Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects
Abstract
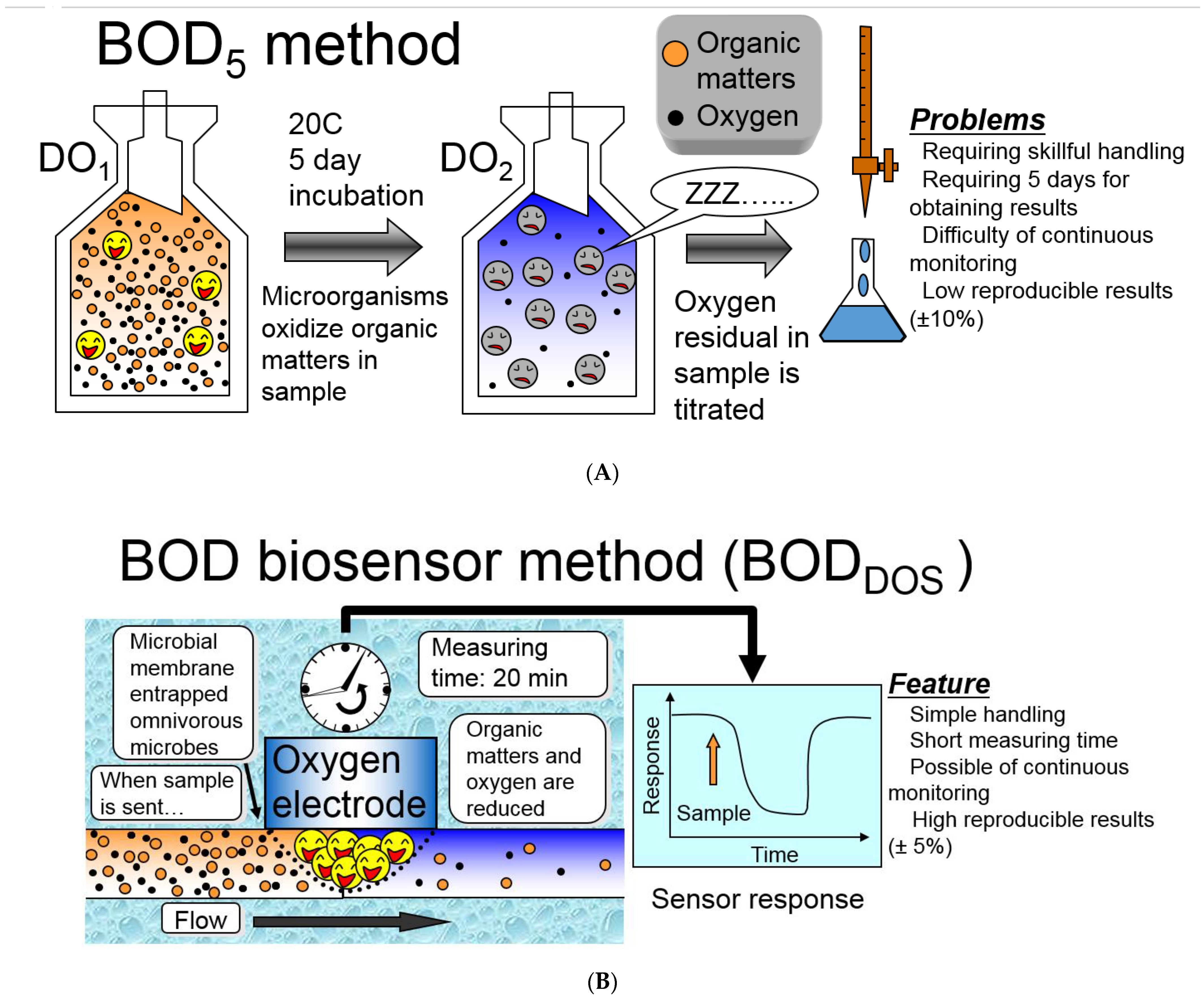
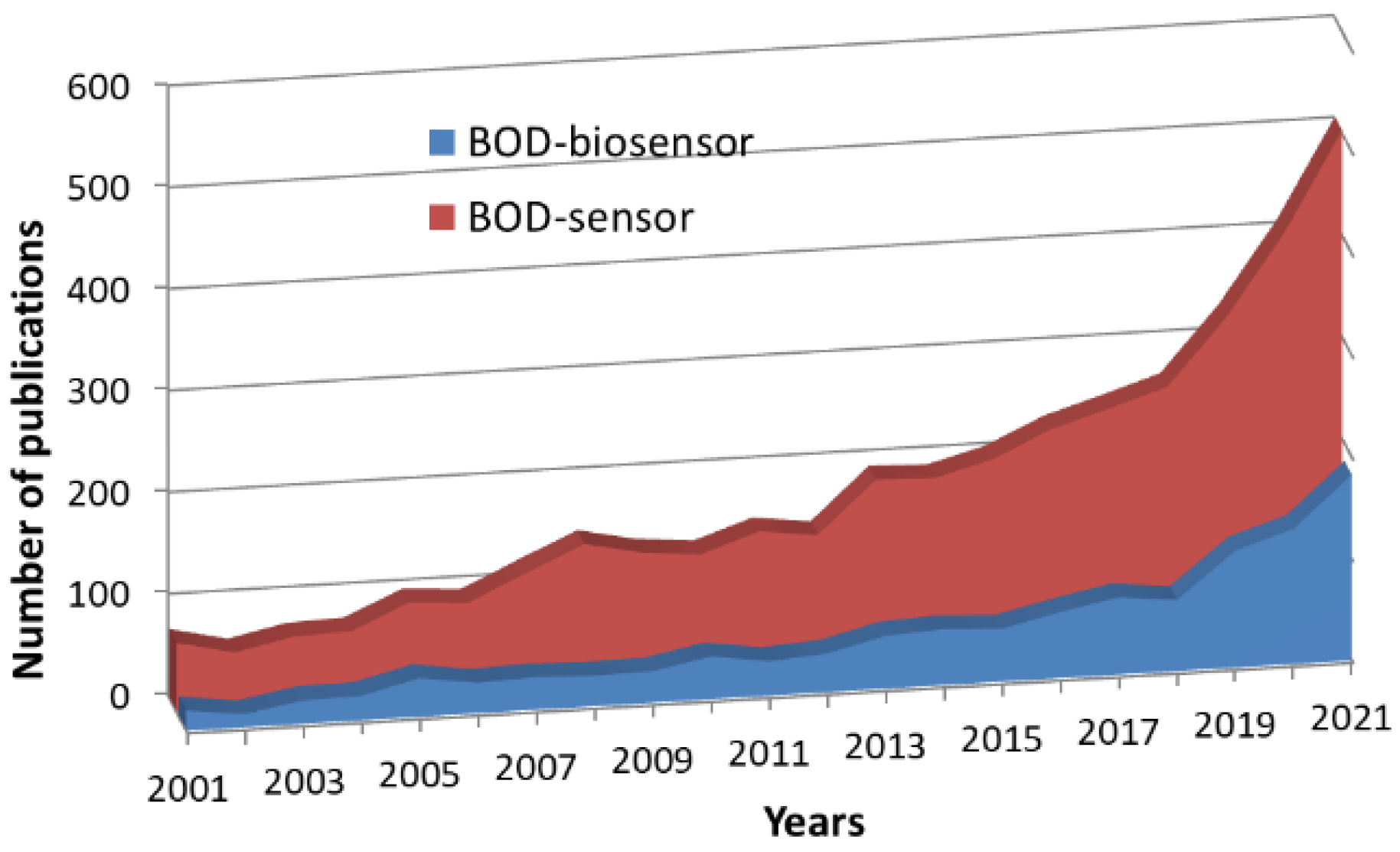
:1. Introduction
2. Microorganisms of BOD Biosensors
3. Types of Transducers
3.1. Electrochemical Systems for Rapid Analysis of BOD
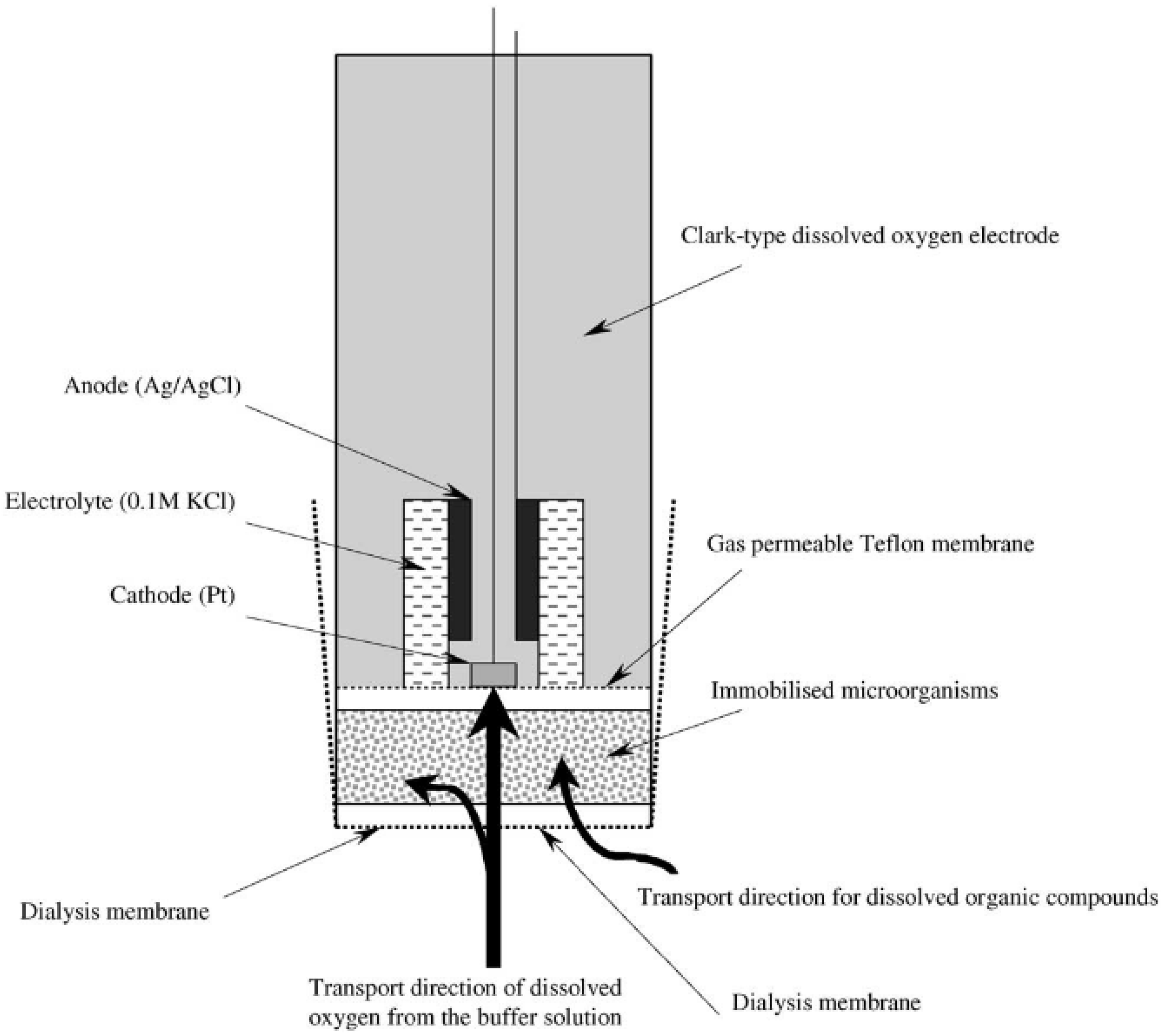
3.1.1. Biosensor Analyzers of BOD Based on the Registration of Oxygen by Clark Electrode
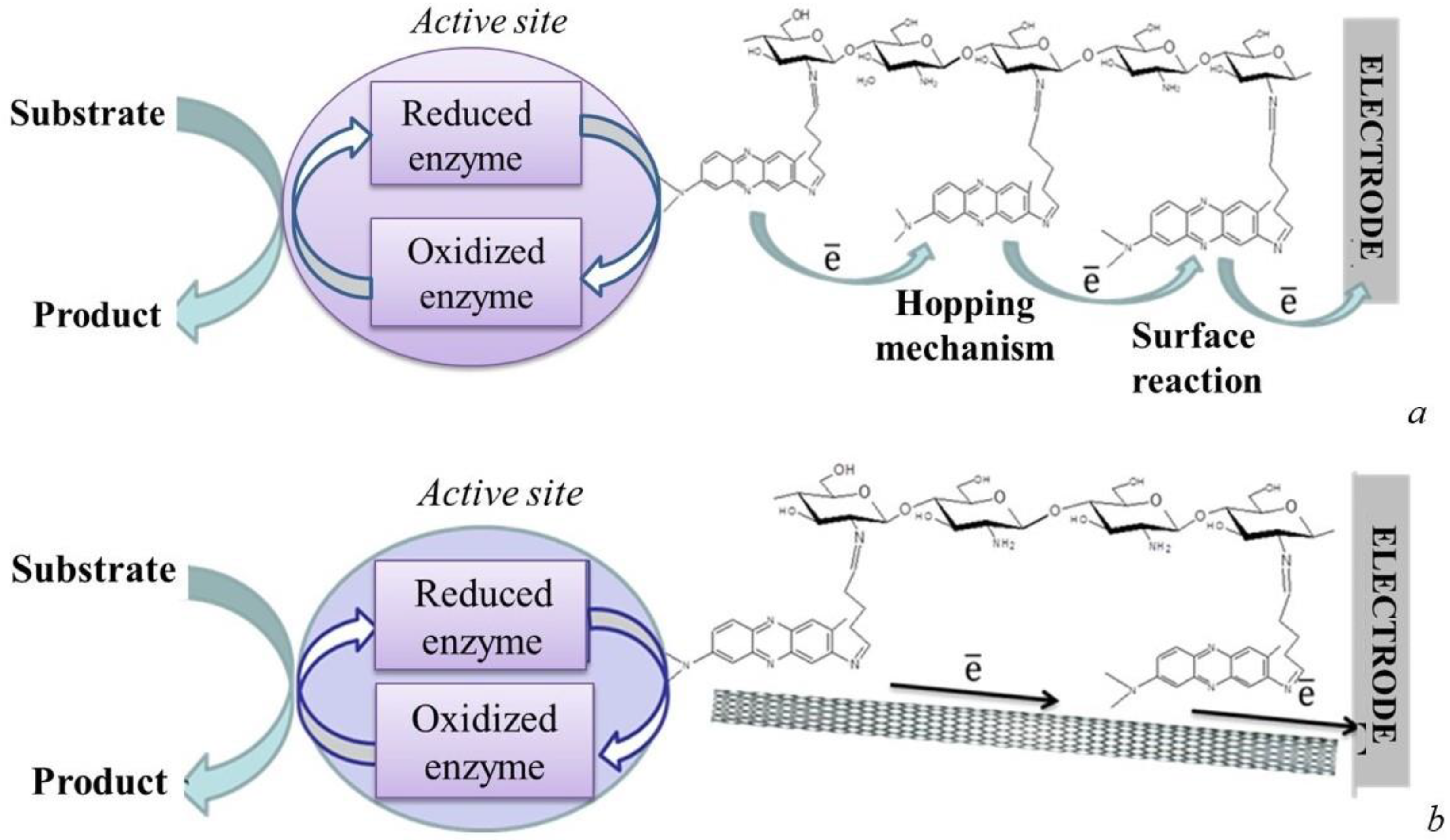
3.1.2. Mediator-Type Biosensor Systems
3.2. Optical Biosensor Systems
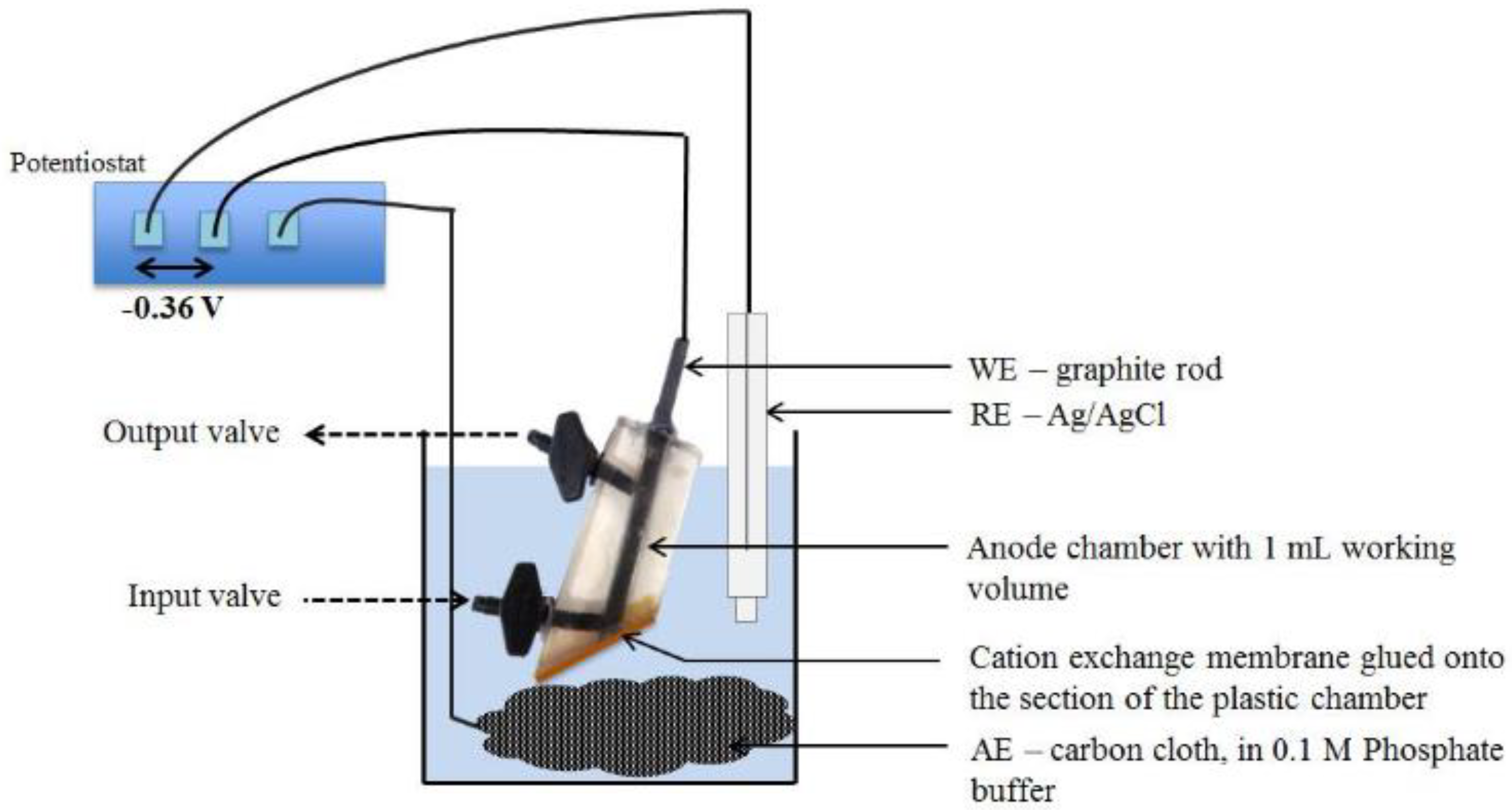
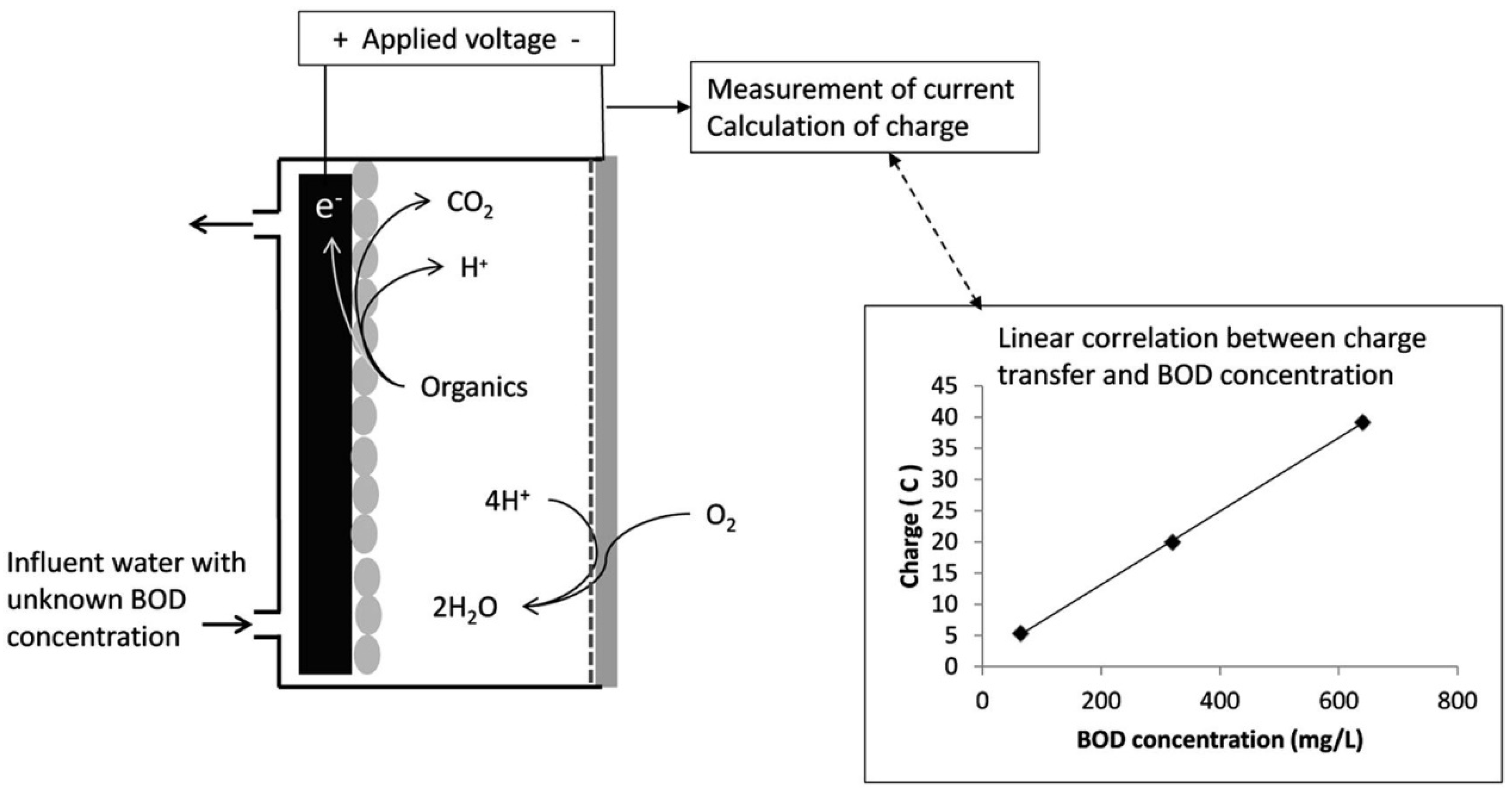
3.3. Microbial Fuel Cells as BOD Biosensors
3.4. Alternative Approaches to Biosensor Determination of BOD
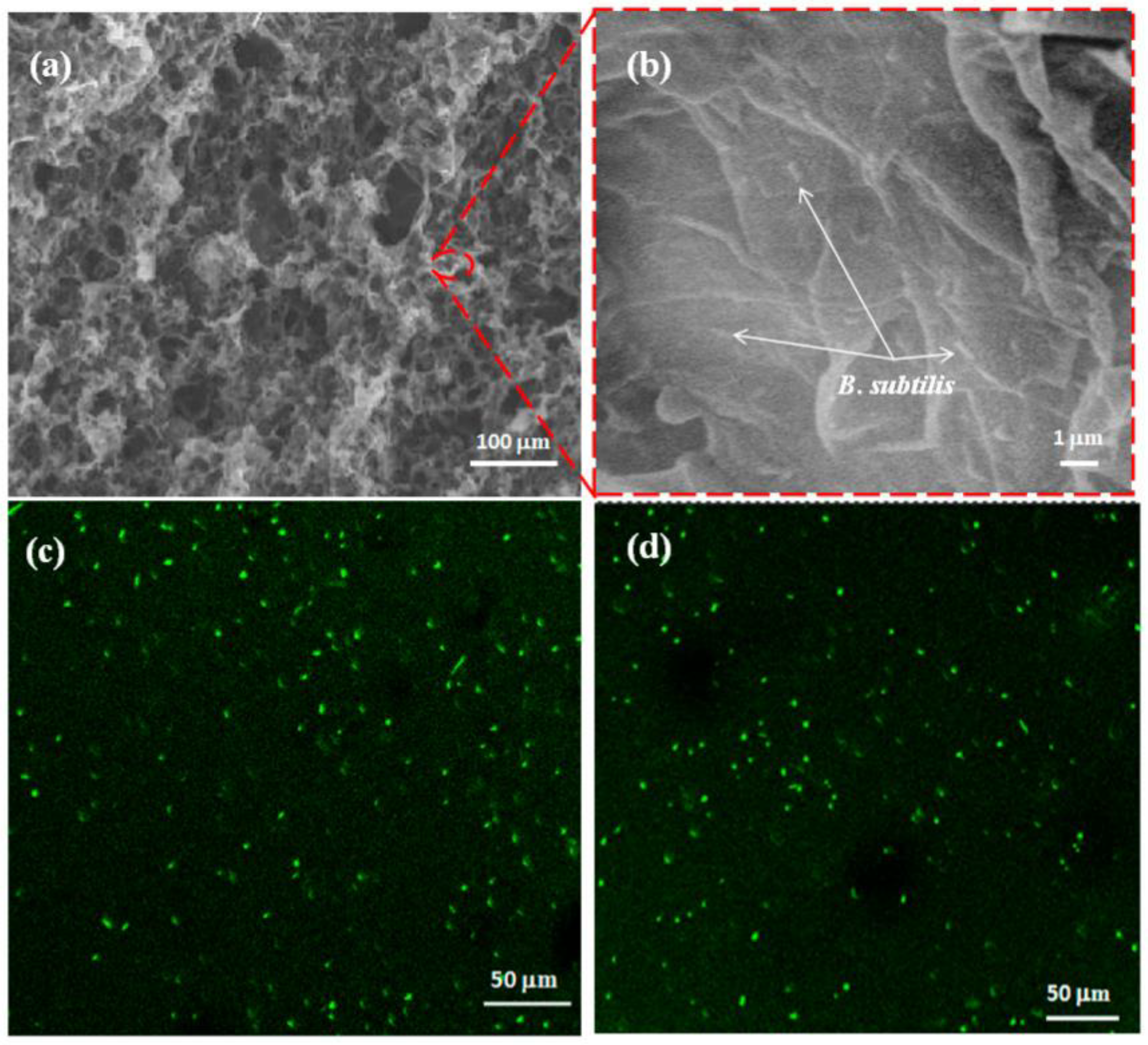
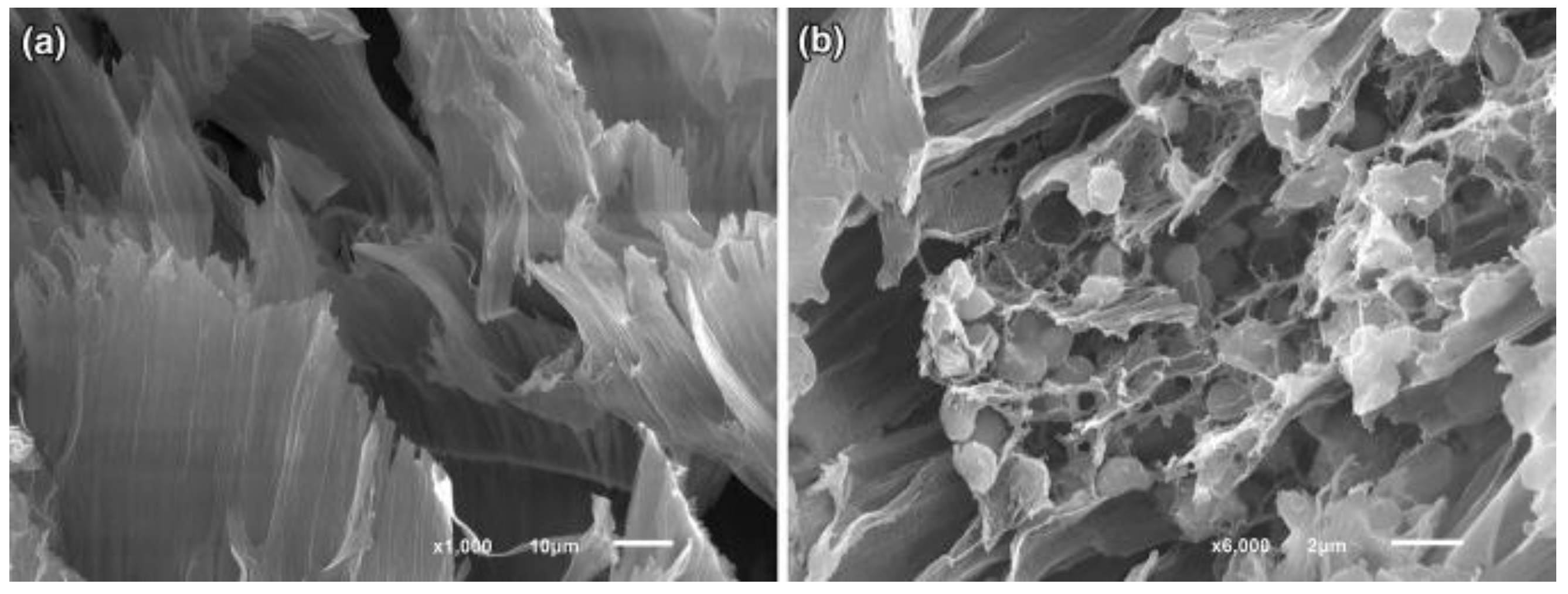
4. Immobilization Techniques
5. Parameters of BOD Biosensors
6. Problems and Achievements
6.1. Standard Solution for Calibration of Biological Devices
6.2. Correlation of Results of Biological Devices and Standard Methods
6.3. Commercialization of BOD Biosensors and MFCs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, G.K. Ecology of Inland Waters and Estuaries; Chapman & Hall: London, UK, 1961; pp. 317–320. [Google Scholar]
- Great Britain Royal Commission on Sewage Disposal. Final Report of the Commissioners Appointed to Inquire and Report What Methods of Treating and Disposing of Sewage; H.M. Stationery Off.: London, UK, 1912. [Google Scholar]
- ISO 5815–1:2003; Water Quality—Determination of Biochemical Oxygen Demand After N Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition. ISO: London, UK, 2003.
- PND F 14. 1:2:3:4. 123-97; Quantitative Chemical Analysis of Waters. Methodology for Measuring Biochemical Oxygen Demand After n Days of Incubation (BODultimate) in Surface Fresh, Underground (ground), Drinking, Sewage and Treated Wastewater. Standartinform: Moscow, Russia, 1997; p. 25.
- Vigiak, O.; Grizzetti, B.; Udias-Moinelo, A.; Zanni, M.; Dorati, C.; Bouraoui, F.; Pistocchi, A. Predicting biochemical oxygen demand in European freshwater bodies. Sci. Total Environ. 2019, 666, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Karube, I. Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Valencia, CA, USA, 2005; Volume 6, pp. 87–126. [Google Scholar]
- Jouanneau, S.; Recoules, L.; Durand, M.J.; Boukabache, A.; Picot, V.; Primault, Y.; Thouand, G. Methods for assessing biochemical oxygen demand (BOD): A review. Water Res. 2014, 49, 62–82. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, B.; Tang, X. Microbial fuel cell-based biosensors. Biosensors 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhall, P.; Kumar, A.; Joshi, A.; Saxsena, T.K.; Manoharan, A.; Makhijani, S.D.; Kumar, R. Quick and reliable estimation of BOD load of beverage industrial wastewater by developing BOD biosensor. Sens. Actuators B Chem. 2008, 133, 478–483. [Google Scholar] [CrossRef]
- Karube, I.; Matsunaga, T.; Mitsuda, S.; Suzuki, S. Microbial electrode BOD sensors. Biotechnol. Bioeng. 1977, 19, 1535–1547. [Google Scholar] [CrossRef]
- Hikuma, M.; Suzuki, H.; Yasuda, T.; Karube, I.; Suzuki, S. Amperometric estimation of BOD by using living immobilized yeasts. Eur. J. Appl. Microbiol. Biotechnol. 1979, 8, 289–297. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Ezugwu, C.I.; Ghosh, P.C. Microbial fuel cell-based biological oxygen demand sensors for monitoring wastewater: State-of-the-art and practical applications. ACS Sens. 2020, 5, 2297–2316. [Google Scholar] [CrossRef]
- Yudina, N.Y.; Arlyapov, V.A.; Chepurnova, M.A.; Alferov, S.V.; Reshetilov, A.N. A yeast co-culture-based biosensor for determination of waste water contamination levels. Enzym. Microb. Technol. 2015, 78, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ponamoreva, O.N.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Microbial biosensors for detection of biological oxygen demand (a review). Appl. Biochem. Microbiol. 2011, 47, 1–11. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, L.L.; Huang, S.; Zhao, L.; Cui, J.S.; Wang, X.H.; Chen, X. Novel BOD optical fiber biosensor based on co-immobilized microorganisms in ormosils matrix. Biosens. Bioelectron. 2006, 21, 1703–11709. [Google Scholar] [CrossRef]
- Chen, H.; Ye, T.; Qiu, B.; Chen, G.; Chen, X. A novel approach based on ferricyanide-mediator immobilized in an ion-exchangeable biosensing film for the determination of biochemical oxygen demand. Anal. Chim. Acta 2008, 612, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.; Kamanin, S.; Ponamoreva, O.; Reshetilov, A. Biosensor analyzer for BOD index express control on the basis of the yeast microorganisms Candida maltosa, Candida blankii, and Debaryomyces hansenii. Enzym. Microb. Technol. 2012, 50, 215–220. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Chung, Y.-C. Measurement of biochemical oxygen demand from different wastewater samples using a mediator-less microbial fuel cell biosensor. Environ. Technol. 2014, 35, 2204–2211. [Google Scholar] [CrossRef]
- Tan, T.C.; Qian, Z. Dead Bacillus subtilis cells for sensing biochemical oxygen demand of waters and wastewaters. Sens. Actuators B Chem. 1997, 40, 65–70. [Google Scholar] [CrossRef]
- Jia, J.; Tang, M.; Chen, X.; Qi, L.; Dong, S. Co-immobilized microbial biosensor for BOD estimation based on sol–gel derived composite material. Biosens. Bioelectron. 2003, 18, 1023–1029. [Google Scholar] [CrossRef]
