Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare
Abstract
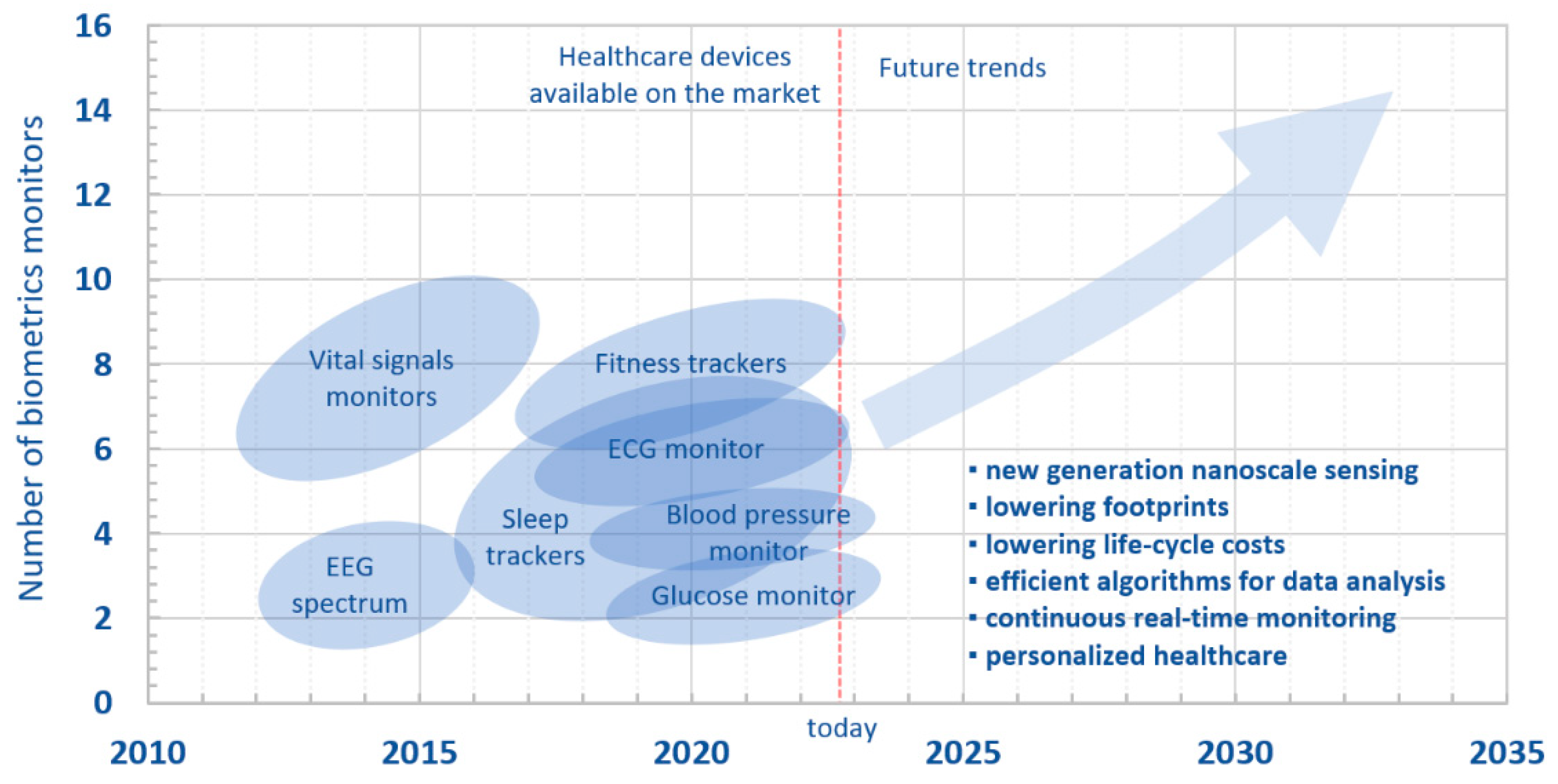
:1. Introduction
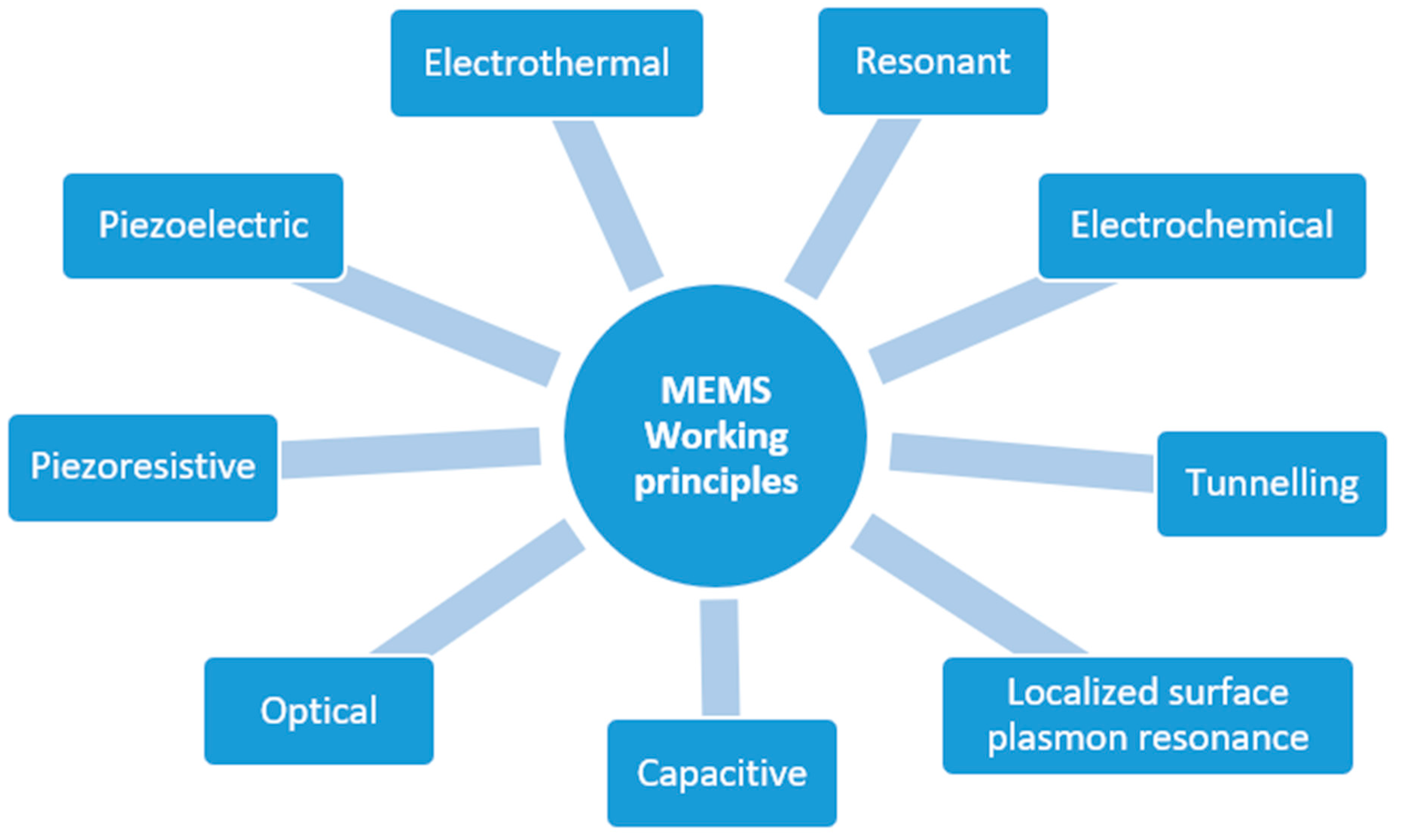
2. MEMS: Classification and Employment
- Automotive, e.g., collision and rollover sensing devices for airbags deployment; fuel level and vapor pressure detection; tire pressure; active suspension and braking monitoring; navigation systems;
- Healthcare, e.g., blood pressure, breathing, glucose and heartbeat sensing; auditory assessment; prosthetics; sleep monitoring; muscle stimulation; drug delivery systems; pacemakers;
- Industrial automation, e.g., machine health monitoring; predictive maintenance; automatic safety mechanisms activation; surveillance of production processes; goods tracking and logistics;
- Consumer electronics, e.g., temperature and vibration monitoring for PC, hard disks, printers, home appliances; gesture recognition for gaming controllers and smartphones; sports training devices;
- Environmental and agriculture, e.g., environmental sensing and weather forecasting, soil fertilization and irrigation planning; crop health monitoring; automated farming;
- Telecommunications, e.g., network devices monitoring; fault detection and localization; electrical and optical signal processing;
- Aerospace and defense, e.g., surveillance; satellite monitoring; UAV and remote system operations; weapons guidance; spacecraft and aircraft navigation.
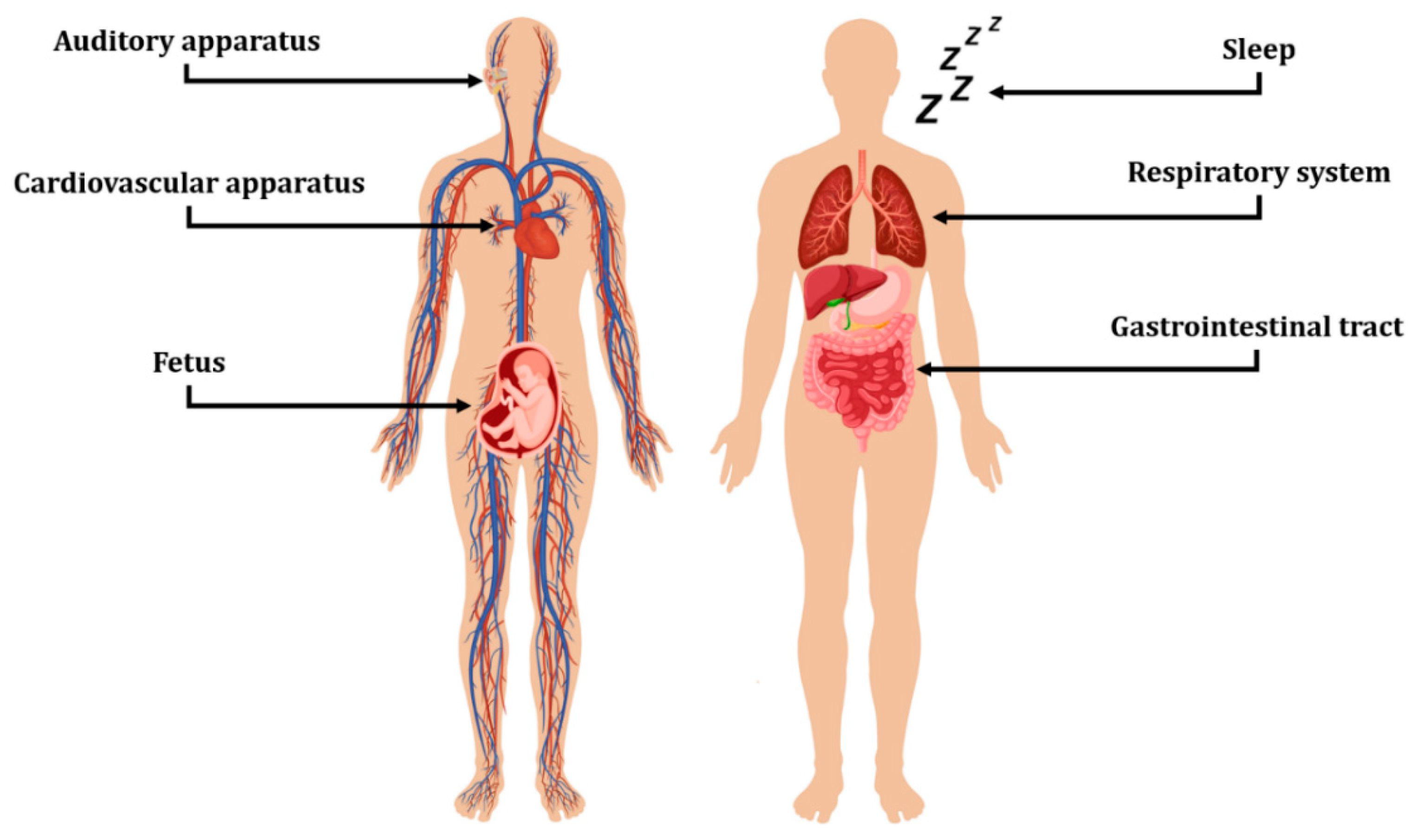
3. Sensing Devices in Healthcare
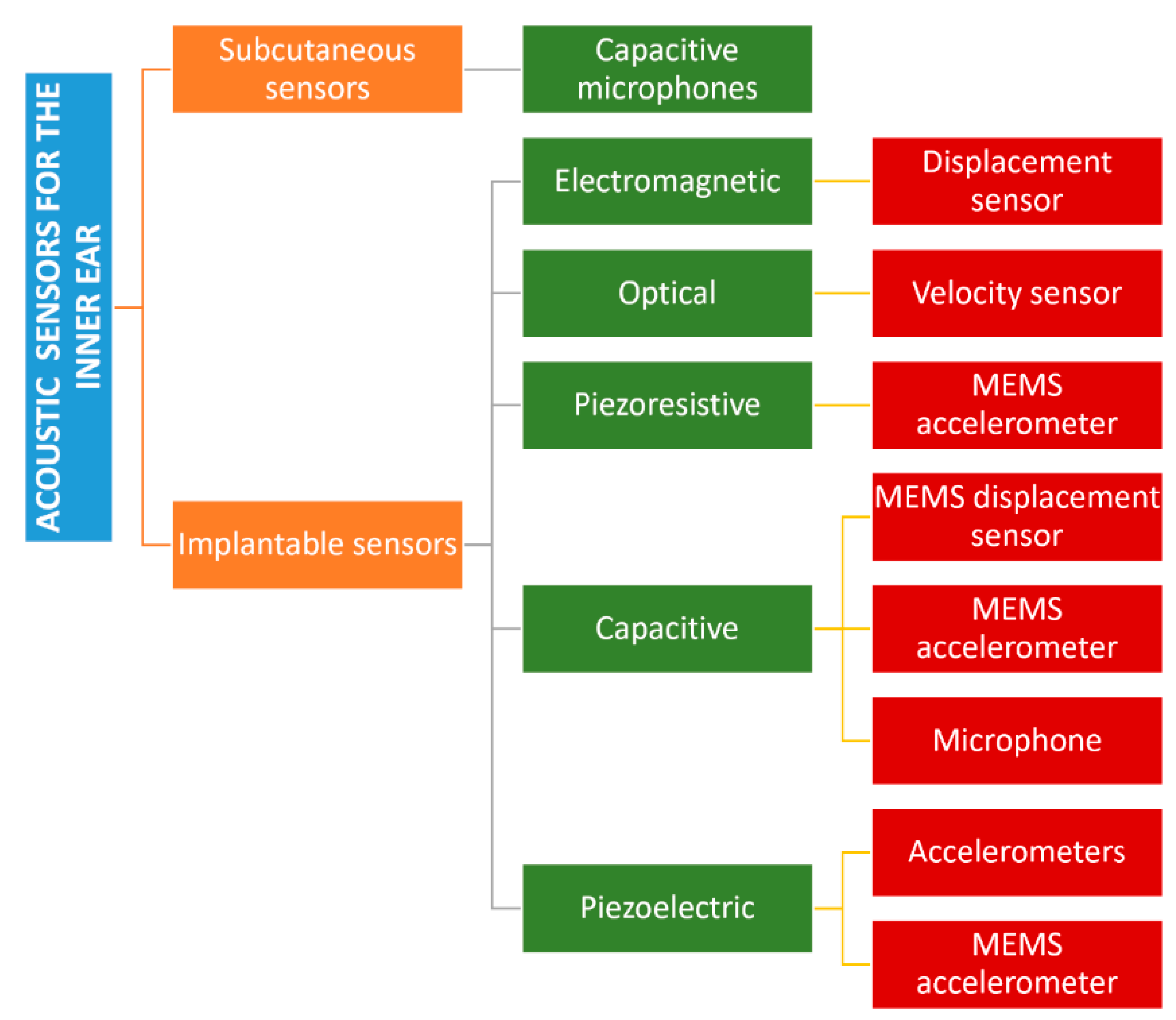
3.1. Auditory Apparatus
3.2. Cardiovascular Apparatus
3.3. Fetus
3.4. Respiratory System
3.5. Gastrointestinal Tract
3.6. Sleep Monitoring
4. Conclusions and Future Overlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, R.R., Jr.; Kurdila, A.J. Fundamentals of Structural Dynamics; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Stenfelt, S. Acoustic and Physiologic Aspects of Bone Conduction Hearing. Implant. Bone Conduct. Hear. Aids 2011, 71, 10–21. [Google Scholar]
- Puria, S. Middle Ear Biomechanics: Smooth Sailing. Acoust. Today 2020, 16, 27–35. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Li, H.; Ren, Y.; Zhang, G.; Wang, R.; Zhang, X.; Zhang, T.; Zhang, L.; Cui, J.; Xu, Q.D.; Duan, S. Design of a High SNR Electronic Heart Sound Sensor Based on a MEMS Bionic Hydrophone. AIP Adv. 2019, 9, 015005. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Kim, E.G.; Cao, G.; Liu, S.; Xu, Y. Physiological Acoustic Sensing Based on Accelerometers: A Survey for Mobile Healthcare. Ann. Biomed. Eng. 2014, 42, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Gad-el-Hak, M. The MEMS Handbook; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Du Plessis, M. Sensors, MEMS and Electro-Optical Systems; SPIE Press: Bellingham, WA, USA, 2014. [Google Scholar]
- Zhu, J.; Liu, X.; Shi, Q.; He, T.; Sun, Z.; Guo, X.; Liu, W.; Sulaiman, O.B.; Dong, B.; Lee, C. Development Trends and Perspectives of Future Sensors and MEMS/NEMS. Micromachines 2019, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Adams, T.M.; Layton, R.A. MEMS Transducers—An Overview of How They Work. Introd. MEMS 2010, 1, 167–210. [Google Scholar]
- Mishra, M.K.; Dubey, V.; Mishra, P.M.; Khan, I. MEMS Technology: A Review. J. Eng. Res. Rep. 2019, 4, 1–24. [Google Scholar] [CrossRef]
- Caliò, R.; Rongala, U.; Camboni, D.; Milazzo, M.; Stefanini, C.; de Petris, G.; Oddo, C. Piezoelectric Energy Harvesting Solutions. Sensors 2014, 14, 4755–4790. [Google Scholar] [CrossRef] [Green Version]
- Piriyanont, B.; Moheimani, S.O.R. MEMS Rotary Microgripper with Integrated Electrothermal Force Sensor. J. Microelectromechan. Syst. 2014, 23, 1249–1251. [Google Scholar] [CrossRef]
- Zhao, C.; Montaseri, M.H.; Wood, G.S.; Pu, S.H.; Seshia, A.A.; Kraft, M. A Review on Coupled MEMS Resonators for Sensing Applications Utilizing Mode Localization. Sens. Actuators A Phys. 2016, 249, 93–111. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- DiLella, D.; Whitman, L.J.; Colton, R.J.; Kenny, T.W.; Kaiser, W.J.; Vote, E.C.; Podosek, J.A.; Miller, L.M. A Micromachined Magnetic-Field Sensor Based on an Electron Tunneling Displacement Transducer. Sens. Actuators A Phys. 2000, 86, 8–20. [Google Scholar] [CrossRef]
- Alim, N.; Uddin, M.N. Surface Plasmon Resonance Biosensor in Healthcare Application. In Proceedings of the 2017 IEEE Region 10 Symposium (TENSYMP), Cochin, India, 14–16 July 2017; pp. 1–4. [Google Scholar]
- Hurtado-Aviles, E.A.; Torres, J.A.; Trejo-Valdez, M.; Urriolagoitia-Sosa, G.; Villalpando, I.; Torres-Torres, C. Acousto-Plasmonic Sensing Assisted by Nonlinear Optical Interactions in Bimetallic Au-Pt Nanoparticles. Micromachines 2017, 8, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrnegar, M.M.; Darbari, S.; Farshi, M.K.M. Simulating a Graphene-Based Acousto-Plasmonic Biosensor to Eliminate the Interference of Surrounding Medium. Opt. Express 2022, 30, 15721–15734. [Google Scholar] [CrossRef]
- Hamza, M.E.; Othman, M.A.; Swillam, M.A. Plasmonic Biosensors. Biology 2022, 11, 621. [Google Scholar] [CrossRef]
- Wang, C.; Chen, F.; Wang, Y.; Sadeghpour, S.; Wang, C.; Baijot, M.; Esteves, R.; Zhao, C.; Bai, J.; Liu, H.; et al. Micromachined Accelerometers with Sub-μg/√ Hz Noise Floor: A Review. Sensors 2020, 20, 4054. [Google Scholar] [CrossRef]
- Dong, Y.; Zwahlen, P.; Nguyen, A.M.; Frosio, R.; Rudolf, F. Ultra-High Precision MEMS Accelerometer. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 695–698. [Google Scholar]
- Ozevin, D. MEMS Acoustic Emission Sensors. Appl. Sci. 2020, 10, 8966. [Google Scholar] [CrossRef]
- Gao, X.; Wen, J.; Wang, J.; Li, K. Broadband Acoustic Sensing with Optical Nanofiber Couplers Working at the Dispersion Turning Point. Sensors 2022, 22, 4940. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, J.; Sataloff, R.T. Hearing Loss; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Mota, C.; Danti, S.; D’Alessandro, D.; Trombi, L.; Ricci, C.; Puppi, D.; Dinucci, D.; Milazzo, M.; Stefanini, C.; Chiellini, F.; et al. Multiscale Fabrication of Biomimetic Scaffolds for Tympanic Membrane Tissue Engineering. Biofabrication 2015, 7, 025005. [Google Scholar] [CrossRef]
- Moscato, S.; Rocca, A.; D’Alessandro, D.; Puppi, D.; Gramigna, V.; Milazzo, M.; Stefanini, C.; Chiellini, F.; Petrini, M.; Berrettini, S.; et al. Tympanic Membrane Collagen Expression by Dynamically Cultured Human Mesenchymal Stromal Cell/Star-Branched Poly (ε-Caprolactone) Nonwoven Constructs. Appl. Sci. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Milazzo, M.; Danti, S.; Inglese, F.; van Vuuren, G.; Gramigna, V.; Bonsignori, G.; De Vito, A.; Bruschini, L.; Stefanini, C.; Berrettini, S. Ossicular Replacement Prostheses from Banked Bone with Ergonomic and Functional Geometry. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Muyshondt, P.G.G.; Carstensen, J.; Dirckx, J.J.J.; Danti, S.; Buehler, M.J. De Novo Topology Optimization of Total Ossicular Replacement Prostheses. J. Mech. Behav. Biomed. Mater. 2020, 103, 103541. [Google Scholar] [CrossRef] [Green Version]
- National Institute of on Deafness and Other Communication Disorders Quick Statistics about Hearing. Available online: https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing (accessed on 20 August 2022).
- Carlson, M.L.; Driscoll, C.L.W.; Gifford, R.H.; McMenomey, S.O. Cochlear Implantation: Current and Future Device Options. Otolaryngol. Clin. N. Am. 2012, 45, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-G. Cochlear Implants: Auditory Prostheses and Electric Hearing; Springer Science & Business Media: New York, NY, USA, 2004; Volume 20. [Google Scholar]
