayedi, Y. Digital Technology Application for Improved Responses to Health Care Challenges: Lessons Learned from COVID-19. Can. J. Cardiol. 2021, 38, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sammour, Y.; Spertus, J.A.; Austin, B.A.; Magalski, A.; Gupta, S.K.; Shatla, I.; Dean, E.; Kennedy, K.F.; Jones, P.G.; Nassif, M.E.; et al. Outpatient Management of Heart Failure during the COVID-19 Pandemic after Adoption of a Telehealth Model. JACC Heart Fail. 2021, 9, 916–924. [Google Scholar] [CrossRef]
- Miller, J.C.; Skoll, D.; Saxon, L.A. Home Monitoring of Cardiac Devices in the Era of COVID-19. Curr. Cardiol. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Bertagnin, E.; Greco, A.; Bottaro, G.; Zappulla, P.; Romanazzi, I.; Russo, M.D.; Lo Presti, M.; Valenti, N.; Sollano, G.; Calvi, V. Remote monitoring for heart failure management during COVID-19 pandemic. Int. J. Cardiol. Heart Vasc. 2021, 32, 100724. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Codina, P.; Abdul-Jawad Altisent, O.; Santiago, E.; Domingo, M.; Cediel, G.; Spitaleri, G.; Lupón, J. Advanced remote care for heart failure in times of COVID-19 using an implantable pulmonary artery pressure sensor: The new normal. Eur. Heart J. Suppl. 2020, 22, P29–P32. [Google Scholar] [CrossRef]
- Tersalvi, G.; Winterton, D.; Cioffi, G.M.; Ghidini, S.; Roberto, M.; Biasco, L.; Pedrazzini, G.; Dauw, J.; Ameri, P.; Vicenzi, M. Telemedicine in Heart Failure during COVID-19: A Step Into the Future. Front. Cardiovasc. Med. 2020, 7, 612818. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Fiuzat, M.; Psotka, M.A.; O’Connor, C.M. Heart Failure Collaboratory Statement on Remote Monitoring and Social Distancing in the Landscape of COVID-19. JACC Heart Fail. 2020, 8, 692–694. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total Population (n = 227) | Lung Congestion | |
|---|---|---|---|
| No (n = 126) | Yes (n = 101) | ||
| Age (years): mean (SD) | 68 (14) | 65 (15) | 73 (12) |
| Gender (males): n (%) | 148 (65%) | 76 (60%) | 72 (71%) |
| Gender (female): n (%) | 79 (35%) | 50 (40%) | 29 (29%) |
| Height (cm): mean (SD) | 167 (10) | 167 (10) | 167 (11) |
| Weight (Kg): mean (SD) | 79 (15) | 79 (16) | 80 (15) |
| BMI (Kg/m2): mean (SD) | 28 (6) | 28 (5) | 29 (6) |
| BMI ≥ 30: n (%) | 72 (32%) | 34 (27%) | 38 (38%) |
| Smoking: n (%) | 34 (15%) | 18 (14%) | 16 (16%) |
| Risk Factors (background): | |||
| Heart failure: n (%) | 154 (68%) | 76 (60%) | 78 (77%) |
| Hypertension: n (%) | 124 (55%) | 53 (42%) | 71 (70%) |
| Diabetes: n (%) | 79 (35%) | 22 (17%) | 57 (56%) |
| Stroke/TIA/thromboembolism: n (%) | 23 (10%) | 7 (6%) | 16 (16%) |
| Vascular disease: n (%) | 114 (50%) | 56 (44%) | 58 (57%) |
| Other Medical Conditions: | |||
| Respiratory conditions (COPD, asthma, pulmonary HTN, pulmonary embolism, lung cancer, etc.) | 33 (15%) | 11 (9%) | 22 (22%) |
| Valvular disorders (any type; mild, moderate or severe) | 91 (40%) | 29 (23%) | 62 (61%) |
| Medications: | |||
| Anticoagulants: n (%) | 71 (31%) | 23 (18%) | 48 (48%) |
| Antiplatelet: n (%) | 129 (57%) | 73 (58%) | 56 (55%) |