- Nakamura, H.; Suzuki, K.; Ishikuro, H.; Kinoshita, S.; Koizumi, R.; Okuma, S.; Gotoh, M.; Karube, I. A new BOD estimation method employing a double-mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae. Talanta 2007, 72, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Alferov, S.V.; Alferov, V.A.; Reshetilov, A.N. BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzym. Microb. Technol. 2013, 53, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Raud, M.; Kikas, T. Bioelectronic tongue and multivariate analysis: A next step in BOD measurements. Water Res. 2013, 47, 2555–2562. [Google Scholar] [CrossRef]
- Kharkova, A.S.; Arlyapov, V.A.; Ilyukhina, A.S.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. A kinetic approach to the formation of two-mediator systems for developing microbial biosensors as exemplified by a rapid biochemical oxygen demand assay. 3 Biotech 2021, 11, 222. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Alferov, S.V.; Kamanina, O.A.; Alferov, V.A.; Rogova, T.V.; Arlyapov, V.A.; Machulin, A.V.; Suzina, N.E.; Ivanova, E.P. Yeast-based self-organized hybrid bio-silica sol–gels for the design of biosensors. Biosens. Bioelectron. 2015, 67, 321–326. [Google Scholar] [CrossRef]
- Zhao, L.; He, L.; Chen, S.; Zou, L.; Zhou, K.; Ao, X.; Liu, S.; Hu, X.; Han, G. Microbial BOD sensors based on Zr (IV)-loaded collagen fiber. Enzym. Microb. Technol. 2017, 98, 52–57. [Google Scholar] [CrossRef]
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Ponamoreva, O.; Podsevalova, E. Application of organosilicate matrix based on methyltriethoxysilane, PVA and bacteria Paracoccus yeei to create a highly sensitive BOD biosensor. 3 Biotech 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhao, T.; Xie, B.; Zang, Y.; Liu, H. Dual detection of biochemical oxygen demand and nitrate in water based on bidirectional Shewanella loihica electron transfer. Bioresour. Technol. 2020, 309, 123402. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, S.; Li, T.; Wang, X.; Jiang, Y.; Zhou, Y.; Zhou, X.; Huang, X.; Liang, P. An electroactive biofilm-based biosensor for water safety: Pollutants detection and early-warning. Biosens. Bioelectron. 2021, 173, 112822. [Google Scholar] [CrossRef] [PubMed]
- Kurbanalieva, S.; Arlyapov, V.; Kharkova, A.; Perchikov, R.; Kamanina, O.; Melnikov, P.; Popova, N.; Machulin, A.; Tarasov, S.; Saverina, E.; et al. Electroactive biofilms of activated sludge microorganisms on a nanostructured surface as the basis for a highly sensitive biochemical oxygen demand biosensor. Sensors 2022, 22, 6049. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Bouvier, S.; Feiler, L.; Karacs, J.; Weld, R.J. Geobacter-dominated biofilms used as amperometric BOD sensors. Biochem. Eng. J. 2016, 109, 88–95. [Google Scholar] [CrossRef]
- Liu, J.; Mattiasson, B. Microbial BOD sensors for wastewater analysis. Water Res. 2002, 36, 3786–3802. [Google Scholar] [CrossRef]
- Modin, O.; Wilén, B.-M. A novel bioelectrochemical BOD sensor operating with voltage input. Water Res. 2012, 46, 6113–6120. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Soozanipour, A.; Low, Z.-X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for wastewater monitoring: A review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Wang, Y.; Xu, R.; Sun, Z.; Jie, Z. An innovative reactor-type biosensor for BOD rapid measurement. Biosens. Bioelectron. 2010, 25, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Z.; Jiang, D.; Jia, J.; Zhang, Y.; Chai, Y.; Cheng, X.; Dong, S. Demonstration study of biofilm reactor based rapid biochemical oxygen demand determination of surface water. Sens. Bio Sens. Res. 2016, 8, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Yu, H.-W.; Chon, K.; Lee, Y.-G.; Eom, H.; Chae, K.-J.; Oh, S.-E. Real-time biomonitoring of oxygen uptake rate and biochemical oxygen demand using a novel optical biogas respirometric system. J. Environ. Manag. 2021, 277, 111467. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Hoashi, J.; Morita, T.; McNiven, S.J.; Nakamura, H.; Karube, I. Improvement of a mediator-type biochemical oxygen demand sensor for on-site measurement. J. Biotechnol. 2001, 88, 269–275. [Google Scholar] [CrossRef]
- Nakamura, H. Recent organic pollution and its biosensing methods. Anal. Methods 2010, 2, 430–444. [Google Scholar] [CrossRef]
- Hu, J.; Gao, G.; Xia, S. A mediated BOD microsensor based on poly (neutral red) and bacteria modified interdigited ultramicroelectrode array. Int. J. Electrochem. Sci. 2016, 11, 6387–6402. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Xing, L.; Zhao, H.; Dong, S. A co-immobilized mediator and microorganism mediated method combined pretreatment by TiO2 nanotubes used for BOD measurement. Talanta 2012, 93, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.H.; Ismail, A.K.; Ahamad, R.; Shahir, R.S. A redox mediated UME biosensor using immobilized Chromobacterium violaceum strain R1 for rapid biochemical oxygen demand measurement. Electrochim. Acta 2015, 176, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Li, Y.; Gao, G.; Xia, S. A mediated BOD biosensor based on immobilized B. subtilis on three-dimensional porous graphene-polypyrrole composite. Sensors 2017, 17, 2594. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, A.S.; Arlyapov, V.A.; Yudina, N.Y.; Nosova, N.M.; Alferov, V.A.; Reshetilov, A.N. A novel BOD-mediator biosensor based on ferrocene and Debaryomyces hansenii yeast cells. Appl. Biochem. Microbiol. 2017, 53, 381–387. [Google Scholar] [CrossRef]