- Olson, E.S. Direct Measurement of Intra-Cochlear Pressure Waves. Nature 1999, 402, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Knisely, K.E. The Application of a Piezoelectric MEMS Cantilever Array as a Completely Implantable Cochlear Implant. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2014. [Google Scholar]
- Calero, D.; Paul, S.; Gesing, A.; Alves, F.; Cordioli, J.A. A Technical Review and Evaluation of Implantable Sensors for Hearing Devices. Biomed. Eng. Online 2018, 17, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenner, H.P.; Leysieffer, H. Total Implantation of the Implex TICA Hearing Amplifier Implant for High-Frequency Sensorineural Hearing Loss: The Tübingen University Experience. Otolaryngol. Clin. N. Am. 2001, 34, 417–446. [Google Scholar] [CrossRef] [Green Version]
- Briggs, R.J.S.; Eder, H.C.; Seligman, P.M.; Cowan, R.S.C.; Plant, K.L.; Dalton, J.; Money, D.K.; Patrick, J.F. Initial Clinical Experience with a Totally Implantable Cochlear Implant Research Device. Otol. Neurotol. 2008, 29, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Pulcherio, J.O.B.; Bittencourt, A.G.; Burke, P.R.; da Monsanto, R.C.; De Brito, R.; Tsuji, R.K.; Bento, R.F. Carina®and Esteem®: A Systematic Review of Fully Implantable Hearing Devices. PLoS ONE 2014, 9, e110636. [Google Scholar] [CrossRef] [Green Version]
- Bruschini, L.; Forli, F.; Santoro, A.; Bruschini, P.; Berrettini, S. Fully Implantable Otologics MET CarinaTM Device for the Treatment of Sensorineural Hearing Loss. Preliminary Surgical and Clinical Results. Acta Otorhinolaryngol. Ital. 2009, 29, 79. [Google Scholar]
- Bruschini, L.; Berrettini, S.; Forli, F.; Murri, A.; Cuda, D. The Carina© Middle Ear Implant: Surgical and Functional Outcomes. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3631–3640. [Google Scholar] [CrossRef]
- Jung, E.S.; Shin, D.H.; Seong, K.W.; Lee, J.H.; Lee, J.W.; Cho, H.S.; Kim, M.N.; Cho, J.H. Measurement of Directivity for the Design of an Implantable Microphone Implanted under an Artificial Skin Model. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 297–300. [Google Scholar]
- Maniglia, A.J.; Murray, G.; Arnold, J.E.; Ko, W.H. Bioelectronic Microphone Options for a Totally Implantable Hearing Device for Partial and Total Hearing Loss. Otolaryngol. Clin. N. Am. 2001, 34, 469–483. [Google Scholar] [CrossRef]
- Vujanic, A.; Pavelka, R.; Adamovic, N.; Kment, C.; Mitic, S.; Brenner, W.; Popovic, G. Development of a Totally Implantable Hearing Aid. In Proceedings of the 2002 23rd International Conference on Microelectronics. Proceedings (Cat. No. 02TH8595), Nis, Yugoslavia, 12–15 May 2002; Volume 1, pp. 235–238. [Google Scholar]
- Park, W.-T.; O’Connor, K.N.; Chen, K.-L.; Mallon, J.R.; Maetani, T.; Dalal, P.; Candler, R.N.; Ayanoor-Vitikkate, V.; Roberson, J.B.; Puria, S.; et al. Ultraminiature Encapsulated Accelerometers as a Fully Implantable Sensor for Implantable Hearing Aids. Biomed. Microdevices 2007, 9, 939–949. [Google Scholar] [CrossRef]
- Acar, C.; Shkel, A.M. Experimental Evaluation and Comparative Analysis of Commercial Variable-Capacitance MEMS Accelerometers. J. Micromech. Microeng. 2003, 13, 634. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.J.; Lu, T.J.; Fleck, N.A.; Spearing, S.M. MEMS Actuators and Sensors: Observations on Their Performance and Selection for Purpose. J. Micromech. Microeng. 2005, 15, S153. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Guo, J.; Megerian, C.A.; Young, D.J.; Ko, W.H. A Laboratory Study on a Capacitive Displacement Sensor as an Implant Microphone in Totally Implant Cochlear Hearing Aid Systems. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5691–5694. [Google Scholar]
- Ko, W.H.; Zhang, R.; Huang, P.; Guo, J.; Ye, X.; Young, D.J.; Megerian, C.A. Studies of MEMS Acoustic Sensors as Implantable Microphones for Totally Implantable Hearing-Aid Systems. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, M.A.; Young, D.J.; Semaan, M.; Megerian, C.A.; Ko, W.H. MEMS Middle Ear Acoustic Sensor for a Fully Implantable Cochlear Prosthesis. In Proceedings of the 2007 IEEE 20th International Conference on Micro Electro Mechanical Systems (MEMS), Hyogo, Japan, 21–25 January 2007; pp. 11–14. [Google Scholar]
- Young, D.J.; Zurcher, M.A.; Semaan, M.; Megerian, C.A.; Ko, W.H. MEMS Capacitive Accelerometer-Based Middle Ear Microphone. IEEE Trans. Biomed. Eng. 2012, 59, 3283–3292. [Google Scholar] [CrossRef] [PubMed]
- Sachse, M.; Hortschitz, W.; Stifter, M.; Steiner, H.; Sauter, T. Design of an Implantable Seismic Sensor Placed on the Ossicular Chain. Med. Eng. Phys. 2013, 35, 1399–1405. [Google Scholar] [CrossRef]
- Woo, S.H.A.; Cho, H.-S.; Park, I.H.L.; Song, B.S. Implementation of Implantable Microphone in the Middle Ear Cavity and Telemetry Module. In Proceedings of the 5th 2012 Biomedical Engineering International Conference, Muang, Thailand, 5–7 December 2012; pp. 1–4. [Google Scholar]
- Javel, E.; Grant, I.L.; Kroll, K. In Vivo Characterization of Piezoelectric Transducers for Implantable Hearing Aids. Otol. Neurotol. 2003, 24, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.-M.; Thill, M.P.; Chantrain, G.; Gersdorff, M.; Deggouj, N. Esteem 2 Middle Ear Implant: Our Experience. Audiol. Neurotol. 2012, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Barbara, M.; Volpini, L.; Monini, S. Delayed Facial Nerve Palsy after Surgery for the Esteem®fully Implantable Middle Ear Hearing Device. Acta Otolaryngol. 2014, 134, 429–432. [Google Scholar] [CrossRef]
- Debeaupte, M.; Decullier, E.; Tringali, S.; Devèze, A.; Mom, T.; Darrouzet, V.; Truy, E. Evolution of the Reliability of the Fully Implantable Middle Ear Transducer over Successive Generations. Otol. Neurotol. 2015, 36, 625–630. [Google Scholar] [CrossRef]
- Koch, M.; Seidler, H.; Hellmuth, A.; Bornitz, M.; Lasurashvili, N.; Zahnert, T. Influence of the Middle Ear Anatomy on the Performance of a Membrane Sensor in the Incudostapedial Joint Gap. Hear. Res. 2013, 301, 35–43. [Google Scholar] [CrossRef]
- Koch, M.; Eβinger, T.M.; Bornitz, M.; Zahnert, T. Examination of a Mechanical Amplifier in the Incudostapedial Joint Gap: FEM Simulation and Physical Model. Sensors 2014, 14, 14356–14374. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-Y.; Na, G.; Chi, F.-L.; Jin, K.; Pan, T.-Z.; Gao, Z. Feasible Pickup from Intact Ossicular Chain with Floating Piezoelectric Microphone. Biomed. Eng. Online 2012, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Chen, Y.Z.; Chi, F.L.; Zhang, T.Y.; Xu, H.D.; Kang, H.Y.; Pan, T.Z. The Frequency Response of a Floating Piezoelectric Microphone for the Implantable Middle Ear Microphone. Laryngoscope 2013, 123, 1506–1513. [Google Scholar] [CrossRef]
- Jia, X.-H.; Gao, N.; Xu, X.; Wu, Y.-Z.; Kang, H.-Y.; Chi, F.-L. A New Floating Piezoelectric Microphone for the Implantable Middle Ear Microphone in Experimental Studies. Acta Otolaryngol. 2016, 136, 1248–1254. [Google Scholar] [CrossRef]
- Beker, L.; Zorlu, Ö.; Göksu, N.; Külah, H. Stimulating Auditory Nerve with MEMS Harvesters for Fully Implantable and Self-Powered Cochlear Implants. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1663–1666. [Google Scholar]
- Yip, M.; Jin, R.; Nakajima, H.H.; Stankovic, K.M.; Chandrakasan, A.P. A Fully-Implantable Cochlear Implant SoC with Piezoelectric Middle-Ear Sensor and Arbitrary Waveform Neural Stimulation. IEEE J. Solid-State Circuits 2014, 50, 214–229. [Google Scholar] [CrossRef]
- Yüksel, M.B.; Koyuncuoğlu, A.; Külah, H. Thin-Film PZT-Based Multi-Channel Acoustic MEMS Transducer for Cochlear Implant Applications. IEEE Sens. J. 2021, 22, 3052–3060. [Google Scholar] [CrossRef]