| ACEi: n (%) | 55 (24%) | 35 (28%) | 20 (20%) |
| ARB: n (%) | 31 (14%) | 11 (9%) | 20 (20%) |
| CCB: n (%) | 39 (17%) | 14 (11%) | 25 (25%) |
| Beta blockers: n (%) | 130 (57%) | 65 (52%) | 65 (64%) |
| Diuretics (any): n (%) | 112 (49%) | 36 (29%) | 76 (75%) |
| Antiarrhythmic: n (%) | 31 (14%) | 6 (5%) | 25 (25%) |
| Variable | Point Estimates (95% CI) | |
|---|---|---|
| Complete Study Population | Subgroup Analysis | |
| Sensitivity | 0.91 (0.86, 0.93) | 0.99 (0.96, 1) |
| Specificity | 0.91 (0.87, 0.94) | 0.93 (0.88, 0.95) |
| PPV Sample | 0.92 (0.88, 0.95) | 0.89 (0.83, 0.93) |
| NPV Sample | 0.90 (0.85, 0.93) | 0.99 (0.97, 1) |
| PPV 32% | 0.82 (0.77, 0.86) | 0.86 (0.80, 0.90) |
| NPV 32% | 0.95 (0.92, 0.97) | 0.99 (0.97, 1) |
| PLR | 10 (6.6, 15) | 9.9 (6.4, 15) |
| NLR | 0.1 (0.07, 0.16) | 0.015 (0.005, 0.049) |
| Covariate | p-Values | |
|---|---|---|
| Complete Study Population | Subgroup Analysis | |
| Gender | 0.430 | 0.313 |
| Age | 0.354 | 0.094 |
| Height | 0.245 | 0.235 |
| BMI | 0.260 | 0.303 |
| BMI ≥ 30 | 0.565 | 0.298 |
| Smoking | 0.552 | 0.626 |
| Risk Factors: | ||
| Heart Failure | 0.548 | 0.577 |
| Hypertension | 0.520 | 0.511 |
| Diabetes | 0.467 | 0.563 |
| Stroke/TIA/Thromboembolism | 0.584 | 0.811 |
| Vascular Disease | 0.456 | 0.552 |
| Other Medical Conditions: | ||
| Respiratory Condition (COPD, Asthma, Pulmonary HTN, Pulmonary Embolism, Lung Cancer, etc.) | 0.587 | 0.620 |
| Valve Disorder (Any Type; Mild, Moderate or Severe) | 0.273 | 0.186 |
| Medications: | ||
| Anticoagulant | 0.551 | 0.556 |
| Antiplatelet | 0.499 | 0.566 |
| ACEi | 0.572 | 0.594 |
| ARB | 0.439 | 0.430 |
| CCB | 0.450 | 0.547 |
| Beta Blockers | 0.365 | 0.399 |
| Diuretics | 0.409 | 0.412 |
| Antiarrhythmic | 0.622 | 0.655 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merdler, I.; Hochstadt, A.; Ghantous, E.; Lupu, L.; Borohovitz, A.; Zahler, D.; Taieb, P.; Sadeh, B.; Zalevsky, Z.; Garcia-Monreal, J.; et al. A Contact-Free Optical Device for the Detection of Pulmonary Congestion—A Pilot Study. Biosensors 2022, 12, 833. https://doi.org/10.3390/bios12100833
Merdler I, Hochstadt A, Ghantous E, Lupu L, Borohovitz A, Zahler D, Taieb P, Sadeh B, Zalevsky Z, Garcia-Monreal J, et al. A Contact-Free Optical Device for the Detection of Pulmonary Congestion—A Pilot Study. Biosensors. 2022; 12(10):833. https://doi.org/10.3390/bios12100833
Chicago/Turabian StyleMerdler, Ilan, Aviram Hochstadt, Eihab Ghantous, Lior Lupu, Ariel Borohovitz, David Zahler, Philippe Taieb, Ben Sadeh, Zeev Zalevsky, Javier Garcia-Monreal, and et al. 2022. "A Contact-Free Optical Device for the Detection of Pulmonary Congestion—A Pilot Study" Biosensors 12, no. 10: 833. https://doi.org/10.3390/bios12100833
APA StyleMerdler, I., Hochstadt, A., Ghantous, E., Lupu, L., Borohovitz, A., Zahler, D., Taieb, P., Sadeh, B., Zalevsky, Z., Garcia-Monreal, J., Shergei, M., Shatsky, M., Beck, Y., Polani, S., & Arbel, Y. (2022). A Contact-Free Optical Device for the Detection of Pulmonary Congestion—A Pilot Study. Biosensors, 12(10), 833. https://doi.org/10.3390/bios12100833