- Bonetto, M.C.; Sacco, N.J.; Ohlsson, A.H.; Cortón, E. Assessing the effect of oxygen and microbial inhibitors to optimize ferricyanide-mediated BOD assay. Talanta 2011, 85, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Welsh, D.T.; John, R.; Catterall, K.; Teasdale, P.R. A sensitive ferricyanide-mediated biochemical oxygen demand assay for analysis of wastewater treatment plant influents and treated effluents. Water Res. 2013, 47, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Welsh, D.T.; Teasdale, P.R. Ubiquity of activated sludge ferricyanide-mediated BOD methods: A comparison of sludge seeds across wastewater treatment plants. Talanta 2014, 125, 293–300. [Google Scholar] [CrossRef]
- Pasco, N.; Baronian, K.; Jeffries, C.; Hay, J. Biochemical mediator demand—A novel rapid alternative for measuring biochemical oxygen demand. Appl. Biochem. Microbiol. 2000, 53, 613–618. [Google Scholar] [CrossRef]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Asawatreratanakul, P.; Kanatharana, P.; Thavarungkul, P. A novel BOD biosensor based on entrapped activated sludge in a porous chitosan-albumin cryogel incorporated with graphene and methylene blue. Sens. Actuators B Chem. 2017, 241, 473–481. [Google Scholar] [CrossRef]
- Farré, M.; Barceló, D. Characterization of wastewater toxicity by means of a whole-cell bacterial biosensor, using Pseudomonas putida in conjunction with chemical analysis. Fresenius J. Anal. Chem. 2001, 371, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Vacchino, J.F.; Beeson, C.; McConnell, H.M. Potentiometric measurement of intracellular redox activity. J. Am. Chem. Soc. 1998, 120, 2464–2473. [Google Scholar] [CrossRef]
- Trosok, S.P.; Driscoll, B.T.; Luong, J.H.T. Mediated microbial biosensor using a novel yeast strain for wastewater BOD measurement. Appl. Biochem. Microbiol. 2001, 56, 550–554. [Google Scholar] [CrossRef]
- Heiskanen, A.; Yakovleva, J.; Spegel, C.; Taboryski, R.; Koudelka-Hep, M.; Emneus, J.; Ruzgas, T. Amperometric monitoring of redox activity in living yeast cells: Comparison of menadione and menadione sodium bisulfite as electron transfer mediators. Electrochem. Commun. 2004, 6, 219–224. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Kharkova, A.S.; Kurbanaliyeva, S.K.; Kuznetsova, L.S.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N.; Machulin, A.V.; Tarasov, S.E.; Melnikov, P.V. Use of biocompatible redox-active polymers based on carbon nanotubes and modified organic matrices for development of a highly sensitive BOD biosensor. Enzym. Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Kamanin, S.S.; Arlyapov, V.A.; Ponamoreva, O.N.; Blokhin, I.V.; Alferov, V.A.; Reshetilov, A.N. Graphite screen-printed electrodes modified with conductive protein hydrogel and bacterial cells as the basis of an amperometric biosensor. Sens. Sist. 2017, 31, 161–171. [Google Scholar]
- Yılmaz, Ö.; Demirkol, D.O.; Gülcemal, S.; Kılınc, A.; Timur, S.; Cetinkaya, B. Chitosan–ferrocene film as a platform for flow injection analysis applications of glucose oxidase and Gluconobacter oxydans biosensors. Colloids Surf. B 2012, 100, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef]
- Naghib, S.M. Two-dimensional functionalised methacrylated graphene oxide nanosheets as simple and inexpensive electrodes for biosensing applications. Micro Nano Lett. 2019, 14, 462. [Google Scholar] [CrossRef]
- Plekhanova, Y.; Tarasov, S.; Bykov, A.; Prisyazhnaya, N.; Kolesov, V.; Sigaev, V.; Signore, M.A.; Reshetilov, A. Multiwalled carbon nanotubes and the electrocatalytic activity of Gluconobacter oxydans as the basis of a biosensor. Biosensors 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Plekhanova, Y.; Tarasov, S.; Reshetilov, A. Use of PEDOT:PSS/Graphene/Nafion composite in biosensors based on acetic acid bacteria. Biosensors 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Morioka, Y.; Yamasaki, M.; Iwanaga, J.; Beppu, K.; Maeda, H.; Morita, Y.; Tamiya, E. Rapid and onsite BOD sensing system using luminous bacterial cells-immobilized chip. Biosens. Bioelectron. 2007, 22, 1345–1350. [Google Scholar] [CrossRef]
- Kashem, M.A.; Suzuki, M.; Kimoto, K.; Iribe, Y. An optical biochemical oxygen demand biosensor chip for environmental monitoring. Sens. Actuators B Chem. 2015, 221, 1594–1600. [Google Scholar] [CrossRef]
- Nakamura, H.; Abe, Y.; Koizumi, R.; Suzuki, K.; Mogi, Y.; Hirayama, T.; Karube, I. A chemiluminescence biochemical oxygen demand measuring method. Anal. Chim. Acta 2007, 602, 94–100. [Google Scholar] [CrossRef]
- Yoshida, N.; McNiven, S.J.; Yoshida, A.; Morita, T.; Nakamura, H.; Karube, I. A compact optical system for multi-determination of biochemical oxygen demand using disposable strips. Field Anal. Chem. Technol. 2001, 5, 222–227. [Google Scholar] [CrossRef]
- Yoshida, N.; McNiven, S.J.; Morita, T.; Nakamura, H.; Karube, I. A simple, multiple simultaneous spectrophotometric method for BOD determination using DCIP as the redox color indicator. Anal. Lett. 2002, 35, 1541–1549. [Google Scholar] [CrossRef]
- Nakamura, H.; Suzuki, M. New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells (part I): Characterization of the phenomenon. Anal. Bioanal. Chem. 2007, 389, 1225–1232. [Google Scholar] [CrossRef]
- Nakamura, H.; Suzuki, M. New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells (part II): Application to analytical toxicology. Anal. Bioanal. Chem. 2007, 389, 1233–1241. [Google Scholar] [CrossRef]