- Hearn, T.C.; Mazumdar, J.; Hubbard, R.; Eyster, G. Temporal and Heart-Size Effects in First-Heart-Sound Spectra. Med. Biol. Eng. Comput. 1979, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Moghimi, M.J.; Jeong, Y.; Gupta, D.; Inan, O.T.; Ayazi, F. Precision Wearable Accelerometer Contact Microphones for Longitudinal Monitoring of Mechano-Acoustic Cardiopulmonary Signals. NPJ Digit. Med. 2020, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, H.K.; Hall, W.D.; Hurst, J. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Santis, D.A. René Théophile Hyacinthe Laënnec (1781–1826). Two Hundred Years of the Stethoscope. A Brief Overview. Arch. Argent. Pediatr. 2020, 118, e444–e448. [Google Scholar] [CrossRef]
- Min, S.D.; Shin, H. A Localization Method for First and Second Heart Sounds Based on Energy Detection and Interval Regulation. J. Electr. Eng. Technol. 2015, 10, 2126–2134. [Google Scholar] [CrossRef]
- Carlson, G.M.; Siejko, K.Z. Third Heart Sound Activity Index for Heart Failure Monitoring. US 10/746874, 24 December 2003. [Google Scholar]
- Drazner, M.H.; Rame, J.E.; Stevenson, L.W.; Dries, D.L. Prognostic Importance of Elevated Jugular Venous Pressure and a Third Heart Sound in Patients with Heart Failure. N. Engl. J. Med. 2001, 345, 574–581. [Google Scholar] [CrossRef]
- Sarkar, M.; Madabhavi, I.; Niranjan, N.; Dogra, M. Auscultation of the Respiratory System. Ann. Thorac. Med. 2015, 10, 158–168. [Google Scholar] [CrossRef]
- Bohadana, A.; Izbicki, G.; Kraman, S.S. Fundamentals of Lung Auscultation. N. Engl. J. Med. 2014, 370, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Sanga, S.H.N. Chitosan Coating on Biodegradable Film Modified Surfaces by Corona Treatment. In Proceedings of the 12th Eco-Energy and Materials Science and Engineering Symposium, Krabi, Thailand, 11–14 June 2015. [Google Scholar]
- Ishmail, A.A.; Wing, S.; Ferguson, J.; Hutchinson, T.A.; Magder, S.; Flegel, K.M. Interobserver Agreement by Auscultation in the Presence of a Third Heart Sound in Patients with Congestive Heart Failure. Chest 1987, 91, 870–873. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.; Yang, D.; Chen, X.; Li, D.; Zhu, K.; Xie, J. Heart Sound Monitoring Based on a Piezoelectric Mems Acoustic Sensor. Proc. IEEE Int. Conf. Micro Electro Mech. Syst. 2021, 2021, 59–63. [Google Scholar] [CrossRef]
- Sharma, P.; Imtiaz, S.A.; Rodriguez-Villegas, E. Acoustic Sensing as a Novel Wearable Approach for Cardiac Monitoring at the Wrist. Sci. Rep. 2019, 9, 20079. [Google Scholar] [CrossRef] [Green Version]
- Giordano, N.; Knaflitz, M. A Novel Method for Measuring the Timing of Heart Sound Components through Digital Phonocardiography. Sensors 2019, 19, 1868. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Imtiaz, S.A.; Rodriguez-Villegas, E. An Algorithm for Heart Rate Extraction from Acoustic Recordings at the Neck. IEEE Trans. Biomed. Eng. 2018, 66, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.; Cheng, C.K.; Gupta, A.; Hsu, P.H.; Hsu, P.Y.; Liu, H.L.; Moffitt, A.; Ren, A.; Tsaur, I.; Wang, S. Cuff-Less Blood Pressure Monitoring with a 3-Axis Accelerometer. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6834–6837. [Google Scholar] [CrossRef]
- Qian, K.; Wu, C.; Xiao, F.; Zheng, Y.; Zhang, Y.; Yang, Z.; Liu, Y. Acousticcardiogram: Monitoring Heartbeats Using Acoustic Signals on Smart Devices. In Proceedings of the IEEE INFOCOM 2018-IEEE conference on computer communications, Honolulu, HI, USA, 15–19 April 2018; pp. 1574–1582. [Google Scholar]
- Cui, J.; Li, Y.; Yang, Y.; Shi, P.; Wang, B.; Wang, S.; Zhang, G.; Zhang, W. Design and Optimization of MEMS Heart Sound Sensor Based on Bionic Structure. Sens. Actuators A Phys. 2022, 333, 113188. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, H.-Y.; Chou, Y.-J.; Huang, N.; Chou, Y.-C.; Li, C.-P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine 2015, 94, e2160. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.; Harder, D. Non-Invasive Determination of Systolic Blood Pressure by Heart Sound Pattern Analysis. Clin. Phys. Physiol. Meas. 1992, 13, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Pibarot, P.; Honos, G.; Durand, L.G. Estimation of pulmonary artery pressure by spectral analysis of the second heart sound. J. Am. Coll. Cardiol. 1996, 78, 785–789. [Google Scholar] [CrossRef]
- Dennis, A.; Michaels, A.D.; Arand, P.; Ventura, D. Noninvasive Diagnosis of Pulmonary Hypertension Using Heart Sound Analysis. Comput. Biol. Med. 2010, 40, 758–764. [Google Scholar] [CrossRef]
- Zhang, X.Y.; MacPherson, E.; Zhang, Y.T. Relations between the Timing of the Second Heart Sound and Aortic Blood Pressure. IEEE Trans. Biomed. Eng. 2008, 55, 1291–1297. [Google Scholar] [CrossRef]
- Gemignani, V.; Bianchini, E.; Faita, F.; Giannoni, M.; Pasanisi, E.; Picano, E.; Bombardini, T. Assessment of Cardiologic Systole and Diastole Duration in Exercise Stress Tests with a Transcutaneous Accelerometer Sensor. Comput. Cardiol. 2008, 35, 153–156. [Google Scholar] [CrossRef]
- Imtiaz, M.S.; Shrestha, R.; Dhillon, T.; Yousuf, K.A.; Saeed, B.; Dinh, A.; Wahid, K. Correlation between Seismocardiogram and Systolic Blood Pressure. In Proceedings of the 2013 26th IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Regina, SK, Canada, 5–8 May 2013; pp. 8–11. [Google Scholar] [CrossRef]
- Browne, R.A.V.; Macêdo, G.A.D.; Cabral, L.L.P.; Oliveira, G.T.A.; Vivas, A.; Fontes, E.B.; Elsangedy, H.M.; Costa, E.C. Initial Impact of the COVID-19 Pandemic on Physical Activity and Sedentary Behavior in Hypertensive Older Adults: An Accelerometer-Based Analysis. Exp. Gerontol. 2020, 142, 111121. [Google Scholar] [CrossRef]
- Sehgal, S.; Chowdhury, A.; Rabih, F.; Gadre, A.; Park, M.M.; Li, M.; Wang, X.; Highland, K.B. Counting Steps: A New Way to Monitor Patients with Pulmonary Arterial Hypertension. Lung 2019, 197, 501–508. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, S.; Shao, S.; Zheng, S. Non-Invasive Diagnosis Methods of Coronary Disease Based on Wavelet Denoising and Sound Analyzing. Saudi J. Biol. Sci. 2017, 24, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Semmlow, J.; Rahalkar, K. Acoustic Detection of Coronary Artery Disease. Annu. Rev. Biomed. Eng. 2007, 9, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.; Nissen, L.; Schmidt, S.E.; Westra, J.; Andersen, I.T.; Nyegaard, M.; Madsen, L.H.; Knudsen, L.L.; Urbonaviciene, G.; Larsen, B.S.; et al. Advanced Heart Sound Analysis as a New Prognostic Marker in Stable Coronary Artery Disease. Eur. Hear. J.-Digit. Health 2021, 2, 279–289. [Google Scholar] [CrossRef]
- Kovács, F.; Kersner, N.; Kádár, K.; Hosszú, G. Computer Method for Perinatal Screening of Cardiac Murmur Using Fetal Phonocardiography. Comput. Biol. Med. 2009, 39, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Nowlan, N.C.; Vaidyanathan, R.; Shaw, C.J.; Lees, C.C. Fetal Movements as a Predictor of Health. Acta Obstet. Gynecol. Scand. 2016, 95, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Manning, F.A.; Morrison, I.; Lange, I.R.; Harman, C. Antepartum determination of fetal health: Composite biophysical profile scoring. Clin. Perinatol. 1982, 9, 285–296. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Balasubramanian, S.; Devasahayam, S.; Vaidyanathan, R.; Cherian, A.; Prasad, J.; Nowlan, N.C. Detection and Analysis of Fetal Movements Using an Acoustic Sensor-Based Wearable Monitor. In Proceedings of the 2020 7th International Conference on Information Science and Control Engineering (ICISCE), Changsha, China, 18–20 December 2020; pp. 512–516. [Google Scholar] [CrossRef]