- Pang, H.-L.; Kwok, N.-Y.; Chan, P.-H.; Yeung, C.-H.; Lo, W.; Wong, K.-Y. High-throughput determination of biochemical oxygen demand (BOD) by a microplate-based biosensor. Environ. Sci. Technol. 2007, 41, 4038–4044. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified nanodiamonds as a means of polymer surface functionalization. From fouling suppression to biosensor design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Aleksandrovskaya, A.Y.; Safonov, A.V.; Popova, N.M.; Spitsin, B.V.; Naumova, A.O.; Zaitsev, N.K. Tuning the wetting angle of fluorinated polymer with modified nanodiamonds: Towards new type of biosensors. Mendeleev Commun. 2020, 30, 453–455. [Google Scholar] [CrossRef]
- Alexandrovskaya, A.Y.; Melnikov, P.V.; Safonov, A.V.; Naumova, A.O.; Zaytsev, N.K. A comprehensive study of the resistance to biofouling of different polymers for optical oxygen sensors. The advantage of the novel fluorinated composite based on core-dye-shell structure. Mater. Today Commun. 2020, 23, 100916. [Google Scholar] [CrossRef]
- Karube, I.; Matsunaga, T.; Tsuru, S.; Suzuki, S. Biochemical fuel cell utilizing immobilized cells of Clostridium butyricum. Biotechnol. Bioeng. 1977, 19, 1727–1760. [Google Scholar] [CrossRef]
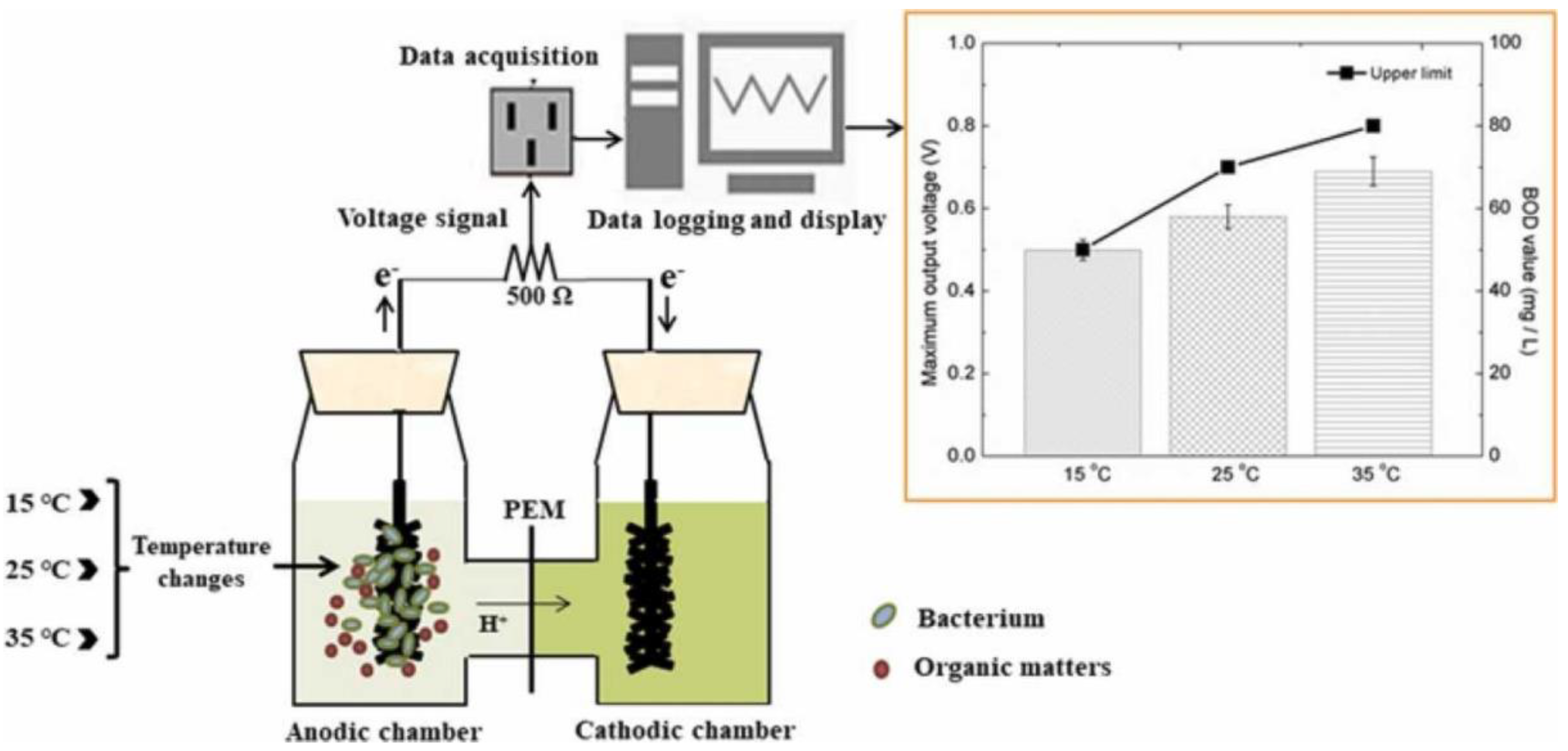
- Ma, Y.; Deng, D.; Zhan, Y.; Cao, L.; Liu, Y. A systematic study on self-powered microbial fuel cell based BOD biosensors running under different temperatures. Biochem. Eng. J. 2022, 180, 108372. [Google Scholar] [CrossRef]
- Kaur, A.; Kim, J.R.; Michie, I.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Microbial fuel cell type biosensor for specific volatile fatty acids using acclimated bacterial communities. Biosens. Bioelectron. 2013, 47, 50–55. [Google Scholar] [CrossRef]
- Tee, P.F.; Abdullah, M.O.; Tan, I.A.W.; Amin, M.A.M.; Nolasco-Hipolito, C.; Bujang, K. Performance evaluation of a hybrid system for efficient palm oil mill effluent treatment via an air-cathode, tubular upflow microbial fuel cell coupled with a granular activated carbon adsorption. Bioresour. Technol. 2016, 216, 478–485. [Google Scholar] [CrossRef]
- Alferov, S.V.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Biofuel cell based on bacteria of the genus Gluconobacter as a sensor for express analysis of biochemical oxygen demand. Appl. Biochem. Microbiol. 2018, 54, 689–694. [Google Scholar] [CrossRef]
- Moon, H.; Chang, I.S.; Kang, K.H.; Jang, J.K.; Kim, B.H. Improving the dynamic response of a mediator-less microbial fuel cell as a biochemical oxygen demand (BOD) sensor. Biotechnol. Lett. 2004, 26, 1717–1738. [Google Scholar] [CrossRef]
- Sun, J.Z.; Kingori, G.P.; Si, R.W.; Zhai, D.D.; Liao, Z.H.; Sun, D.Z.; Yong, Y.C. Microbial fuel cell-based biosensors for environmental monitoring: A review. Water Sci. Technol. 2015, 71, 801–809. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Varjani, S.; Kumar, M. Microbial fuel cell-based biosensor for online monitoring wastewater quality: A critical review. Sci. Total Environ. 2020, 712, 135612. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Nguyen, T.V. Performance of mediator-less double chamber microbial fuel cell-based biosensor for measuring biological chemical oxygen. J. Environ. Manag. 2020, 276, 111279. [Google Scholar] [CrossRef]
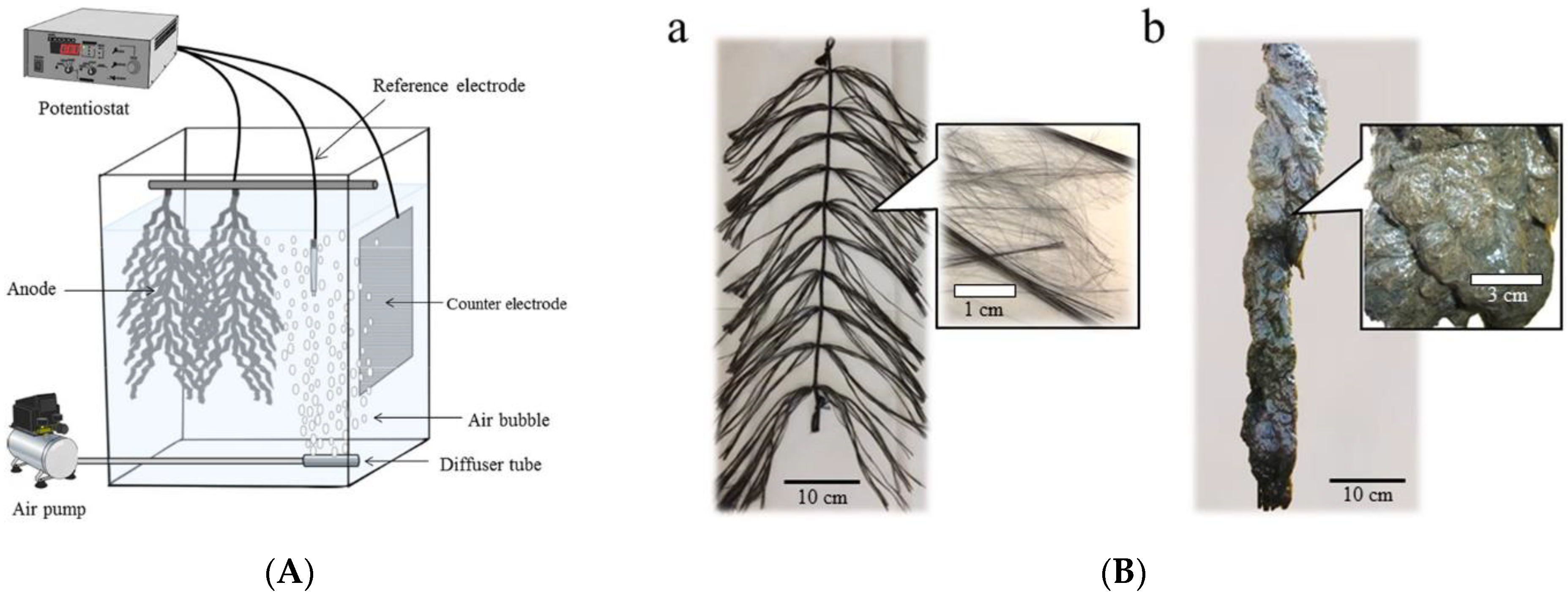
- Yamashita, T.; Ookawa, N.; Ishida, M.; Kanamori, H.; Sasaki, H.; Katayose, Y.; Yokoyama, H. A novel open-type biosensor for the in-situ monitoring of biochemical oxygen demand in an aerobic environment. Sci. Rep. 2016, 6, 38552. [Google Scholar] [CrossRef] [Green Version]
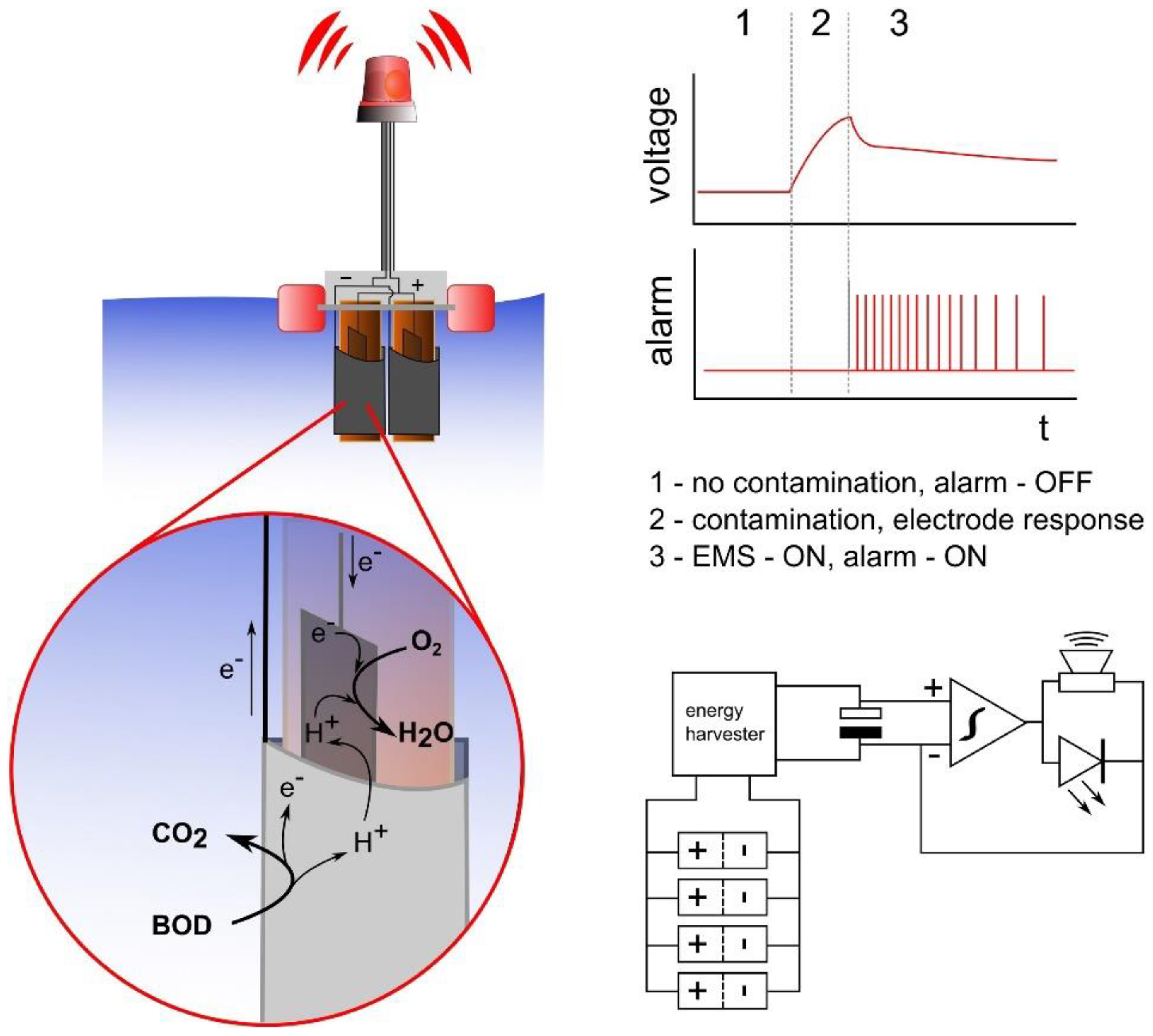
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Self-powered, autonomous Biological Oxygen Demand biosensor for online water quality monitoring. Sens. Actuators B 2017, 244, 815–822. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Melidis, P.; Kampragou, E.; Aivasidis, A. On-line load monitoring of wastewaters with a respirographic microbial sensor. Biosens. Bioelectron. 2005, 21, 365–371. [Google Scholar] [CrossRef]
- Tønning, E.; Sapelnikova, S.; Christensen, J.; Carlsson, C.; Winther-Nielsen, M.; Dock, E.; Solna, R.; Skladal, P.; Nørgaard, L.; Ruzgas, T.; et al. Chemometric exploration of an amperometric biosensor array for fast determination of wastewater quality. Biosens. Bioelectron. 2005, 21, 608–617. [Google Scholar] [CrossRef]
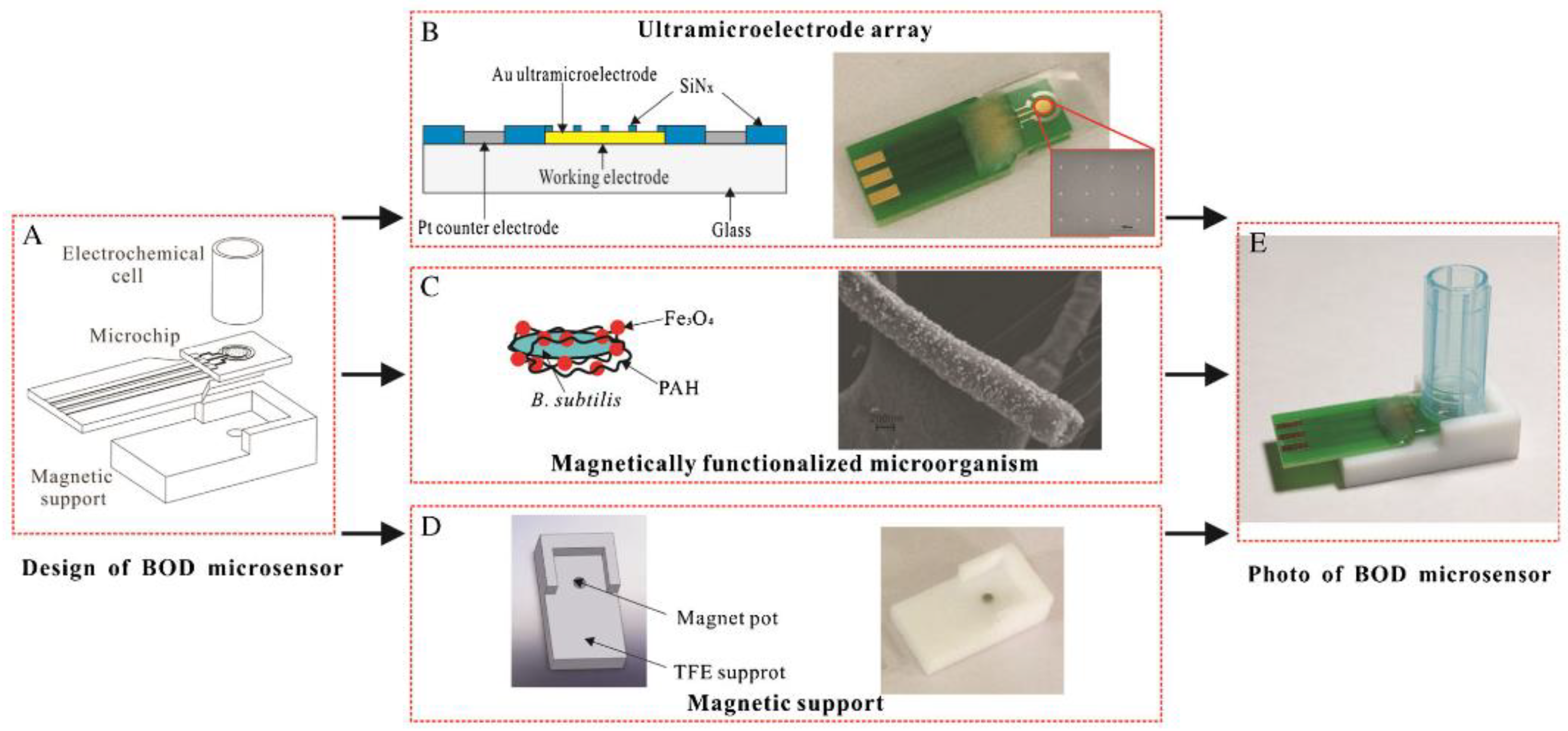
- Wang, J.; Li, Y.; Bian, C.; Tong, J.; Fang, Y.; Xia, S. Ultramicroelectrode array modified with magnetically labeled Bacillus subtilis, palladium nanoparticles and reduced carboxy graphene for amperometric determination of biochemical oxygen demand. Microchim. Acta 2017, 184, 763–771. [Google Scholar] [CrossRef]
- Mattiasson, B.; Larsson, P.O.; Mosbach, K. The microbe thermistor. Nature 1977, 268, 519–520. [Google Scholar] [CrossRef]
- Gotovtsev, A.V. Evaluating BOD and the coefficient of oxidation rate: Monitoring, direct and inverse problems, formulas, calculations and tables. Water Resour. 2016, 43, 885–898. [Google Scholar] [CrossRef]
- Gotovtsev, A.V. Determination of the biochemical oxygen demand and of the oxidation rate based on a modified Streeter–Phelps system. Dokl. Akad. Nauk 2015, 460, 713. [Google Scholar] [CrossRef]
- Streeter, H.W.; Phelps, E.B. A Study of the Pollution and Natural Purification of the Ohio River. In U.S. Public Health Services Bull; U.S. Department of Health, Education, & Welfare: Washington, DC, USA, 1925; Volume 146, pp. 1–75. [Google Scholar]
- Ahmad, H.A.; Ni, S.-Q.; Ahmad, S.; Zhang, J.; Ali, M.; Ngo, H.H.; Guo, W.; Tan, Z.; Wang, Q. Gel immobilization: A strategy to improve the performance of anaerobic ammonium oxidation (anammox) bacteria for nitrogen-rich wastewater treatment. Bioresour. Technol. 2020, 313, 123642. [Google Scholar] [CrossRef]
- Liu, C.; Ma, C.; Yu, D.; Jia, J.; Liu, L.; Zhang, B.; Dong, S. Immobilized multi-species based biosensor for rapid biochemical oxygen demand measurement. Biosens. Bioelectron. 2011, 26, 2074–2079. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Yudina, N.Y.; Alferov, V.A.; Ponamoreva, O.N.; Machulin, A.V.; Reshetilov, A.N. A biosensor based microorganisms immobilized in layer-by-layer films for the determination of biochemical oxygen demand. Appl. Biochem. Microbiol. 2021, 57, 133–141. [Google Scholar] [CrossRef]
- Raud, M.; Tenno, T.; Jõgi, E.; Kikas, T. Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzym. Microb. Technol. 2012, 50, 221–226. [Google Scholar] [CrossRef]
- Ponamoreva, O.N.; Afonina, E.L.; Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Boronin, A.M. The yeast Debaryomyces hansenii in an organosilicon shell as the basis of a heterogeneous biocatalyst. Biotekhnologiya 2017, 33, 44–53. [Google Scholar] [CrossRef]
- Tag, K.; Lehmann, M.; Chan, C.; Renneberg, R.; Riedel, K.; Kunze, G. Measurement of biodegradable substances with a mycelia-sensor based on the salt tolerant yeast Arxula adeninivorans LS3. Sens. Actuators B Chem. 2000, 67, 142–148. [Google Scholar] [CrossRef]
- Chan, C.; Lehmann, M.; Chan, K.; Chan, P.; Chan, C.; Gruendig, B.; Kunze, G.; Renneberg, R. Designing an amperometric thick-film microbial BOD sensor. Biosens. Bioelectron. 2000, 15, 343–353. [Google Scholar] [CrossRef]
- Liang, Q.; Yamashita, T.; Yamamoto-Ikemoto, R.; Yokoyama, H. Flame-oxidized stainless-steel anode as a probe in bioelectrochemical system-based biosensors to monitor the biochemical oxygen demand of wastewater. Sensors 2018, 18, 607. [Google Scholar] [CrossRef] [Green Version]
- Guisan, J.M. (Ed.) Immobilization of Enzyme and Cells, 2nd ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; p. 449. [Google Scholar]
- Aleshina, E.Y.; Yudanova, T.N.; Skokova, I.F. Production and properties of polyvinyl alcohol spinning solutions containing protease C and polyhexamethylene guanidine. Fibre Chem. 2001, 33, 421–423. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Saverina, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. Preparation of hybrid sol-gel materials based on living cells of microorganisms and their application in nanotechnology. Nanomaterials 2022, 12, 1086. [Google Scholar] [CrossRef]
- Yang, Z.; Sasaki, S.; Karube, I.; Suzuki, H. Fabrication of oxygen electrode arrays and their incorporation into sensors for measuring biochemical oxygen demand. Anal. Chim. Acta 1997, 357, 41–50. [Google Scholar] [CrossRef]
- Tan, T.C.; Lim, E.W.C. Thermally killed cells of complex microbial culture for biosensor measurement of BOD of wastewater. Sens. Actuators B Chem. 2005, 107, 546–551. [Google Scholar] [CrossRef]
- Raudkivi, K.; Tutt, M.; Talpsep, E.; Kikas, T. Pseudomonas putida P67. 2 and Pseudomonas flourescens P75 based microbial sensors for biochemical oxygen demand (BOD) measurements in phenolic wastewaters of oil shale industry. Oil Shale 2008, 25, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.S.; Choo, K.H.; Chang, H.N.; Park, J.K. A flow injection analysis system with encapsulated high-density Saccharomyces cerevisiae cells for rapid determination of biochemical oxygen demand. Appl. Microbiol. Biotechnol. 2009, 83, 217–223. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Zhong, L.; Liu, C.; Jia, J.; Xu, X.; Liu, L.; Dong, S. A biofilm reactor-based approach for rapid on-line determination of biodegradable organic pollutants. Biosens. Bioelectron. 2012, 34, 77–82. [Google Scholar] [CrossRef]
- Chee, G.-J. Development and characterization of microbial biosensors for evaluating low biochemical oxygen demand in rivers. Talanta 2013, 117, 366–370. [Google Scholar] [CrossRef]
- Liu, L.; Zhai, J.; Zhu, C.; Gao, Y.; Wang, Y.; Han, Y.; Dong, S. One-pot synthesis of 3-dimensional reduced graphene oxide-based hydrogel as support for microbe immobilization and BOD biosensor preparation. Biosens. Bioelectron. 2015, 63, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ponamoreva, O.N.; Afonina, E.L.; Kamanina, O.A.; Lavrova, D.G.; Arlyapov, V.A.; Alferov, V.A.; Boronin, A.M. Yeast debaryomyces hansenii within ormosil shells as a heterogeneous biocatalyst. Appl. Biochem. Microbiol. 2018, 54, 736–742. [Google Scholar] [CrossRef]
- Rybochkin, P.V.; Kamanina, O.A.; Lantsova, E.A.; Arlyapov, V.A.; Saverina, E.A. Characterization of the catalytic ability and surface properties of a heterogeneous biocatalyst obtained by the sol-gel method. J. Sol Gel Sci. Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- Oota, S.; Hatae, Y.; Amada, K.; Koya, H.; Kawakami, M. Development of mediated BOD biosensor system of flow injection mode for shochu distillery wastewater. Biosens. Bioelectron. 2010, 26, 262–266. [Google Scholar] [CrossRef]
- Liu, L.; Bai, L.; Yu, D.; Zhai, J.; Dong, S. Biochemical oxygen demand measurement by mediator method in flow system. Talanta 2015, 138, 36–39. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wang, J.; Bian, C.; Tong, J.; Li, Y.; Xia, S. A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem. Eng. J. 2016, 112, 219–225. [Google Scholar] [CrossRef]
- Zaitseva, A.S.; Arlyapov, V.A.; Yudina, N.Y.; Alferov, S.V.; Reshetilov, A.N. Use of one- and two-mediator systems for developing a BOD biosensor based on the yeast Debaryomyces hansenii. Enzym. Microb. Technol. 2017, 98, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Reshetilov, A.N.; Avtukh, A.N.; Starodumova, I.P. Mediator BOD biosensor based on cells of microorganisms isolated from activated sludge. Appl. Biochem. Microbiol. 2019, 55, 189–197. [Google Scholar] [CrossRef]