- Kovács, F.; Horváth, C.; Balogh, Á.T.; Hosszú, G. Fetal Phonocardiography-Past and Future Possibilities. Comput. Methods Programs Biomed. 2011, 104, 19–25. [Google Scholar] [CrossRef]
- Urdal, J.; Engan, K.; Eftestøl, T.; Yarrot, L.B. Noise and Contraction Detection Using Fetal Heart Rate and Accelerometer Signals During Labour. In Proceedings of the 17th Scandinavian Conference on Health Informatics, Oslo, Norway, 12–13 November 2019; pp. 12–13. [Google Scholar]
- Ghosh, A.K.; Burniston, S.F.; Krentzel, D.; Roy, A.; Sheikh, A.S.; Siddiq, T.; Trinh, P.M.P.; Velazquez, M.M.; Vielle, H.T.; Nowlan, N.C.; et al. A Novel Fetal Movement Simulator for the Performance Evaluation of Vibration Sensors for Wearable Fetal Movement Monitors. Sensors 2020, 20, 6020. [Google Scholar] [CrossRef]
- Zakaria, N.D.; Numan, P.E.; Malarvili, M.B. Fetal Movements Recording System Using Accelerometer Sensor. ARPN J. Eng. Appl. Sci. 2018, 13, 1022–1032. [Google Scholar]
- Altini, M.; Rossetti, E.; Rooijakkers, M.; Penders, J.; Lanssens, D.; Grieten, L.; Gyselaers, W. Variable-Length Accelerometer Features and Electromyography to Improve Accuracy of Fetal Kicks Detection during Pregnancy Using a Single Wearable Device. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Orlando, FL, USA, 16–19 February 2017; pp. 221–224. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, X.; Koehl, L.; Tartare, G.; De Jonckheere, J.; Song, K. An IoT-Based Wearable System Using Accelerometers and Machine Learning for Fetal Movement Monitoring. In Proceedings of the 2019 IEEE International Conference on Industrial Cyber Physical Systems (ICPS), Taipei, Taiwan, 6–9 May 2019; pp. 299–304. [Google Scholar] [CrossRef]
- Yusenas, N.; Intaravichai, J.; Tirasuwannarat, P.; Ouypornkochagorn, T. Preliminary Study to Detect Fetal Movement by Using Acceleration Sensor and MEMS Microphone. In Proceedings of the 2018 15th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology (ECTI-CON), Chiang Rai, Thailand, 18–21 July 2018; pp. 290–292. [Google Scholar] [CrossRef]
- Lai, J.; Woodward, R.; Alexandrov, Y.; Ain Munnee, Q.; Lees, C.C.; Vaidyanathan, R.; Nowlan, N.C. Performance of a Wearable Acoustic System for Fetal Movement Discrimination. PLoS ONE 2018, 13, e0195728. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Lin, B.S.; Tsai, C.H.; Yang, C.T.; Lin, B.S. Design of Wearable Breathing Sound Monitoring System for Real-Time Wheeze Detection. Sensors 2017, 17, 171. [Google Scholar] [CrossRef] [Green Version]
- Schreur, H.J.W.; Sterk, P.J.; Vanderschoot, J.; Van Klink, H.C.J.; Van Vollenhoven, E.; Dijkman, J.H. Lung Sound Intensity in Patients with Emphysema and in Normal Subjects at Standardised Airfiows. Thorax 1992, 47, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baughman, R.P.; Loudon, R.G. Lung Sound Analysis for Continuous Evaluation of Airflow Obstruction in Asthma. Chest 1985, 88, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, N. Monitoring and analysis of lung sounds remotely. Int. J. COPD 2011, 6, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amper-West, M.; Saatchi, R.; Barker, N.; Elphick, H. Respiratory Sound Analysis as a Diagnosis Tool for Breathing Disorders. In Proceedings of the 32nd International Congress and Exhibition on Condition Monitoring and Diagnostic Engineering Management, Huddersfield, UK, 3–5 September 2019. [Google Scholar]
- Alsmadi, S.; Kahya, Y.P. Design of a DSP-Based Instrument for Real-Time Classification of Pulmonary Sounds. Comput. Biol. Med. 2008, 38, 53–61. [Google Scholar] [CrossRef]
- Kandaswamy, A.; Kumar, C.S.; Ramanathan, R.P.; Jayaraman, S.; Malmurugan, N. Neural Classification of Lung Sounds Using Wavelet Coefficients. Comput. Biol. Med. 2004, 34, 523–537. [Google Scholar] [CrossRef]
- Troncoso, Á.; Ortega, J.A.; Seepold, R.; Madrid, N.M. Non-Invasive Devices for Respiratory Sound Monitoring. Procedia Comput. Sci. 2021, 192, 3040–3048. [Google Scholar] [CrossRef]
- Rodgers, G.W.; Lau Young, J.B.; Desaive, T.; Shaw, G.M.; Chase, J.G. A Proof of Concept Study of Acoustic Sensing of Lung Recruitment during Mechanical Ventilation. Biomed. Signal Process. Control 2017, 32, 130–142. [Google Scholar] [CrossRef]
- Lozano-García, M.; Fiz, J.A.; Martínez-Rivera, C.; Torrents, A.; Ruiz-Manzano, J.; Jané, R. Novel Approach to Continuous Adventitious Respiratory Sound Analysis for the Assessment of Bronchodilator Response. PLoS ONE 2017, 12, e0171455. [Google Scholar] [CrossRef] [Green Version]
- Aras, S.; Öztürk, M.; Gangal, A. Automatic Detection of the Respiratory Cycle from Recorded, Single-Channel Sounds from Lungs. Turkish J. Electr. Eng. Comput. Sci. 2018, 26, 11–22. [Google Scholar] [CrossRef]
- Macias-Montero, J.G.; Sarraj, M.; Chmeissani, M.; Martinez, R.; Puigdengoles, C. A 2D 4 × 4 Channel Readout ASIC for Pixelated CdTe Detectors for Medical Imaging Applications. IEEE Trans. Nucl. Sci. 2015, 62, 2327–2333. [Google Scholar] [CrossRef] [Green Version]
- Emmanouilidou, D.; McCollum, E.D.; Park, D.E.; Elhilali, M. Computerized Lung Sound Screening for Pediatric Auscultation in Noisy Field Environments. IEEE Trans. Biomed. Eng. 2018, 65, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Y. An Ultra-Sensitive Wearable Accelerometer for Continuous Heart and Lung Sound Monitoring. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2012, 2012, 694–697. [Google Scholar] [CrossRef]
- Yuasa, Y.; Suzuki, K. Wearable Device for Monitoring Respiratory Phases Based on Breathing Sound and Chest Movement. Adv. Biomed. Eng. 2019, 8, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Mansour, K.B.; Guesneau, M.; Mansour, K.B. Monitoring of Various Breathing Rate with an Accelerometer. In Proceedings of the Jetsan2021: Telehealth and Biomedical Devices Study Conference 2021, Toulouse, France, 20–21 May 2021. [Google Scholar]
- He, C.; Tan, J.; Jian, X.; Zhong, G.; Cheng, L.; Lin, J. A Smart Flexible Vital Signs and Sleep Monitoring Belt Based on MEMS Triaxial Accelerometer and Pressure Sensor. IEEE Internet Things J. 2022, 9, 14126–14136. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Liu, H.; Liu, J.; Xiao, Y.; Wu, D.; Pan, X.; Zhou, C.; Lei, Y.; Liu, S. A Two-Stage Amplified PZT Sensor for Monitoring Lung and Heart Sounds in Discharged Pneumonia Patients. Microsyst. Nanoeng. 2021, 7, 55. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Okamoto, Y.; Takeshita, T.; Takei, Y.; Okada, H.; Nguyen, K.; Phan, H.-P.; Ichiki, M. Highly Sensitive Low-Frequency Acoustic Sensor Using Piezoresistive Cantilever. In Proceedings of the 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS), Tokyo, Japan, 9–13 January 2022; pp. 841–844. [Google Scholar] [CrossRef]
- Ni, X.; Ouyang, W.; Jeong, H.; Kim, J.T.; Tzaveils, A.; Mirzazadeh, A.; Wu, C.; Lee, J.Y.; Keller, M.; Mummidisetty, C.K.; et al. Automated, Multiparametric Monitoring of Respiratory Biomarkers and Vital Signs in Clinical and Home Settings for COVID-19 Patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2026610118. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, H.; Zeng, Y.; Xue, J.; Cao, X.; Wang, N.; Wang, Z. Intelligent Facemask Based on Triboelectric Nanogenerator for Respiratory Monitoring. Nano Energy 2022, 91, 106612. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Reyes, B.A.; Chon, K.H. Estimation of Respiratory Rates Using the Built-in Microphone of a Smartphone or Headset. IEEE J. Biomed. Health Inform. 2016, 20, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hirayama, K.; Sadamitsu, Y.; Toshimitsu, A.; Fujita, H.; Shin, S.; Tanigawa, T. Monitoring Sound to Quantify Snoring and Sleep Apnea Severity Using a Smartphone: Proof of Concept. J. Clin. Sleep Med. 2014, 10, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bokov, P.; Mahut, B.; Flaud, P.; Delclaux, C. Wheezing Recognition Algorithm Using Recordings of Respiratory Sounds at the Mouth in a Pediatric Population. Comput. Biol. Med. 2016, 70, 40–50. [Google Scholar] [CrossRef]