- Kwok, N.-Y.; Dong, S.; Lo, W.; Wong, K.-Y. An optical biosensor for multi-sample determination of biochemical oxygen demand (BOD). Sens. Actuators B Chem. 2005, 110, 289–298. [Google Scholar] [CrossRef]
- Wang, S.; Zu, L.; Miao, Y.; Fei, C.; Zhang, H.; Li, B.; Zhang, K.; Liu, F. Simultaneous measurement of the BOD concentration and temperature based on a tapered microfiber for water pollution monitoring. Appl. Opt. 2020, 59, 7364–7370. [Google Scholar] [CrossRef]
- Chang, I.S.; Moon, H.; Jang, J.K.; Kim, B.H. Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors. Biosens. Bioelectron. 2005, 20, 1856–1859. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Curtis, T.P.; Head, I.M.; Scott, K. A single-chamber microbial fuel cell as a biosensor for wastewaters. Water Res. 2009, 43, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Min, B.; Martins, G.; Brito, A.G.; Kroff, P.; Parpot, P.; Angelidaki, I.; Nogueira, R. In situ microbial fuel cell-based biosensor for organic carbon. Bioelectrochemistry 2011, 81, 99–103. [Google Scholar] [CrossRef]
- Yi, Y.; Xie, B.; Zhao, T.; Liu, H. Comparative analysis of microbial fuel cell based biosensors developed with a mixed culture and Shewanella loihica PV-4 and underlying biological mechanism. Bioresour. Technol. 2018, 265, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, S.; Zhang, P.; Ye, J.; Tao, X.; Li, F.; Zhou, Z.; Nabi, M. Enhancement of biological oxygen demand detection with a microbial fuel cell using potassium permanganate as cathodic electron acceptor. J. Environ. Manag. 2019, 252, 109682. [Google Scholar] [CrossRef]
- Guo, F.; Liu, H. Impact of heterotrophic denitrification on BOD detection of the nitrate-containing wastewater using microbial fuel cell-based biosensors. Chem. Eng. J. 2020, 394, 125042. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, R.; Huang, J.J.; Selvaganapathy, P.R. Development of a xurographically fabricated miniaturized low-cost, high-performance microbial fuel cell and its application for sensing biological oxygen demand. Sens. Actuators B Chem. 2020, 304, 127432. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Liu, H. Hibernations of electroactive bacteria provide insights into the flexible and robust BOD detection using microbial fuel cell-based biosensors. Sci. Total Environ. 2021, 753, 142244. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kobayashi, S.; Hirata, Y.; Suzuki, K.; Mogi, Y.; Karube, I. A spectrophotometric biochemical oxygen demand determination method using 2,6-dichlorophenolindophenol as the redox color indicator and the eukaryote Saccharomyces cerevisiae. Anal. Biochem. 2007, 369, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Melidis, P.; Vaiopoulou, E.; Aivasidis, A. Development and implementation of microbial sensors for efficient process control in wastewater treatment plants. Bioprocess. Biosyst. Eng. 2008, 31, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.P.F.; Cunha, E.; Azevedo, A.M.O.; Pereira, S.A.P.; Neves, A.F.D.C.; Vilaranda, A.G.; Araujo, A.R.T.S.; Passos, M.L.C.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Microfluidic chemiluminescence system with yeast saccharomyces cerevisiae for rapid biochemical oxygen demand measurement. ACS Sustain. Chem. Eng. 2018, 6, 6094–6101. [Google Scholar] [CrossRef]
- JIS K3602; Testing Methods for Industrial Waste Water. Japanese Industrial Standard Committee: Tokyo, Japan, 1990.
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992; pp. 5.1–5.6. [Google Scholar]
- Liu, J.; Bjornsson, L.; Mattiasson, B. Immobilised activated sludge based biosensor for biochemical oxygen demand measurement. Biosens. Bioelectron. 2000, 14, 883–893. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Kitagawa, K.; Ando, T.; Murakami, Y.; Morita, Y.; Yamamura, A.; Yokoyama, K.; Tamiya, E. A rapid BOD sensing system using luminescent recombinants of Escherichia coli. Biosens. Bioelectron. 2003, 19, 115–121. [Google Scholar] [CrossRef]
- Thévenot, R.D.; Toth, K.; Durst, A.D.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Kim, M.-N.; Park, K.-H. Klebsiella BOD sensor. Sens. Act. B 2001, 80, 9–14. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, E.; Minamiyama, Y.; Toyoda, T. BOD biosensor for secondary effluent from wastewater treatment plants. Water Sci. Technol. 1994, 30, 215–227. [Google Scholar] [CrossRef]
- Organization for Economic Corporation and Development (OECD). OECD Guidelines Testing Chem; Organization for Economic Corporation and Development: Paris, France, 1991; Volume 209, p. 1.
- Lóránt, B.; Gyalai-Korpos, M.; Goryanin, I.; Tardy, G.M. Single chamber air–cathode microbial fuel cells as biosensors for determination of biodegradable organics. Biotechnol. Lett. 2019, 41, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Tardy, G.M.; Lóránt, B.; Gyalai-Korpos, M.; Bakos, V.; Simpson, D.; Goryanin, I. Microbial fuel cell biosensor for the determination of biochemical oxygen demand of wastewater samples containing readily and slowly biodegradable organics. Biotechnol. Lett. 2021, 43, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Spurr, M.W.A.; Yu, E.H.; Scott, K.; Head, I.M. Extending the dynamic range of biochemical oxygen demand sensing with multi-stage microbial fuel cells. Environ. Sci. Water Res. Technol. 2018, 4, 2029–2040. [Google Scholar] [CrossRef] [Green Version]
- BOD Analyzer for Controlling WWTPS. Available online: https://lar.com/products/lar/bod-toxicity-analysis/bod-analyzer-biomonitor/ (accessed on 30 September 2022).