- Reyes, B.A.; Olvera-Montes, N.; Charleston-Villalobos, S.; González-Camarena, R.; Mejía-ávila, M.; Aljama-Corrales, T. A Smartphone-Based System for Automated Bedside Detection of Crackle Sounds in Diffuse Interstitial Pneumonia Patients. Sensors 2018, 18, 3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faezipour, M.; Abuzneid, A. Smartphone-Based Self-Testing of COVID-19 Using Breathing Sounds. Telemed. E-Health 2020, 26, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Chen, X.; Yang, D.; Li, D.; Zhu, K.; Guo, X.; Xie, J. Monitoring of Physiological Sounds with Wearable Device Based on Piezoelectric MEMS Acoustic Sensor. J. Micromech. Microeng. 2022, 32, 014001. [Google Scholar] [CrossRef]
- Mamun, K.A.A.; McFarlane, N. Integrated Real Time Bowel Sound Detector for Artificial Pancreas Systems. Sens. Bio-Sens. Res. 2016, 7, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.K.; Nowak, R.; Radzikowski, K.; Grulkowski, I.; Walkowiak, J. Automated Bowel Sound Analysis: An Overview. Sensors 2021, 21, 5294. [Google Scholar] [CrossRef]
- Du, X.; Allwood, G.; Mary Webberley, K.; Inderjeeth, A.J.; Osseiran, A.; Marshall, B.J. Noninvasive Diagnosis of Irritable Bowel Syndrome via Bowel Sound Features: Proof of Concept. Clin. Transl. Gastroenterol. 2019, 10, e00017. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, D.; Jin, P.; Zhang, Y.; Yang, Y.; Ma, Y.; Yang, A.; Fu, J.; Feng, X. A Flexible Skin-Mounted Wireless Acoustic Device for Bowel Sounds Monitoring and Evaluation. Sci. China Inf. Sci. 2019, 62, 202402. [Google Scholar] [CrossRef] [Green Version]
- Dagdeviren, C.; Javid, F.; Joe, P.; Von Erlach, T.; Bensel, T.; Wei, Z.; Saxton, S.; Cleveland, C.; Booth, L.; McDonnell, S.; et al. Flexible Piezoelectric Devices for Gastrointestinal Motility Sensing. Nat. Biomed. Eng. 2017, 1, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Baronetto, A.; Graf, L.S.; Fischer, S.; Neurath, M.F.; Amft, O. GastroDigitalShirt: A Smart Shirt for Digestion Acoustics Monitoring. In Proceedings of the ISWC ‘20: Proceedings of the 2020 International Symposium on Wearable Computers, Virtual, 12–16 September 2020; pp. 17–21. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Chen, S.; Fu, J.; Wu, D.; Yang, A.; Ma, Y.; Feng, X. Flexible Dual-Channel Digital Auscultation Patch with Active Noise Reduction for Bowel Sound Monitoring and Application. IEEE J. Biomed. Health Inform. 2022, 26, 2951–2962. [Google Scholar] [CrossRef]
- Moorcroft, W.H.; Belcher, P. Understanding Sleep and Dreaming; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Crivello, A.; Barsocchi, P.; Girolami, M.; Palumbo, F. The Meaning of Sleep Quality: A Survey of Available Technologies. IEEE Access 2019, 7, 167374–167390. [Google Scholar] [CrossRef]
- Lee-Chiong, T.L. Sleep: A Comprehensive Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [Green Version]
- Fourati, H.; Belkhiat, D.E.C. Multisensor Attitude Estimation: Fundamental Concepts and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Devani, N.; Pramono, R.X.A.; Imtiaz, S.A.; Bowyer, S.; Rodriguez-Villegas, E.; Mandal, S. Accuracy and Usability of AcuPebble SA100 for Automated Diagnosis of Obstructive Sleep Apnoea in the Home Environment Setting: An Evaluation Study. BMJ Open 2021, 11, e046803. [Google Scholar] [CrossRef]
- Narayan, S.; Shivdare, P.; Niranjan, T.; Williams, K.; Freudman, J.; Sehra, R. Noncontact Identification of Sleep-Disturbed Breathing from Smartphone-Recorded Sounds Validated by Polysomnography. Sleep Breath. 2019, 23, 269–279. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Deak, M.C.; Khosla, S.; Goldstein, C.A.; Yurcheshen, M.; Chiang, A.; Gault, D.; Kern, J.; O’Hearn, D.; Ryals, S.; et al. Evaluating Consumer and Clinical Sleep Technologies: An American Academy of Sleep Medicine Update. J. Clin. Sleep Med. 2021, 17, 2275–2282. [Google Scholar] [CrossRef]
- Kortelainen, J.M.; Mendez, M.O.; Bianchi, A.M.; Matteucci, M.; Cerutti, S. Sleep Staging Based on Signals Acquired through Bed Sensor. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 776–785. [Google Scholar] [CrossRef]
- Tuominen, J.; Peltola, K.; Saaresranta, T.; Valli, K. Sleep Parameter Assessment Accuracy of a Consumer Home Sleep Monitoring Ballistocardiograph Beddit Sleep Tracker: A Validation Study. J. Clin. Sleep Med. 2019, 15, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Luís-Ferreira, F.; Gião, J.; Sarraipa, J.; Jardim-Goncalves, R.; McManus, G.; O’Brien, P. Sleeping Movement Detection Towards Mental Health Indicators-A Review. In Proceedings of the 2020 IEEE International Conference on Engineering, Technology and Innovation (ICE/ITMC), Cardiff, UK, 15–17 June 2020; pp. 1–6. [Google Scholar]
- Henriksen, A.; Mikalsen, M.H.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Others Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e9157. [Google Scholar] [CrossRef]
- Altini, M.; Kinnunen, H. The Promise of Sleep: A Multi-Sensor Approach for Accurate Sleep Stage Detection Using the Oura Ring. Sensors 2021, 21, 4302. [Google Scholar] [CrossRef]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Grandner, M.A.; Rosenberger, M.E. Chapter 12: Actigraphic Sleep Tracking and Wearables: Historical Context, Scientific Applications and Guidelines, Limitations, and Considerations for Commercial Sleep Devices. In Sleep and Health; Grandner, M.A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ho, M.L.; Brass, S.D. Obstructive Sleep Apnea. Neurol. Int. 2011, 3, e15. [Google Scholar] [CrossRef] [Green Version]
- Roebuck, A.; Monasterio, V.; Gederi, E.; Osipov, M.; Behar, J.; Malhotra, A.; Penzel, T.; Clifford, G.D. A Review of Signals Used in Sleep Analysis. Physiol. Meas. 2013, 35, R1. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.E.; Cretu, E. Simple Heart Rate Monitoring System with a MEMS Gyroscope for Sleep Studies. In Proceedings of the 2018 IEEE 9th Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON), Vancouver, BC, Canada, 1–3 November 2018; pp. 61–67. [Google Scholar]
- Walch, O.; Huang, Y.; Forger, D.; Goldstein, C. Sleep Stage Prediction with Raw Acceleration and Photoplethysmography Heart Rate Data Derived from a Consumer Wearable Device. Sleep 2019, 42, zsz180. [Google Scholar] [CrossRef] [Green Version]
- Klum, M.; Urban, M.; Tigges, T.; Pielmus, A.-G.; Feldheiser, A.; Schmitt, T.; Orglmeister, R. Wearable Cardiorespiratory Monitoring Employing a Multimodal Digital Patch Stethoscope: Estimation of ECG, PEP, LVET and Respiration Using a 55 Mm Single-Lead ECG and Phonocardiogram. Sensors 2020, 20, 2033. [Google Scholar] [CrossRef] [Green Version]
- Yüzer, A.H.; Sümbül, H.; Polat, K. A Novel Wearable Real-Time Sleep Apnea Detection System Based on the Acceleration Sensor. Irbm 2020, 41, 39–47. [Google Scholar] [CrossRef]
- Jin, H.; Tao, X.; Dong, S.; Qin, Y.; Yu, L.; Luo, J.; Deen, M.J. Flexible Surface Acoustic Wave Respiration Sensor for Monitoring Obstructive Sleep Apnea Syndrome. J. Micromech. Microeng. 2017, 27, 115006. [Google Scholar] [CrossRef]
- Matar, G.; Lina, J.-M.; Carrier, J.; Kaddoum, G. Unobtrusive Sleep Monitoring Using Cardiac, Breathing and Movements Activities: An Exhaustive Review. IEEE Access 2018, 6, 45129–45152. [Google Scholar] [CrossRef]
- Jaworski, D.J.; Park, A.; Park, E.J. Internet of Things for Sleep Monitoring. IEEE Instrum. Meas. Mag. 2021, 24, 30–36. [Google Scholar] [CrossRef]
- Arlotto, P.; Grimaldi, M.; Naeck, R.; Ginoux, J.-M. An Ultrasonic Contactless Sensor for Breathing Monitoring. Sensors 2014, 14, 15371–15386. [Google Scholar] [CrossRef]
- Nandakumar, R.; Gollakota, S.; Watson, N. Contactless Sleep Apnea Detection on Smartphones. In Proceedings of the 13th Annual International Conference on Mobile Systems, Applications, and Services, Florence, Italy, 18–22 May 2015; pp. 45–57. [Google Scholar]
- LaBerge, S. Physiological Studies of Lucid Dreaming. In The Neuropsychology of Sleep and Dreaming; Antrobus, J.S., Bertini, M., Eds.; Lawrence Erlbaum Associates Location: Hillsdale, NH, USA, 1992. [Google Scholar]
- Mota-Rolim, S.A.; Pavlou, A.; Nascimento, G.C.; Fontenele-Araujo, J.; Ribeiro, S. Portable Devices to Induce Lucid Dreams—Are They Reliable? Front. Neurosci. 2019, 13, 428. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Auerbach, S.; Casey, K.R.; Kristo, D.; Maganti, R.; Ramar, K.; Zak, R.; Kartje, R. Position Paper for the Treatment of Nightmare Disorder in Adults: An American Academy of Sleep Medicine Position Paper. J. Clin. Sleep Med. 2018, 14, 1041–1055. [Google Scholar] [CrossRef] [Green Version]
- Rozo Forero, F.A.; Others Aurora Headband. Technical Report Design Project 2 - IBIO 3870 Biomedical Engineering Department; Universidad de los Andes: Bogotá, CO, USA, 20 May 2018. [Google Scholar]
- Comini, E. Achievements and Challenges in Sensor Devices. Front. Sens. 2021, 1, 607063. [Google Scholar] [CrossRef]
- Lazareck, L.J.; Moussavi, Z.M.K. Classification of Normal and Dysphagic Swallows by Acoustical Means. IEEE Trans. Biomed. Eng. 2004, 51, 2103–2112. [Google Scholar] [CrossRef] [Green Version]
- Posatskiy, A.O.; Chau, T. The Effects of Motion Artifact on Mechanomyography: A Comparative Study of Microphones and Accelerometers. J. Electromyogr. Kinesiol. 2012, 22, 320–324. [Google Scholar] [CrossRef]


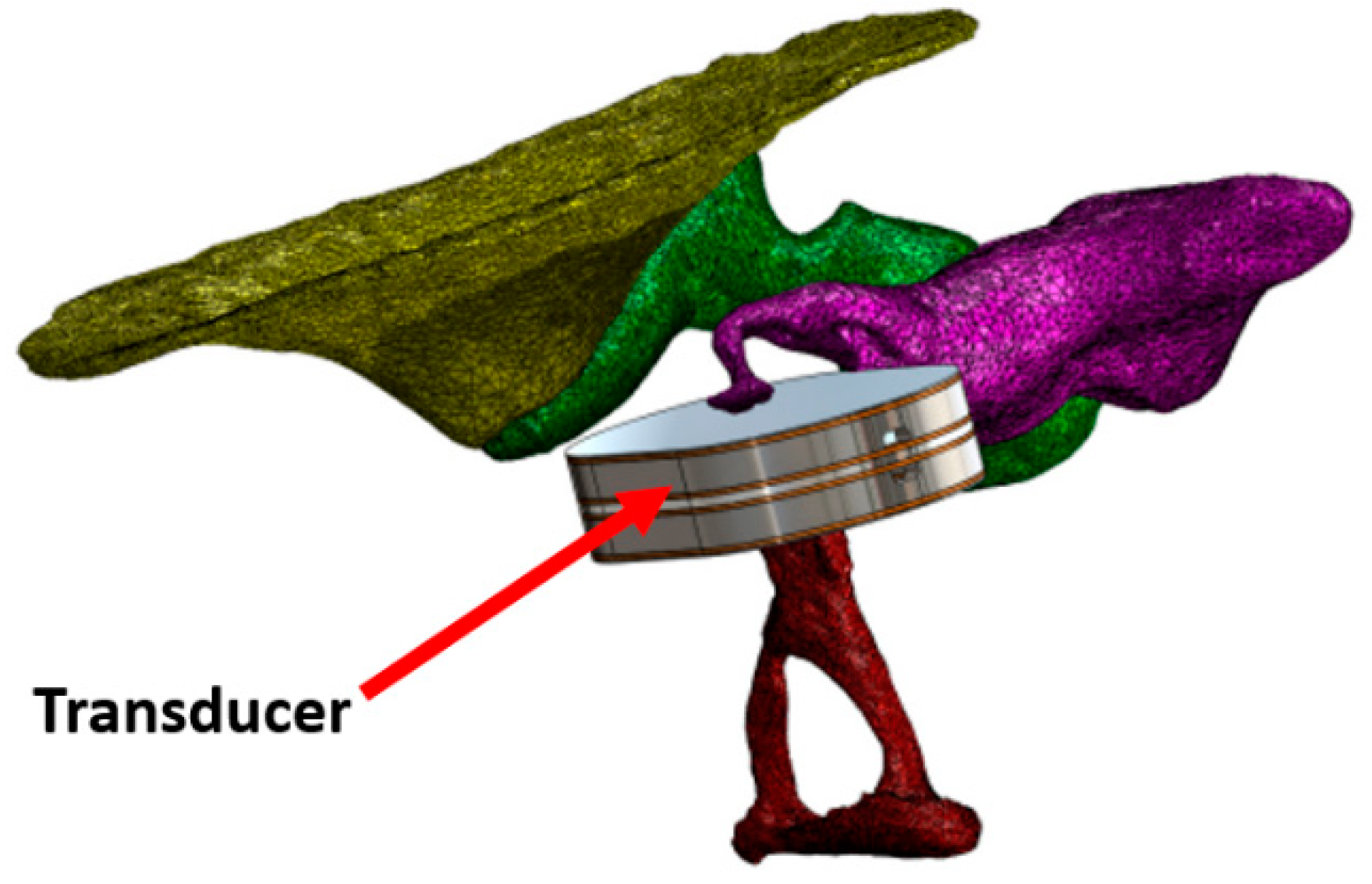
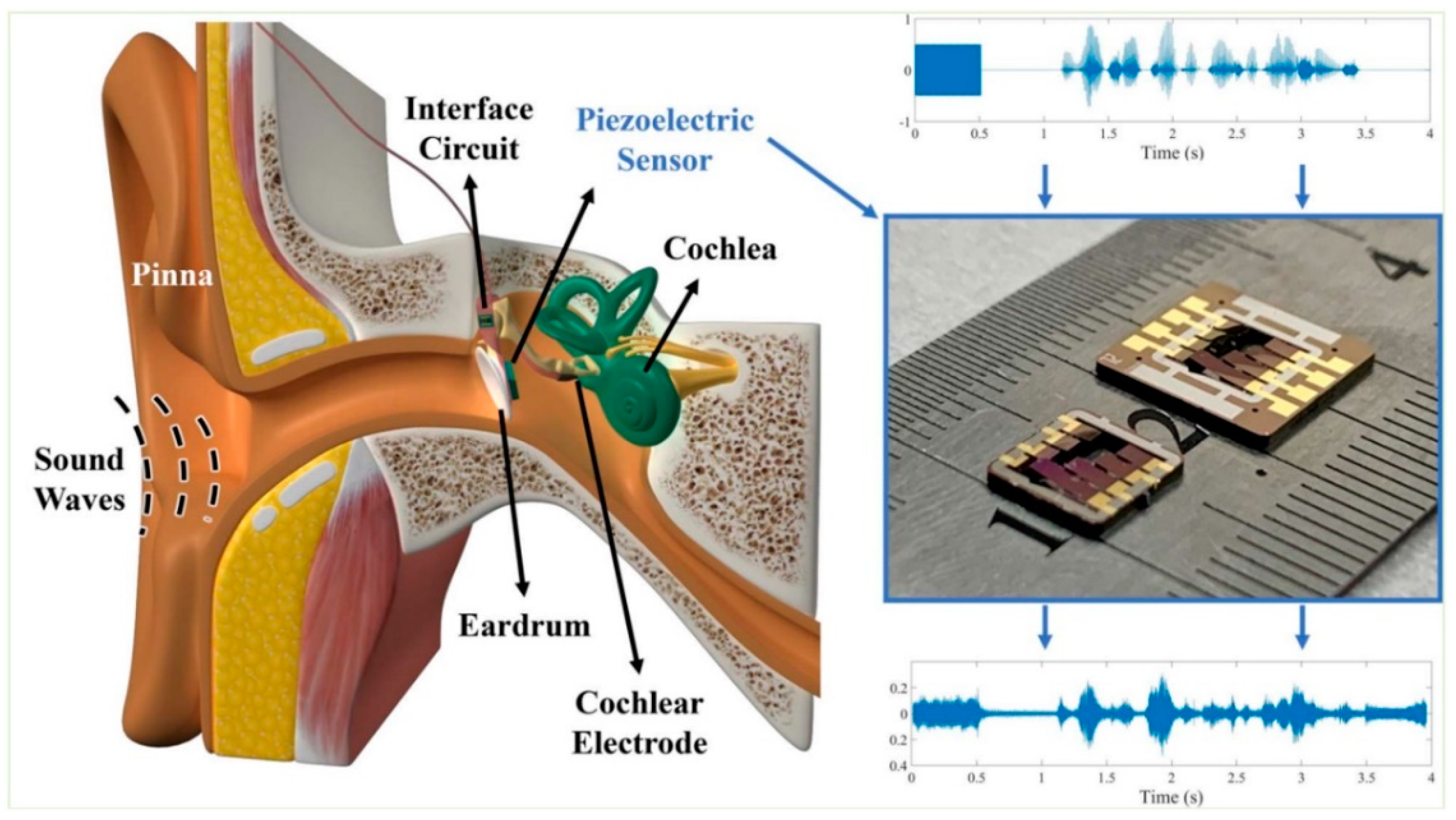
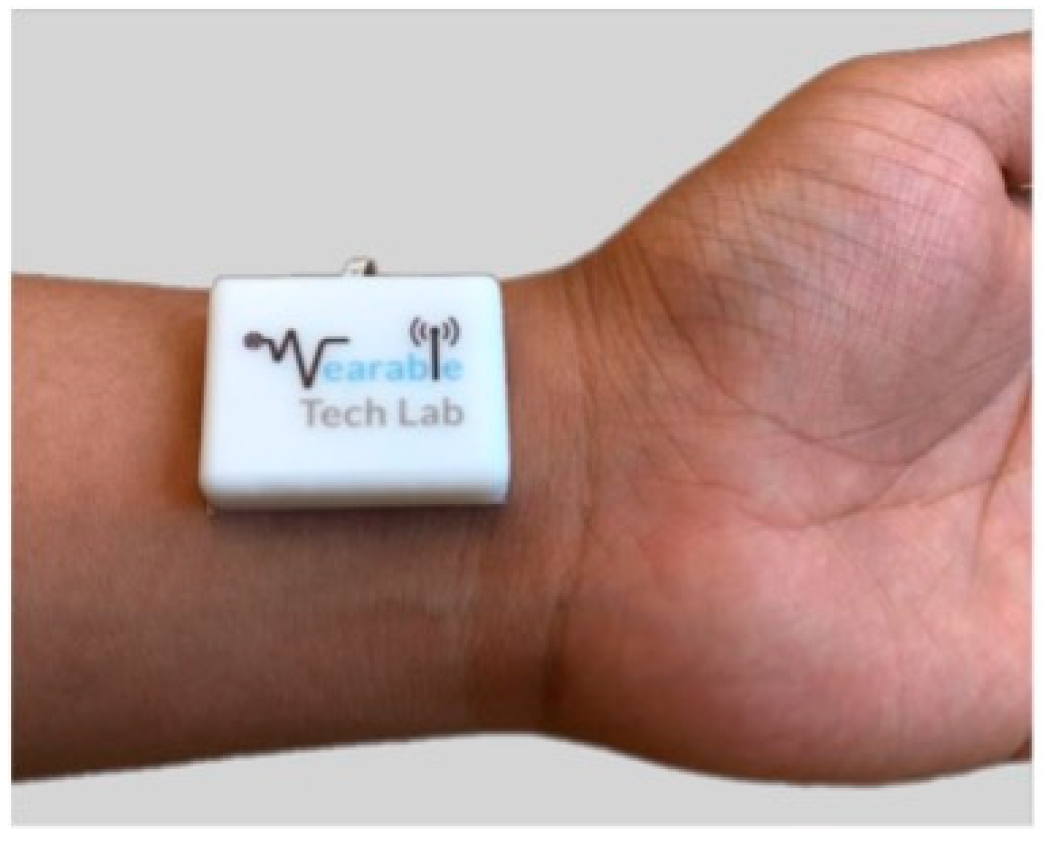
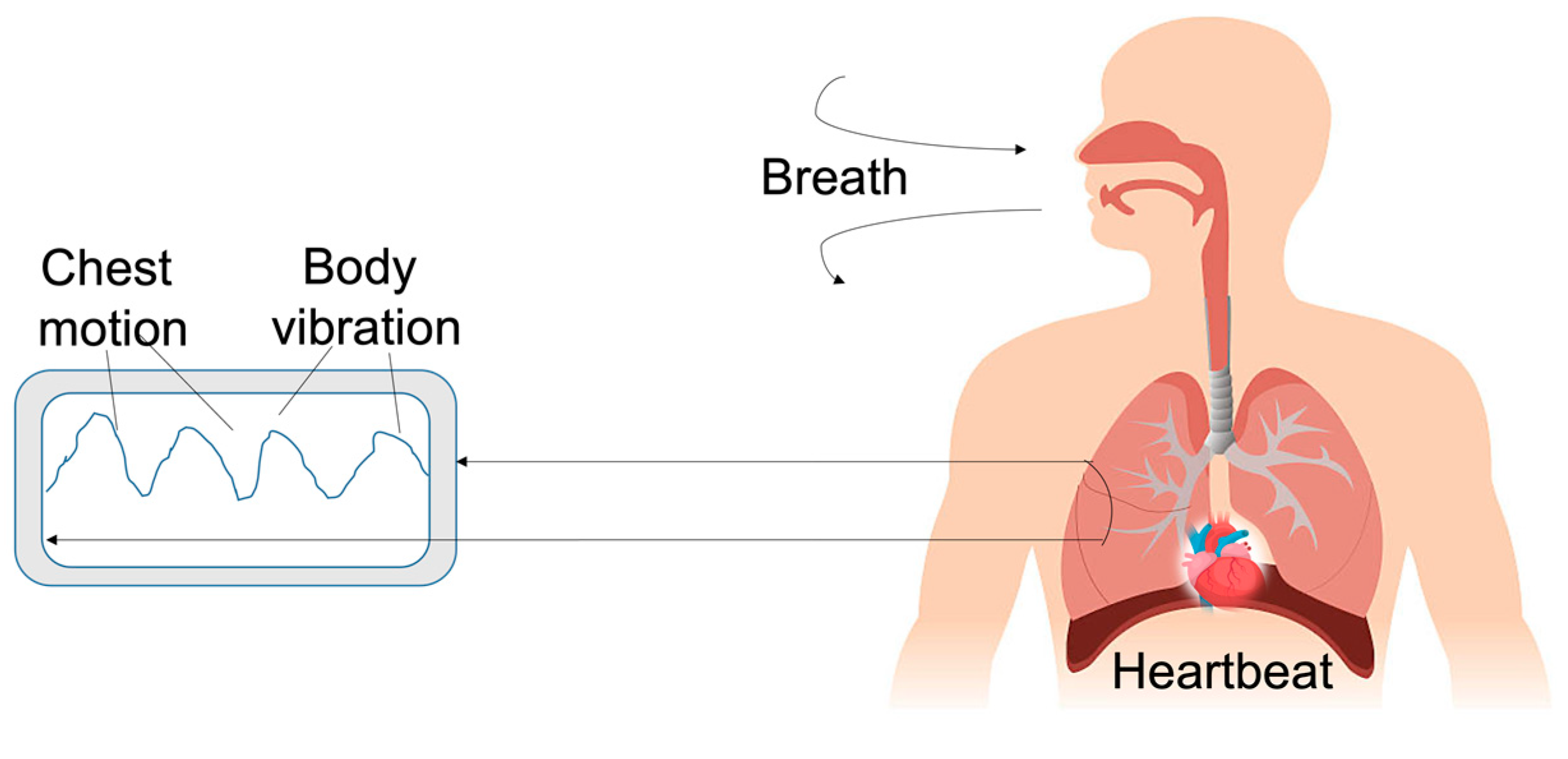

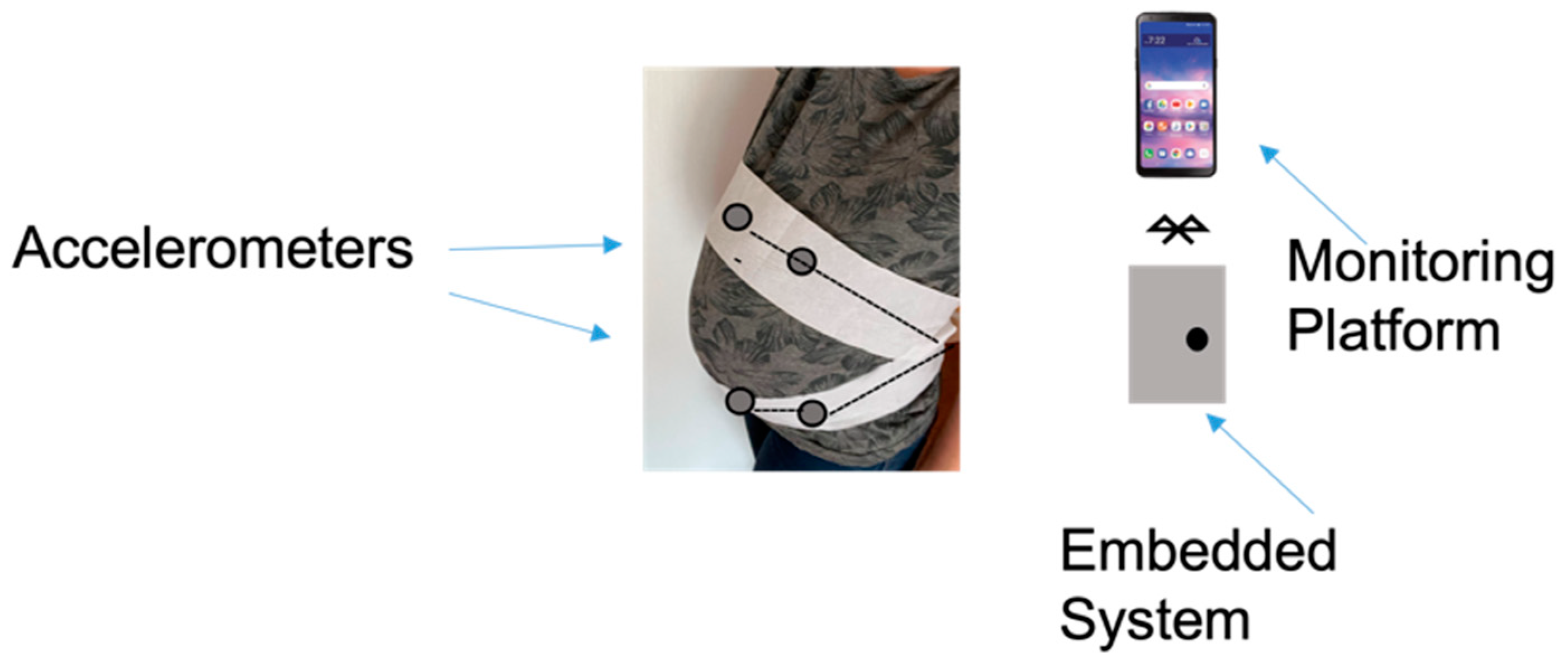
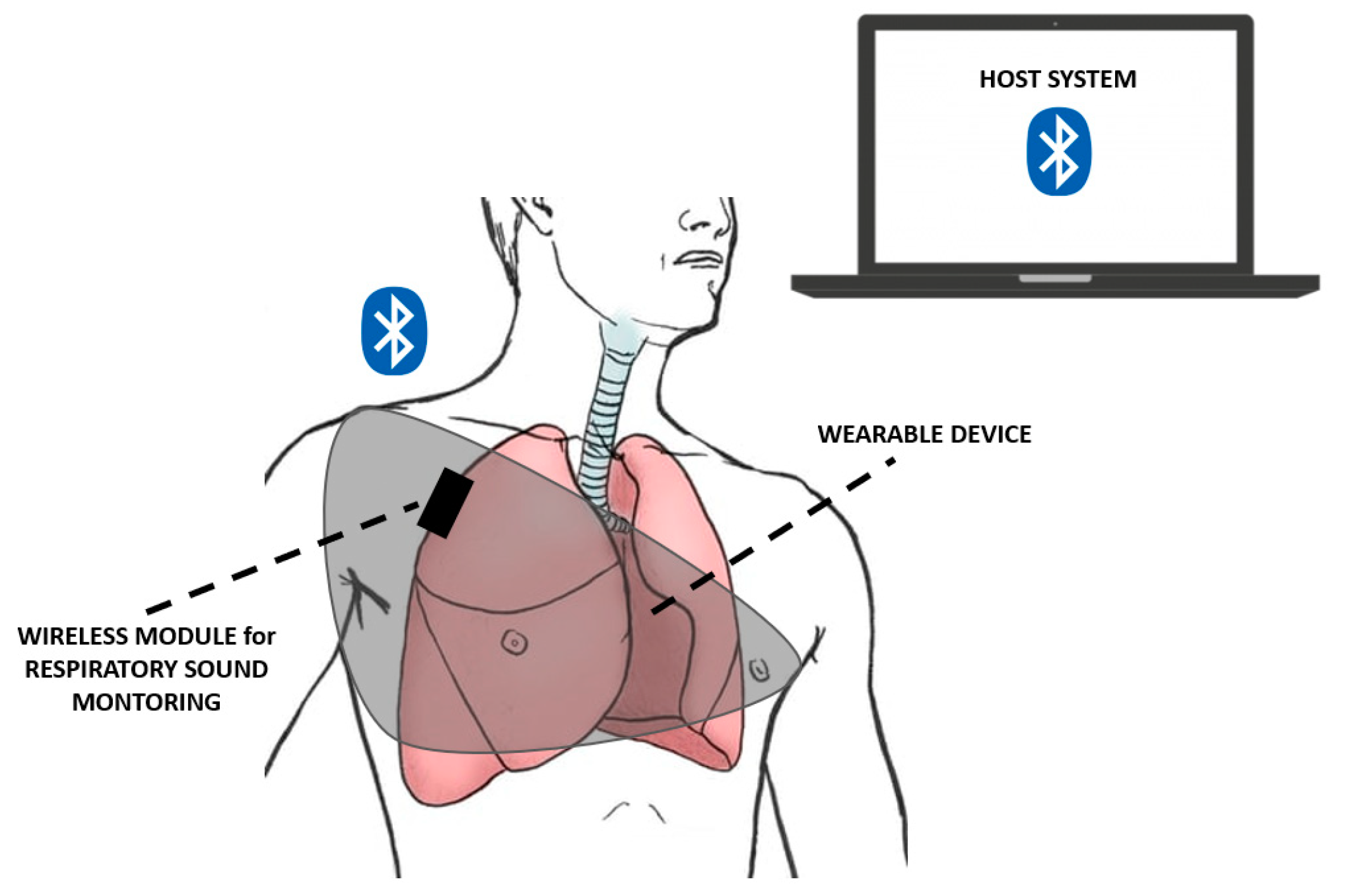
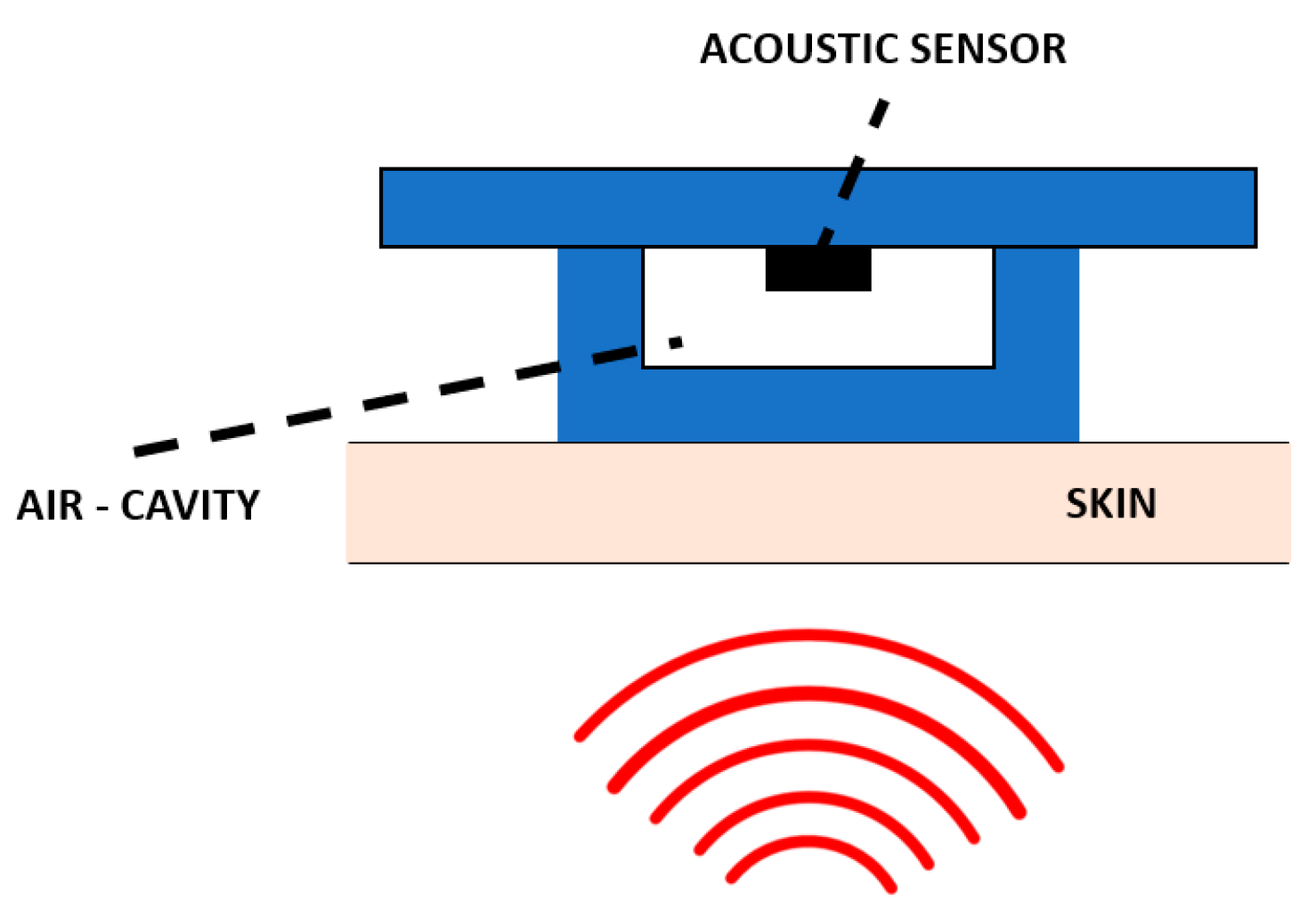
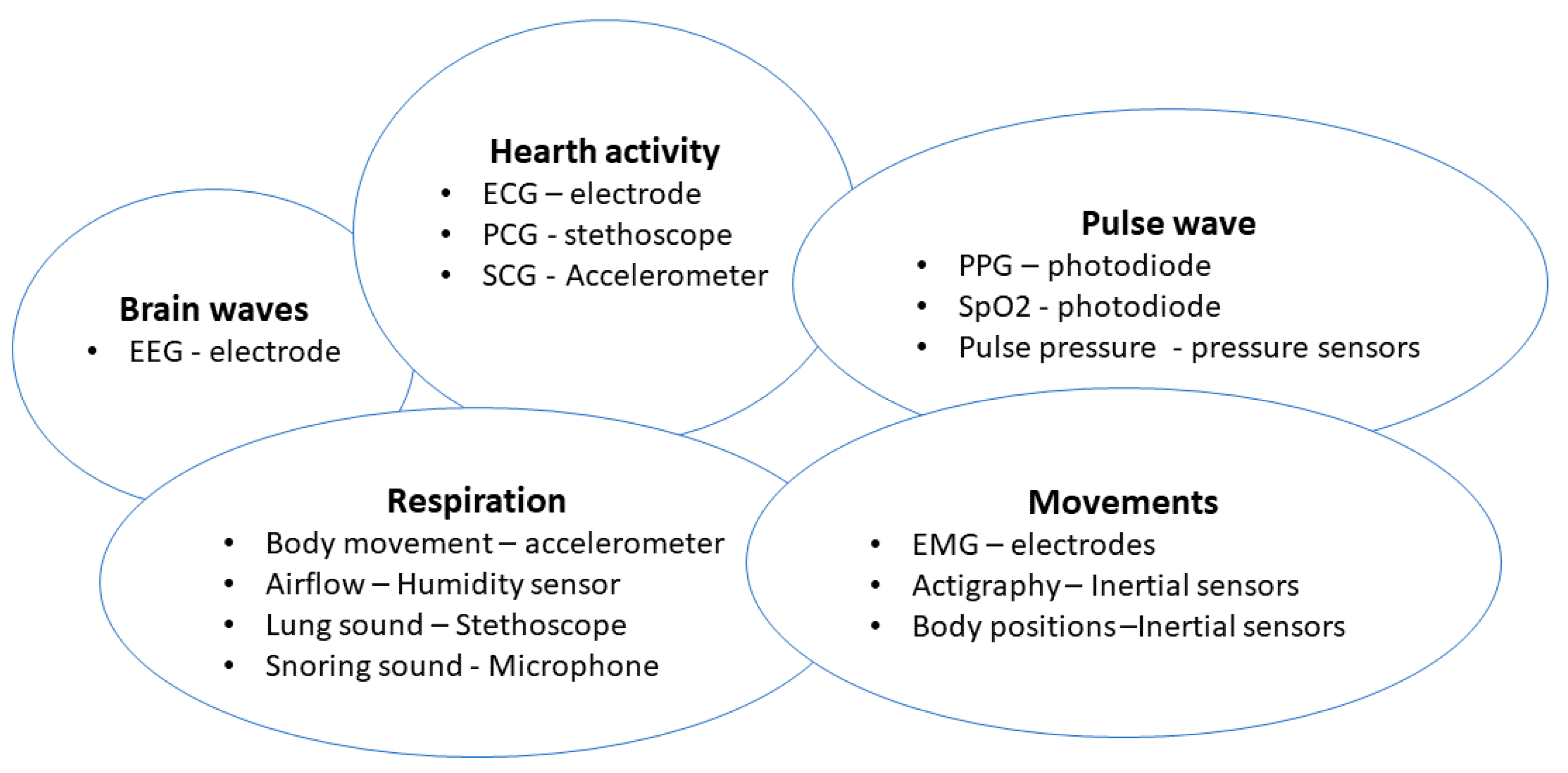
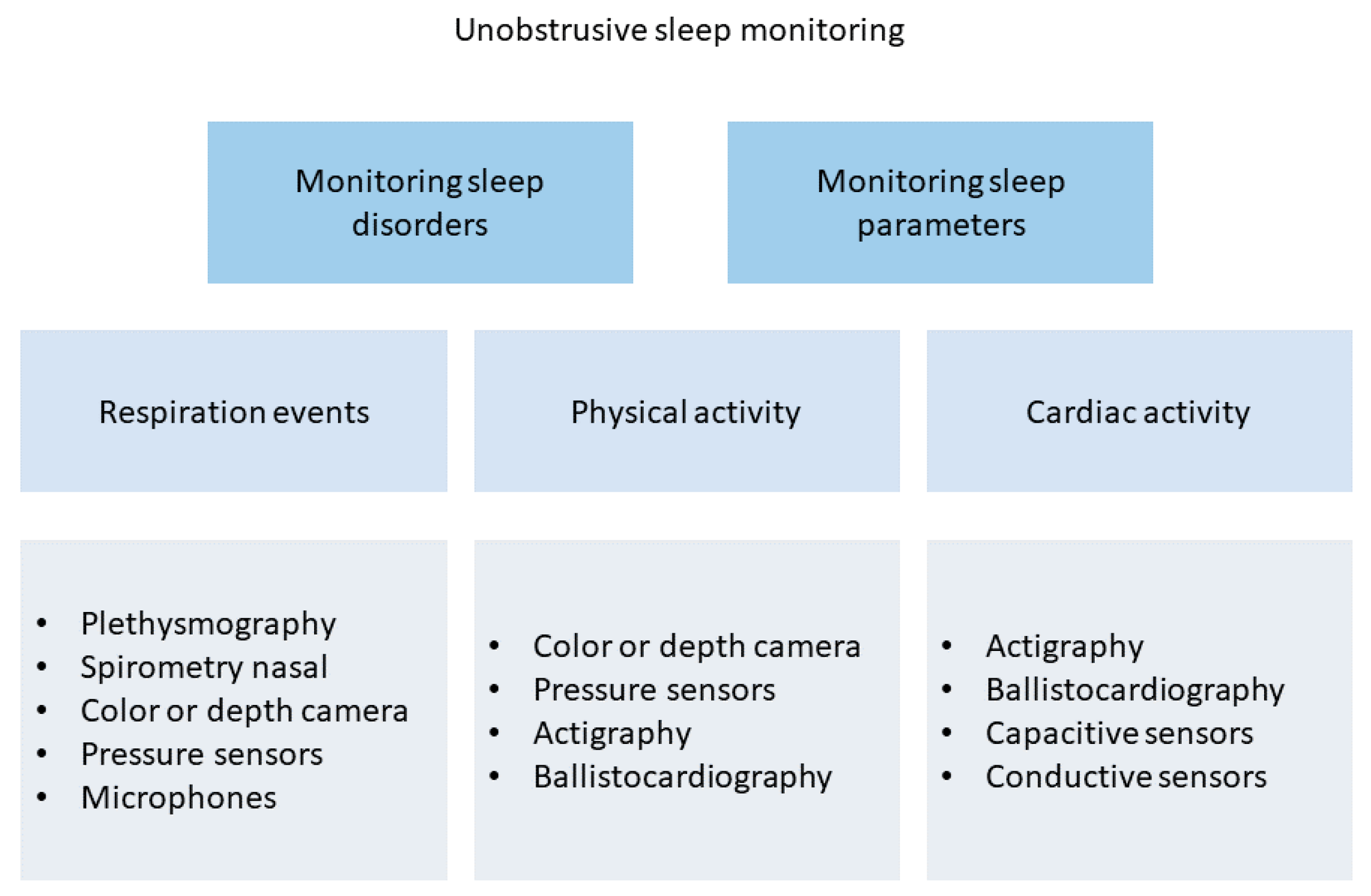
| Device | Size | Weight | Bandwidth | Sensitivity | Power Consumption | Tests | Ref. |
|---|---|---|---|---|---|---|---|
| TICA: subcutaneous microphone based on a capacitive membrane | Diameter: 4.5 mm | Mass: 0.4 g | 0.1–10 kHz | 5 dB ref. 1 mV/Pa | 0.05–0.5 mW | Implanted in 20 patients | [37] |
| TIKI: two capacitive microphones (one subcutaneous and one external) | Volume: 7.5 × 28 × 28 mm3 | - | 0.2–6 kHz | 10 dB ref. 1 mV/Pa | 0.05–0.5 mW | Implanted in 3 patients | [38] |
| Carina™: subcutaneous microphone with condenser microphone | - | - | 0.25–5 kHz | - | 0.05–0.5 mW | Implanted in 110 patients, but a full integration was not achieved | [40] |
| Subcutaneous microphone with a titanium membrane | Diameter: 12 mm | - | 0.1–8 kHz | 35 dB ref. 1 mV/Pa | 0.05–0.5 mW | Tested in the laboratory with a silicone-made skin | [42] |
| Electromagnetic sensor: interaction between a magnet fixed on the malleus and a fixed coil | - | - | 0.25–3 kHz | 30 dB ref. 1 mV/Pa | ≈1 mW | Tested in the laboratory | [43] |
| Optical sensor based on the reflection of a laser beam on the tympanic membrane (or one of the ossicles) | - | - | 0.5–10 kHz | 46 dB ref. 1 mV/Pa | 6.4 mW | Tested in the laboratory | [44] |
| Piezoresistive MEMS that measured the acceleration of the incus | Volume: 387 × 800 × 230 µm3 | - | 0.9–7 kHz | 46 dB ref. 1 mV/Pa | >1 mW | Tested in the laboratory with temporal bones and the LDV | [45] |
| Capacitive MEMS displacement sensor based on a coiled spring that transferred the displacement of the umbo to the condenser | - | - | 0.5–5 kHz | 20 dB ref. 1 mV/Pa | ≈4.5 mW | Tested on one temporal bone and the LDV | [48] |
| Capacitive MEMS displacement sensor fixed on the umbo through springs | - | 25 mg | 0.8–8 kHz | 20 dB ref. 1 mV/Pa | ≈4.5 mW | Tested on temporal bones | [49] |
| Capacitive MEMS acceleration sensor: a plate moved between fixed walls to generate capacitance-related voltage | Volume: 2.5 × 6.2 × 1 mm3 | 25 mg | 0.2–6 kHz | 19 dB ref. 1 mV/Pa | ≈4.5 mW | Tested in the laboratory | [50] |
| Capacitive MEMS acceleration sensor | - | - | 0.5–6 kHz | 9 dB ref. 1 mV/Pa | - | Optimization through modeling; tested in the laboratory with human temporal bones | [52] |
| Capacitive MEMS that measured the middle ear pressure variation due to the motion of the eardrum | Diameter: 10 mm; thickness: 20 µm | - | 0.1–10 kHz | 28 dB ref. 1 mV/Pa | ≈1 mW | Tested in the laboratory | [53] |
| Piezoelectric bimorph material in the shape of a cantilever beam positioned on the malleus of adult cats | - | - | 0.5–10 kHz | 45 dB ref. 1 mV/Pa | - | An acousto–mechanical assessment was carried out by measuring the vibration of the sensor with an LDV | [54] |
| Esteem®: a piezoelectric acoustic sensor to detect the vibration of the middle ear ossicles | - | - | 0.25–8 kHz | - | - | Tested on 134 patients | [55,56,57] |
| Piezoelectric force sensor: a bidirectional membrane transducer fixed at the incudostapedial joint to measure the force passing through the joint | Volume: 4 × 2.5 × 1 mm3 | 35 mg | 0.25–8 kHz | - | - | Tests in silico and on a test bench | [58,59] |
| Piezoelectric accelerometer sensor: made of a ceramic bimorph element and an electronic chip enclosed in a titanium case | Volume: 4.5 × 1 × 0.3 mm3 | 38.4 mg | 0.4–4 kHz | 15 dB ref. 1 mV/Pa | - | Tests in the laboratory on the incus of cats and with a finite element model that included the human middle ear | [60,61] |
| Piezoelectric accelerometer sensor: placed on the long process of the incus | Volume: | 67 mg | 0.4–4 kHz | 15 dB ref. 1 mV/Pa | ≈1 mW | Tested on seven temporal bones | [62] |
| Piezoelectric accelerometer sensor: to harvest energy from the umbo movement for powering cochlear implants | - | - | 0.5–2.5 kHz | 62 dB ref. 1 mV/Pa | - | Finite element model and tested in the laboratory | [63] |
| Piezoelectric (PZT) accelerometer sensor: for powering cochlear implants | - | - | 0.3–6 kHz | 20 dB ref. 1 mV/Pa | 0.01 mW | Tested on temporal bones to minimize energy consumption | [64] |
| Piezoelectric (PZT) accelerometer sensor: for powering cochlear implants | Volume: 5 × 5 × 0.62 mm3 | 4.8 mg | 0.25–5.5 kHz | 26 dB ref. 1 mV/Pa | - | Tested in the laboratory | [65] |
| Device | Mechanism | Application | Results | Ref. |
|---|---|---|---|---|
| New miniature, battery-operated wearable device | Wearable device to monitor the heart rate at the wrist | An algorithm analyzed the acoustic pulse signal to detect S1 sounds | Removed the artifacts for an accurate heartbeat detection, with an accuracy of 98.7% and an error lower than 0.28 bpm, compared with a commercial photoplethysmography (ppg) device | [78] |
| Wearable device | Wearable device placed on the suprasternal notch at neck | The algorithm determined the heart rate, avoiding the external noise | The results showed an accuracy of 94.34% for the heart rate determination | [80] |
| Portable acoustic device | The device was mounted at the fourth intercostal space | Dedicated CAD score algorithm that included both acoustic features and clinical risk factors | A negative predicted value of 96%, this device could reduce the demand for more advanced and costly diagnostic tools | [95] |