- Logroño, W.; Guambo, A.; Pérez, M.; Kadier, A.; Recalde, C. A terrestrial single chamber microbial fuel cell-based biosensor for biochemical oxygen demand of synthetic rice washed wastewater. Sensors 2016, 16, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial In-telligence (AI) and Internet of Medical Things (IoMT) assisted biomedical systems for intelligent healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef] [PubMed]








| Microorganisms, Immobilization | Measured Samples | Major Characteristics | References, Year |
|---|---|---|---|
| Oxygen Electrode-Based Amperometric BOD Biosensors | |||
| A preparation of activated sludge (BODseed), the cells in which are killed by heating at 300 °C for 1.75 min. Immobilization by adsorption onto a polycarbonate membrane | Glucose glutamic acid (GGA) mix/wastewaters | Range of measured BOD5 values, up to 40 mg O2/dm3; measurement time, 40–45 min; sensitivity, 0.412 μA/(mg BOD /L) | [107], 2005 |
| Association of microorganisms immobilized by adsorption on a nylon membrane | GGA/wastewaters | Response time, 90 min; long-term stability, 60 days; lower detection limit, 1 mg O2 mg O2/dm3; range of measured BOD5 values, up to 75 mg O2/L; correlation between BODbiosens and BOD5 (deviation, 10%), convergence, 3.49–4.45%; sensitivity, 2.8 nA/(mg BOD/L) | [9], 2008 |
| P. putida bacteria immobilized in agarose gel | GGA/synthetic wastewaters (OECD). Effluents with increased content of phenol | Range of measured BOD5 values, 0–50 mg O2/dm3; measurement time, 15–45 min; sensitivity, 0.0136 n.r./(mg BOD/L) | [108], 2008 |
| S. cerevisiae yeast immobilized in calcium alginate | GGA/wastewaters | Response time, 90 min; R = 0.95; long-term stability, 30 days; range of measured BOD5 values, 2–30 mg O2/dm3; sensitivity, 0.06 ppm of dissolved oxygen/(mg BOD/L) | [109], 2009 |
| Commercial preparation of microorganisms of activated sludge (BODseed). Immobilization into an organosilicon sol–gel based on silicic acid and grafted copolymer of polyvinyl alcohol and 4-vinylpyridine | GGA/ synthetic wastewaters (OECD) | Lower boundary of detected BOD5 values, 0.5 mg O2/dm3; measurement time, 10 min; long-term stability, 50 days; R = 0.9851; range of measured BOD5 values, up to 50 mg O2/dm3; sensitivity, 19.7 mV/(mg BOD5/L) | [95], 2011 |
| Microorganisms of D. hansenii VKM Y-2482. Immobilization using a dialysis membrane | GGA/wastewaters of treatment plants and glucose treacle plant | Range of measured BOD5 values, 2.2–177 mg O2/dm3; measurement time, 10–17 min; reproducibility, 4%; long-term stability, >30 days; R = 0.98; sensitivity, 0.21 nA dm3/min mg | [17], 2012 |
| Biofilm of a natural community of microorganisms from local wastewaters grown on a glass substrate | GGA, synthetic wastewaters (OECD). Wastewaters of treatment plants, food industry and surface waters | Range of measured BOD5 values, 0.5–30 mg O2/dm3; measurement time, 6–8 min; reproducibility, 3.9%; long-term stability, 17 months; R = 0.983; sensitivity, 14.7 nA/(mg GGA/L) | [110], 2012 |
| Ps. fluorescens P75 bacteria immobilized in agarose gel | Synthetic wastewaters (OECD), wastewaters of meat processing plants | Linear range of measured BOD7 values, 5–40 mg O2/dm3; long-term stability, 80 days; measurement time, up to 20 min | [98], 2012 |
| Aeromonas hydrophila P69.1 bacteria immobilized in agarose gel | Synthetic wastewaters (OECD), wastewaters of meat processing plants | Linear range of measured BOD7 values, 5–45 mg/dm3; long-term stability, 90 days; measurement time, up to 20 min; sensitivity, 0.019 n.r./(mg BOD7/L) | [98], 2012 |
| Yeast D. hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone | GGA, wastewaters of treatment plants and food productions | Range of measured BOD5 values, 0.7–206.7 mg O2/dm3; measurement time, 5–7 min; reproducibility, 4.2%; long-term stability, 30 days; R2 = 0.9911; sensitivity 0.0045 min−1 | [22], 2013 |
| Individual biosensors based on P. fluorescens, A. hydrophila, P. putida, E. coli, B. subtilis, Paenibacillus sp., Microbacterium phyllosphaerae bacteria, immobilized in agarose gel. Mathematical processing of the data of 7 sensors for accurate determination of BOD | Synthetic wastewaters (OECD) and waters modelling the composition of effluents of meat, milk, oil shale and paper industries | Standard deviation of the results, less than 3.4% | [23], 2013 |
| P. putida bacteria immobilized on a nitrocellulose membrane | GGA/synthetic wastewaters. River water samples | Range of measured BOD5 values, 0.5–10 mg O2/dm3; measurement time, 15 min; long-term stability, 10 days; sensitivity, 0.175 μA/(mg O2/L) | [111], 2013 |
| Biofilm of microorganisms from wastewater treatment plants on a hydrogel of reduced graphene oxide | GGA/synthetic wastewaters (OECD) | Range of measured BOD5 values, 2–64 mg O2/dm3; BOD5 detection limit, 0.4 mg O2/dm3; measurement time, 10 min; long-term stability, 2 months; R = 0.981; sensitivity, 4.8 nA/(mg O2/L) | [112], 2015 |
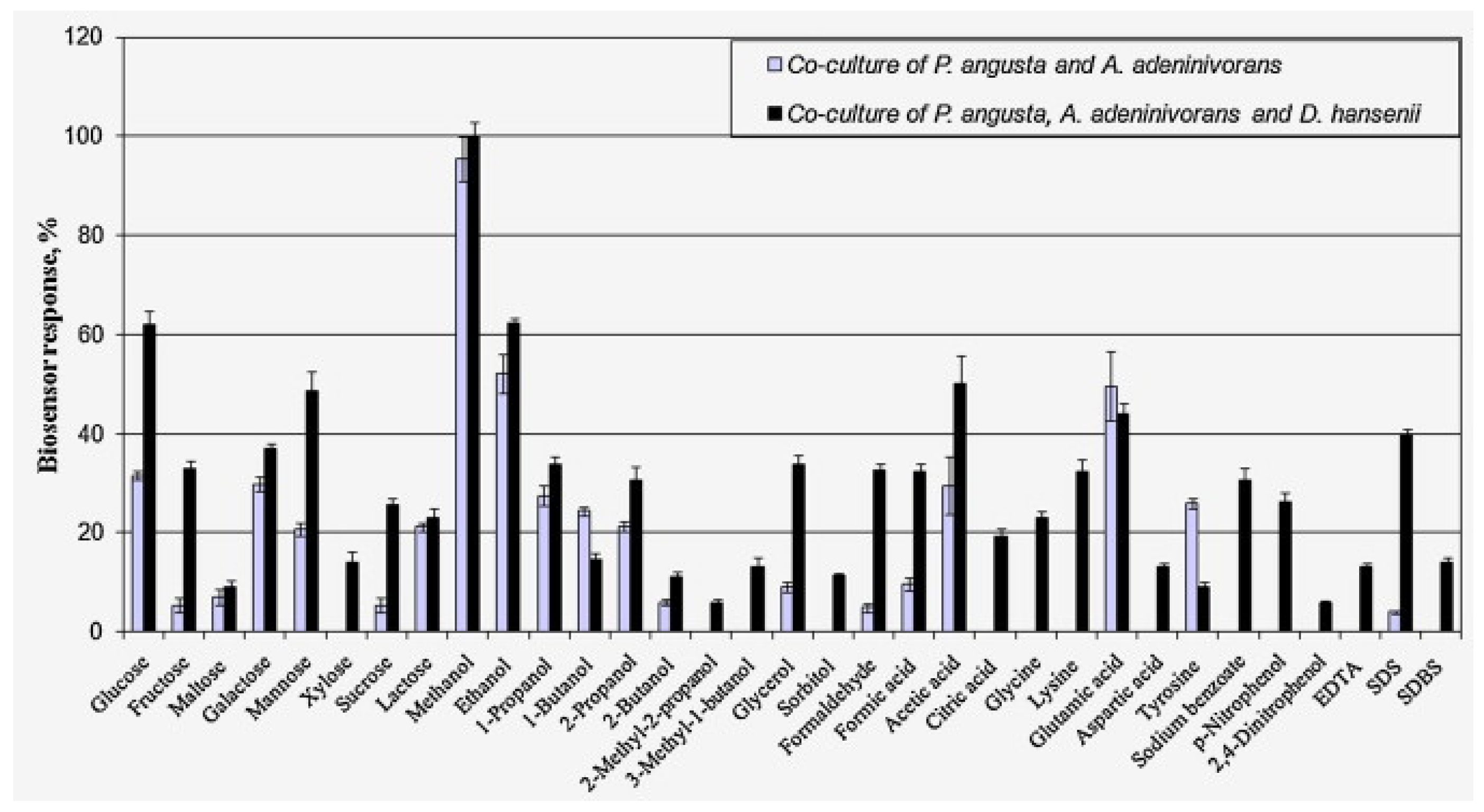
| Co-cultures of the yeasts Pichia angusta, A. adeninivorans and D. hansenii immobilized in N-vinylpyrrolidone-modified poly(vinyl alcohol) | GGA/river and swamp waters, sewage from urban sewage facilities, meltwater, fermentation products of starchy raw materials (distillery stillage) | Range of measured BOD5 values, 2.4–80 mg O2/dm3; measurement time, 3 min; reproducibility, 8.9%; long-term stability, 17 days; R2 = 0.9988; sensitivity coefficient, 10 s−1 × 10−5 | [13], 2015 |
| Biofilm of wastewater microorganisms. Biosensor of bioreactor type | GGA/samples of surface waters | Long-term stability, over 30 days | [38], 2016 |
| E. coli bacteria immobilized in collagen fiber with Zr(IV) | GGA/synthetic wastewaters (OECD). Samples of river water | Range of measured BOD5 values, up to 225 mg O2/dm3; long-term stability, 42 days; sensitivity 0.0085 (mg/L of dissolved oxygen)/(mg BOD/L) | [26], 2017 |
| Yeast D. hansenii in an organosilicate matrix based on polyethylene glycol, tetraethoxysilane and methyltriethoxysilane | GGA/ samples of river and well water, as well as sewage and meltwater | Range of measured BOD5 values, 0.5–5 mg O2/dm3; reproducibility, 6.7%; long-term stability, 40 days; R2 = 0.9988; sensitivity coefficient, 0.40 nA L/mg O2 | [113], 2018 |
| P. yeei VKM B-3302 bacteria isolated from activated sludge and immobilized in an N-vinylpyrrolidone-modified poly(vinyl alcohol) matrix | GGA/wastewaters of municipal treatment facilities, effluents of food industry plants, natural waters, including those of ponds polluted with fuels and lubricants | Range of measured BOD5 values, 0.05–5.0 mg O2/dm3; reproducibility, 7%; measurement time, 4–6 min; long-term stability, 45 days; R2 = 0.9990; sensitivity coefficient, 110 10−5 × s−1 | [96], 2020 |
| Activated sludge bacteria P. yeei, P. veronii and B. proteolyticus immobilized layer-by-layer in a hydrogel of polyvinyl alcohol modified with N-vinylpyrrolidone | GGA/ wastewaters of municipal treatment facilities, effluents of food industry plant, waters from ponds and rivers within the municipal area | Range of measured BOD5 values, 0.51–3.80 mg O2/dm3; measurement time, 5–12 min; reproducibility, 9.5%; long-term stability, 52 days; R = 0.9956; sensitivity coefficient, 120 10−5 × s−1 | [97], 2021 |
| Activated sludge bacteria P. yeei in an organosilica sol–gel matrix consisting of tetraethoxysilane, methyltriethoxysilane and polyvinyl alcohol as a structure-modifying agent | GGA/ samples of natural surface waters and industrial wastewaters | Range of measured BOD5 values, 0.01–20 mg O2/dm3; measurement time, 4–6 min; reproducibility, 3%; long-term stability, 31 days; R = 0.9983; sensitivity coefficient, 30 10−3 × min−1 | [27], 2021 |
| Mixture of the bacteria P. yeei VKM B-3302 and the yeast D. hansenii VKM Y-2482 in a tetraethoxysilane / methyltriethoxysilane sol–gel matrix | GGA/surface waters of rivers and ponds | Range of measured BOD5 values, 0.5–2.9 mg O2/dm3; reproducibility, 3%; long-term stability, 42 days; R2 = 0.9980; sensitivity coefficient, 43 10−3 × min−1 | [114], 2022 |
| Mediator-type amperometric BOD biosensors | |||
| Yeast S. cerevisiae immobilized in polyethylene terephthalate. Two-mediator system with ferricyanide and lipophilic mediator menadione | GGA/samples of river and sea water | Range of measured BOD5 values, 6.6–220 mg O2/dm3; R = 0.999; relative standard deviation, 1.3%; reduction of sensor response after the storage at 4 °C for 14 days, 93%; sensitivity, 0.33 μA/(mg O2/L) | [21], 2007 |
| Bacteria Exiguobacterium marius, B. horikoshii and Halomonas marina immobilized in an organosilicon sol–gel matrix with PVA. Mediator of potassium hexacyanoferrate (III) | GGA/samples of sea water | Range of measured BOD5 values, 1.2–40 mg O2/dm3; BOD5 detection limit, 0.8 mg O2/dm3; standard deviation of the results, 3.8%; R = 0.988; sensitivity, 0.04 μA/(mg O2/L) | [16], 2008 |
| Yeast Candida krusei applied to silica gel particles. Mediator of potassium hexacyanoferrate (III) | GGA, synthetic wastewaters (OECD), distillery wastewaters | Range of measured BOD5 values, 0–140 mg O2/dm3; measurement time, 20 min; sensitivity, 0.00625 mC/(mg BOD/L) | [115], 2010 |
| Bacteria Klebsiella pneumoniae, mediator of potassium hexacyanoferrate (III) | GGA/synthetic wastewaters (OECD), municipal wastewaters | Linear dependence of BOD5 values, 30–500 mg O2/dm3 or 30–200 mg O2/dm3, using GGA and synthetic wastewaters, respectively; R = 0.9962 and 0.9934, respectively; sensitivity, 0.0016 μA/(mg BOD/L) | [47], 2011 |
| E. coli bacteria immobilized in PVA–polyvinylpyridine copolymer. Mediator, neutral red | GGA/synthetic wastewaters (OECD), urea and real wastewaters | Range of measured BOD5 values, 50–1000 mg O2/dm3; relative standard deviation, 2.8%; measurement time, 15–60 min; sensitivity, 0.94 nA/(mg GGA/L) | [43], 2012 |
| Activated sludge. Mediator, potassium hexacyanoferrate (III) | GGA/synthetic wastewaters (OECD), wastewaters of various origin | Range of measured BOD5 values, 2.1–40 mg O2/dm3; measurement time, 240 min; R = 0.96 | [48], 2013 |
| Biofilm based on wastewater microorganisms. Mediator, potassium hexacyanoferrate (III) | GGA/samples of treatment plant effluents | Range of measured BOD5 values, 2–200 mg O2/dm3; measurement time, 30 min; long-term stability, 60 days; sensitivity, 4.32 nA/mg BOD | [116], 2015 |