| Device for blood pressure monitoring | The device was endowed with a 3-axis accelerometer, positioned on the upper chest | Estimated the systolic and diastolic pressures and monitored the blood pressure | Low cost and cuff-less, able to monitor the blood pressure at 1 Hz | [81] |
| Non-contact device to control the heartbeat and the heart rate | A microphone and a speaker on a device, e.g., smartphones or laptops | ACG discriminated the heart rate and the heartbeat by using frequency-modulated sound signals to identify the heart signal from the external noise | Results showed a median heart rate error of 0.6 bpm, and median heartbeat interval error of 19 ms | [82] |
| Bionic MEMS | Device based on the pick-up mechanism of the three-dimensional ciliary bundle structure of human ear hair cells | The acoustic sound was analyzed using analytical and simulation methods, and experimental test | Small size, high sensitivity to monitor the heart sounds—189.5 db @ 500 Hz, a bandwidth 10–800 Hz, and low interference with environmental noises | [83] |
| Heart sound sensor attached on the chest | Piezoelectric MEMS acoustic sensor: a low noise amplification circuit, and silicone polymers | The device recorded different heart sounds while at rest and after training activities | Low cost, light-weight, skin compatible device, unsusceptible to external environmental sounds; good stability | [77] |
| Device | Mechanism | Application | Result | Ref. |
|---|---|---|---|---|
| A cheap acoustic sensor-based device used by pregnant women at home | Wearable acoustic sensor monitor | Analysis of fetal movements | Developed a thresholding-based signal processing algorithm to detect fetal sounds by removing artefacts due to maternal movements | [99] |
| A comparative study of an acoustic sensor, accelerometer, and piezoelectric diaphragm as candidate vibration sensors for a wearable FM monitor | A silicon-based membrane similar to the abdomen to mimic the vibrations due to fetal kicks | Captured fetal sounds produced by the kicks | Better determined the durations, intensities, and locations of fetal kick movements | [102] |
| Six accelerometer sensors and ARDUINO microcontroller | MATLAB signal process tool to record, display and store relevant fetal movement | The sensors were placed on the maternal abdomen to record and process the signals from the fetal | Performed better in identifying episodes of fetal activity and episodes of inactivity | [103] |
| IoT-based wearable system for fetal movement monitoring using accelerometers and machine learning | Internet of Things (IoT) applied on the system to connect all terminal monitoring units to a control center; the system consisted of two parts: the local monitoring unit and the remote health evaluation unit | Local monitoring unit | E- health home care, Internet of Things (IoT) was applied on the system to connect all the terminal monitoring units to a control center | [105] |
| Acceleration sensors and MEMS microphones | The devices were used to detect three actions performed on the subject’s abdomen: flicking, tapping, and knocking | The noise was removed using the sampled heart rate; the three-axes accelerometer set near the Doppler sensor, evaluated the contraction | The accuracy of using acceleration sensors was 69.96% for the tapping action; while for MEMS microphones was 71.11% for the flicking action | [106] |
| A multi-crystal strap-on low-cost Doppler device, including an accelerometer | Developed methods to increase FHR Doppler signals by reducing noise and estimating uterine contractions using accelerometers | - | Noise present in the FHR signal was reduced, for good detection of contractions when the maternal movement was low | [101] |
| Wearable system | Combination of accelerometers and bespoke acoustic | Local monitoring unit on the maternal abdomen | Successfully discriminated fetal and maternal movements, and also movements when the mother was active | [107] |
| Single wearable system | Variable length accelerometer, combination data with electromyography | Single wearable device placed on the abdomen | Decreased false-positive kick detection, and separation from the maternal noise | [106] |
| Device | Mechanism | Application | Result | Ref. |
|---|---|---|---|---|
| Hybrid-based aspiration and respiration sensing (HARS) | Elastic flexible cover, microphone (MEMS) and photoreactor | Monitoring of asthma attacks and detection of the breathing phases | Breathing phase identification of patient condition, such as wheezing | [123] |
| Single-axis accelerometer accessorized with a third-order low pass Butterworth filter | Wearable elastic belt, worn around the subject’s abdomen | Non-intrusive method of screening for sleep disorders and patient follow-up | Testing of three different breathing modes: normal, slow and fast, with an accuracy of breathing frequency evaluation <1% | [124] |
| Digital signal processor (DSP) circuit and a flexible sensor film | Belt packaged by the micro-fiber cloth of a PET-flexible sensor film consisting of a pressure sensor array and a MEMS triaxial accelerometer | Detection of heart and respiration rates, snoring recognition, and sleep stages classification | Accuracies of heart rate and respiration rate reached by the belt were about 1.5 bpm and 0.7 bpm, respectively. Accuracy of 97.2% in the snoring recognition method | [125] |
| Small-sized and ultra-sensitive accelerometer | A sound sensor made of an asymmetric-gapped cantilever structure with a ceramic piezoelectric beam in zirconium titanate (PZT) as the top layer | Lung and heart sound monitoring in discharged pneumonia patients | Tracking of the recovery course of pneumonia patients with a rapid, simple and highly sensitive detection of lung and heart sounds with a great potential for clinical use and home-use health monitoring | [126] |
| MEMS-based microphone | Sensing piezoresistive cantilever with ultra-high acoustic compliance | Applications in healthcare, monitoring | Small size device with a SNR of ~80 dB in the range of 2 to 200 Hz and a high SNR in low-frequency range | [127] |
| MA sensors | Wearable real-time monitoring system | Monitoring of COVID-19 infections due to a continuous record of coughing frequency and intensity | Detection of decay trend of coughing frequency and intensity through the course of disease recovery | [128] |
| Intelligent facemask | An ultrathin FEP film and Al foil as triboelectric layers and a conductive cloth tape as electrode | Respiratory sensing (RS-TENG) for coronavirus disease (COVID-19), integrated with facemask | High sensitivity and feasibility in respiratory monitoring in diagnosing many respiratory diseases of human bodies, able to give timely alarm after breathing stops | [129] |
| Built-in microphone of a smartphone | Recording, by a built-in microphone and the headset microphone of an iPhone 4S (Apple, Inc., Cupertino, CA, USA) placed on the subject’s neck and nose | Detecting nasal airflow and tracheal breath sounds | Accuracy with median errors of less than 1% for the nasal sound, for all breathing ranges even if the smartphone’s microphone was as far as 30 cm away from the nose | [130] |
| Microphone of a smartphone | Smartphone (SH-12C, Sharp Corp., Osaka, Japan) attached to the anterior chest wall over the sternum using adhesive tape | Detection of snoring condition as a marker of obstructive sleep apnea (OSA) and vascular risk | Diagnostic sensitivity and specificity of 0.70 and 0.94, respectively | [131] |
| Microphone of a smartphone | Microphone (SP0410HR5H-PB, distributed by Knowles Electronics, Itasca, IL, USA) of the Nexus 4™ smartphone (Google, Mountain View, CA, USA) placed near the mouth | Monitoring of wheezing respiratory diseases in children as an outpatient objective tool for recognition of wheezing | Achieved 71.4% sensitivity and 88.9% specificity in wheezing detection | [132] |
| Smartphone-based system | Electret subminiature microphone (BT-2159000, Knowles Electronics, Itasca, IL, USA) encapsulated in a plastic bell and a smartphone device containing the developed mobile app governing sounds acquisition, display and processing | Recording, storage and analysis of respiratory sounds with crackle detection | Accuracy ranged from 84.86 to 89.16%, sensitivity ranged from 93.45 to 97.65%, and specificity ranged from 99.82 to 99.84% | [133,134] |
| Piezoelectric MEMS acoustic sensor | A wearable mechano–acoustic sensing device consisting of a piezoelectric MEMS acoustic sensor, a low noise amplification circuit and silicone polymers for the package | Monitoring of heart sound and detection of speech and voice | Light-weight, low cost and skin-compatible device to enable mechano–acoustic sensing including heartbeat and speaking | [135] |
| Device | Mechanism | Application | Result | Ref. |
|---|---|---|---|---|
| Integrated real time bowel sound detector | Wearable piezoelectric sensor | The physiological measure of meal instances in artificial pancreas devices | Remote real-time monitoring was achievable with wireless technologies in an easy-to-fabricate, low-cost, light-weight and wearable device based on a piezoelectric MEMS acoustic sensor | [136] |
| Flexible printed circuit board (fPCB) | Polyimide fPCB film, equipped with two auscultation and an overall structure of silicone packaging | Bowel sound monitoring | Continuous wearable monitoring of BS for patients with postoperative ileus (POI) from pre-operation (POD0) to postoperative day 7 (POD7) | [140] |
| Smart Shirt | Slim-fit T-shirt as a substrate for the microphone system, in elastane, and with an embedded microphone matrix | The capture of abdominal sounds produced during digestion | Substantial data collected with the accurate detection of 4 BS types, as reported in the literature | [141] |
| Flexible piezoelectric devices | A PZT GI-S encapsulated with a 1.2 µm-thick layer of polyimide and a 10 µm-thick layer of ultraviolet curable epoxy (LOCTITE 5055; Henkel), equipped with an electrical connection and computer-controllable USB multimeter | Gastrointestinal motility sensing | The ingestible device, sensing mechanical deformation within the gastric cavity, was able to quantify the behaviors of the gastrointestinal tract using computational modelling | [142] |
| Device | Mechanism | Application | Result | Ref. |
|---|---|---|---|---|
| SimpleLink multi-standard sensorTag CC2650STK which includes an inertial measuring unit (IMU) | An HR monitoring system that uses the angular rate data from a single axis of a MEMS gyroscope to detect heartbeats. IMU was secured to the chest using an elastic fabric belt | Detection of HR in real-time for sleep physicians | Real-time monitoring, but method still required further testing with a larger pool of participants in real-life scenarios | [160] |
| Apple Watch | Apple Watch with a mobile application was applied to the wrist, containing a digital sleep diary and psychomotor vigilance test, sending data remotely in real-time. Mobile application needed for accessing the accelerometer and heart rate data was in the Apple Watch | Distinguished sleep from wake, and determined sleep stages, compared with gold-standard PSG | The sleep/wake classification (without motion) was consistent. However, the wake/NREM/REM neural net classifier achieved a best accuracy of 69% | [161] |
| Patch system composed of ECG, stethoscope, impedance, 9-axial magnetic, angular rate and gravity (MARG) sensors, a digital stethoscope and ambient sound recording | Using the derived standard ECG leads, authors classified the ECG fiducial points and peaks in the stethoscope signal. Respiratory signals and rates were estimated using ECG-derived respiration techniques combined with a novel phonocardiogram-derived respiration approach | Estimation of heart rate and heart rate variability, detailed ECG activity, and respiratory monitoring | Application on long-term home sleep monitoring | [162] |
| MEMS-based 3-axis accelerometer with digital output | Accelerometer placed on the space between the 7th rib and the region above the diaphragm (the solar plexus). If apnea was detected, a signal was sent to the wristband, and a vibration started until the patient started breathing again | Detection of apnea events in real-time and alerting the patient | A study group of 10 patients who had sleep apnea (SA). All apnea events were detected, and all patients were successfully alerted | [163] |
| Surface acoustic wave (SAW) sensor (piezo-electric based) | The flexible and passive sensor was placed around the nostrils, monitoring respiration using its sensitivity to humidity change | Nasal airflow monitoring and sleep apnea detection | SAW sensors were suitable for OSAS monitoring with good sensitivity, reliability, and response time | [164] |
| Device | Mechanism | Application | Result | Ref. |
|---|---|---|---|---|
| Embletta®X100 PSG: IoT sleep tracking platform, including ballistocardiography, environmental sensors, and actigraphy. | BCG sensor used was the SCA11H bed sensor from Murata. Environmental data loggers, including humidity, temperature, light and sound. | Portable and long-term sleep monitoring | The model could distinguish between sleep and wake states, but could not classify each sleep stage as accurately as PSG | [166] |
| Ultrasonic transducers | A 40 kHz ultrasound transmitter illuminated an area including the subject’s head. One receiver, tuned to the same frequency, recovered the signal reflected from the scene. | Breath monitoring with a focus on sleep apnea detection | A validation on a subject equipped with a pressure sensor connected to a nasal cannula showed the synchronization of the pressure signal provided by the nasal cannula with the signal spectrogram. | [167] |
| Microphone | The reflections from the human body arrived at a specific time depending on the distance from the phone speaker. Focusing on the corresponding frequency allowed authors to reliably extract the amplitude changes due to breathing. | Detection of apneas and estimation of total sleep time | Results from a clinical study with 37 patients showed good performances on detecting apnea events. The apneas–hypopneas index could be improved. Other respiratory-related events or physiological information were not detected. | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallegni, N.; Molinari, G.; Ricci, C.; Lazzeri, A.; La Rosa, D.; Crivello, A.; Milazzo, M. Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare. Biosensors 2022, 12, 835. https://doi.org/10.3390/bios12100835
Mallegni N, Molinari G, Ricci C, Lazzeri A, La Rosa D, Crivello A, Milazzo M. Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare. Biosensors. 2022; 12(10):835. https://doi.org/10.3390/bios12100835
Chicago/Turabian StyleMallegni, Norma, Giovanna Molinari, Claudio Ricci, Andrea Lazzeri, Davide La Rosa, Antonino Crivello, and Mario Milazzo. 2022. "Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare" Biosensors 12, no. 10: 835. https://doi.org/10.3390/bios12100835
APA StyleMallegni, N., Molinari, G., Ricci, C., Lazzeri, A., La Rosa, D., Crivello, A., & Milazzo, M. (2022). Sensing Devices for Detecting and Processing Acoustic Signals in Healthcare. Biosensors, 12(10), 835. https://doi.org/10.3390/bios12100835