| Chromobacterium violaceum bacteria immobilized in calcium alginate. Mediator, potassium hexacyanoferrate (III) | GGA/synthetic wastewaters (OECD), wastewaters from the food, textile and processing industries; wastewaters from landfills, river water | Range of measured BOD5 values, 20–230 mg O2/dm3; measurement time, 30 min; repeatability, 4.1%; R = 0.9663; sensitivity, 0.0538 nA/(mg O2/L) | [44], 2015 |
| P. aeruginosa bacteria immobilized in polypyrrole. Mediator, potassium hexacyanoferrate (III) | GGA | Range of measured BOD5 values, 5–100 mg O2/dm3; BOD5 detection limit, 2 mg O2/dm3; repeatability, 3.6%; measurement time, 15 min; sensitivity, 1.58 nA/(mg O2/L) | [117], 2016 |
| P. aeruginosa bacteria immobilized in the polypyrrole–alginate matrix. Mediator, semineutral red | Samples of river water | Range of measured BOD5 values, 5–100 mg O2/dm3; BOD5 detection limit, 3 mg O2/dm3; measurement time, 20 min; sensitivity, 3.67 nA/(mg O2/L) | [42], 2016 |
| B. subtilis bacteria covalently bound to a gold electrode | GGA | Range of measured BOD5 values, 5–30 mg O2/dm3; BOD5 detection limit, 1.65 mg O2/dm3; R = 0.978; sensitivity, 1.58 nA/(mg O2/L) | [117], 2016 |
| Yeast D. hansenii immobilized by adsorption on a graphite-paste electrode. Mediator, ferrocene | GGA/swamp water, wastewaters of municipal sewage treatment facilities, wastewaters of fermentation products | Range of measured BOD5 values, 25.2–320 mg O2/dm3; measurement time, 8–20 min; reproducibility, 2.2%; long-term stability, 39 days; R = 0.9971; sensitivity, 0.5 nA dm3 /mg | [46], 2017 |
| B. subtilis bacteria in a three-dimensional porous graphene–polypyrrole (rGO-PPy) composite material. Mediator, potassium hexacyanoferrate (III) | Real samples of lake and river water, domestic wastewaters of Beijing | Range of measured BOD5 values, 4–60 mg O2/dm3; measurement time, 15 min; BOD5 detection limit, 1.8 mg O2/dm3; long-term stability, 60 days; sensitivity, 0.155 μA/(mg O2/L) | [45], 2017 |
| Yeast D. hansenii immobilized by adsorption on a graphite-paste electrode. Two-mediator system, ferrocene–methylene blue | GGA, swamp and river water, wastewaters from municipal wastewater treatment plants | Range of measured BOD5 values, 2.5–7.2 mg O2/dm3; measurement time, 3.5–6 min; reproducibility, 1.2%; long-term stability, 43 days; R = 0.9913; sensitivity coefficient, 1.1 nA dm3/mg O2 | [118], 2017 |
| Activated sludge immobilized in a chitosan–bovine serum albumin (Chi–BSA) cryogel. Mediator, methylene blue in combination with graphene | GGA/wastewaters of various origins | Range of measured BOD5 values, 1–100 mg O2/dm3; BOD5 detection limit, 0.1 mg O2/dm3; operational stability, 2.9%; measurement time, 9 min; long-term stability, 65 days; sensitivity, 0.19 μA/(mg O2/L) | [51], 2017 |
| P. yeei bacteria isolated from activated sludge immobilized by adsorption on a graphite-paste electrode. Mediator, ferrocene | GGA, river water | Range of measured BOD5 values, 1.3–200 mg O2/dm3; measurement time, 4–6 min; reproducibility, 2.9%; long-term stability, 22 days; R = 0.9934; sensitivity, 4.7 nA/(mg O2/dm3) | [119], 2019 |
| Electroactive biofilm of Sh. loihica. Bidirectional extra-cellular electron transfer | Synthetic samples | Range of measured BOD5 values, 0–435 mg O2/dm3; assay time, less than 1 h; R = 0.99; sensitivity, 1.4 μA/(mg O2/dm3) | [28], 2020 |
| Yeast Blastobotrys adeninivorans immobilized by adsorption on a graphite-paste electrode. Two-mediator system, ferrocene–neutral red | GGA/samples of surface waters | Range of measured BOD5 values, 0.16–2.7 mg O2/dm3; measurement time, 4–5 min; reproducibility, 1.5%; long-term stability, 26 days; R = 0.9693; sensitivity coefficient, 52 nA dm3/mg O2 | [24], 2021 |
| P. yeei bacteria isolated from activated sludge, immobilized in a redox-active polymer based on chitosan covalently bound to ferrocene and including carbon nanotubes | GGA/samples of surface waters | Range of measured BOD5 values, 0.1–4.5 mg O2/dm3; measurement time, 5 min; reproducibility, 1.8%; long-term stability, 50 days; R = 0.9916; sensitivity coefficient, 0.028 μA/(mg O2/dm3) | [57], 2021 |
| Electroactive biofilms of activated sludge grown on the surface of a graphite-paste electrode modified with carbon nanotubes | GGA/samples of surface waters | Range of measured BOD5 values, 0.41–23 mg O2/dm3; measurement time, 5 min; reproducibility, 5.96%; long-term stability, 53 days; R = 0.9901; sensitivity coefficient, 0.04 μA/(mg O2/dm3) | [30], 2022 |
| BOD biosensors based on optical transducers | |||
| B. subtilis bacteria immobilized in a composite tetramethoxysilane sol–gel and chemically modified polyvinyl alcohol. Oxygen-sensitive film of tris(4,7-diphenyl-1,10-phenanthroline) ruthenium(II) | GGA/synthetic and household wastewaters | Range of measured BOD5 values, up to 25 mg O2/dm3; assay time, 15–30 min; long-term stability, over 45 days; R = 0.9808; sensitivity, 0.008 (mg/L min of dissolved oxygen)/(mg BOD/L) | [120], 2005 |
| Microorganisms B. licheniformis, Dietzia maris and Marinobacter marinus from sea water, immobilized in polyvinyl alcohol and organosilicon sol–gel matrix. Sensitive film from organically modified silicate film with embedded oxygen-sensitive ruthenium complex | GGA/samples of sea water | Range of measured BOD5 values, from 0.2 up to 40 mg O2/dm3; measurement time, 3.2 min; stable operation time, up to 1 year; reproducibility, 2%; R = 0.9933 | [15], 2006 |
| Photobacterium phosphoreum bacteria immobilized in sodium alginate gel. Measurement of bioluminescence intensity | GGA/wastewaters of treatment plants | Assay time, 20 min; range of measured BOD5 values, 0 and 16 ppm; sensitivity, 522.75 conventional units/(mg BOD/L) | [65], 2007 |
| Yeast S. cerevisiae immobilized by inclusion in PVA–styrilpyridinium gel. Optical sensor based on fluorescence quenching of a complex compound of ruthenium | GGA | Range of measured BOD5 values, 1–20 mg O2/dm3; R = 0.99; sensitivity, 0.001 conventional units/(mg GGA/L) | [66], 2015 |
| Optical sensor based on tapered microfiber | Range of measured BOD5 values, 0.25–1 mg O2/dm3; BOD detection limit, 0.0016 mg O2/dm3 | [121], 2020 | |
| BOD biosensors based on microbial fuel cells | |||
| Activated sludge of sewage treatment plants | Synthetic wastewaters, real-time wastewater monitoring | Range of measured BOD5 values, up to 100 mg O2/dm3; assay time, ~60 min; reproducibility, 10% at the detection of BOD at a concentration of 100 mg O2/dm3 | [122], 2005 |
| Electrochemically active bacteria | Synthetic and real wastewaters | Linear dependence on BOD, up to 350 mg O2/dm3; stable operation time, 7 months; assay time, ~40 min; sensitivity coefficient, 0.001 mA/ppm COD | [123], 2009 |
| Biofilm of wastewater microorganisms | Wastewaters | Range of measured BOD5 values, 17–78 mg O2/dm3; assay time, from 30 min up to 10 h; sensitivity, 3 (mA/m2)/(mg O2/L) | [124], 2011 |
| Electroactive biofilm | Acetate, propionate, glucose, ethanol | Range of measured BOD5 values, 32–1280 mg O2/dm3; assay time, up to 20 h; sensitivity, 0.04 C/(mg O2/L) | [34], 2012 |
| Electroactive biofilm of a mixture of six bacterial strains: Thermincola carboxydiphila, P. aeruginosa, Ochrobactrum intermedium, Sh. frigidimarina, Citrobacter freundii, Clostridium acetobutylicum. | Wastewaters | Range of measured BOD5 values, 8–240 mg O2/dm3; sensitivity, 0.7498 mV/(mg O2/L) | [18], 2014 |
| Electroactive biofilm with a high content of Geobacter bacteria | Synthetic wastewaters | Range of measured BOD5 values, 174–1200 mg O2/dm3; assay time, 17.5 h; sensitivity, 0.0031 C/(mg O2/L) | [32], 2016 |
| Electroactive biofilm of Sh. loihica | Synthetic wastewaters | Range of measured BOD5 values, 0–43.5 mg O2/dm3; sensitivity, 4.13 mV/(mg O2/L) | [125], 2018 |
| Gluconobacter oxydans bacteria immobilized in N-vinylpyrrolidone-modified vinyl alcohol | GGA/wastewaters from food and biotechnological plants | Range of measured BOD5 values, 0.34–9.6 mg O2/dm3; measurement time, 30–130 min; reproducibility, 4.1%; long-term stability, 7 days; sensitivity coefficient, 8.3 mV dm3/mg | [80], 2018 |
| Electroactive biofilm | Synthetic wastewaters | Range of measured BOD5 values, 25–500 mg O2/dm3; R = 0.995; sensitivity, 0.014 C/(mg O2/L) | [126], 2019 |
| Electroactive biofilm | GGA/wastewaters with a high content of nitrates | Range of measured BOD5 values, 20–500 mg O2/dm3; sensitivity, 0.0355 C/(mg O2/L) | [127], 2020 |
| Electroactive biofilm of Sh. loihica | GGA | Range of measured BOD5 values, 0–130.5 mg O2/dm3; R = 0.99; sensitivity, 2 μA/(mg O2/L) | [28], 2020 |
| Electroactive biofilm | Synthetic wastewaters | Range of measured BOD5 values, up to 300 mg O2/dm3; long-term stability, over 30 days; R = 0.9950; sensitivity, 0.82 mA/(mg O2/L) | [84], 2020 |
| Electroactive biofilm | Wastewaters from treatment facilities | Range of measured BOD5 values, 20–490 mg O2/dm3; assay time, 1.1 min; sensitivity, 2.9 μA/(g NaAc/L) | [128], 2020 |
| Electroactive biofilm | GGA | Range of measured BOD5 values, 20–500 mg O2/dm3; long-term stability, about 30 days; R = 0.9950 | [129], 2021 |
| BOD biosensors based on other methods of signal registration | |||
| Measurement of the concentration of CO2 produced by degradation by microorganisms of the carbon component of effluents. CO2 monitoring using an infrared spectrometer | Real-time measurements—determination of BOD in treatment facilities | R = 0.97457 (for CO2 and BOD concentrations); sensitivity, 12.99 ppm CO2/(mg BOD/L) | [87], 2005 |
| Amperometric bioelectric “language” in a group cell using mathematical methods of data processing. Modification of electrodes by tyrosinase, horseradish peroxidase, acetylcholinesterase and butyrylcholinesterase | Wastewater samples | – | [88], 2005 |
| Yeast S. cerevisiae. Photocolorimetric method in the presence of 2,6-dichlorophenolindophenol | GGA/samples of river water | Range of measured BOD5 values, 1.1–22 mg O2/dm3; reproducibility, 1.77%; long-term stability, 36 days; measurement time, 30 min; sensitivity, 0.0118 a.d./(mg O2/L) | [130], 2007 |
| Activated sludge, pH converter. Determination of CO2 under aerobic conditions and NaOH under anaerobic conditions | Monitoring of the degree of contamination with organic compounds and toxicity | Sensitivity, 12.99 ppm CO2/(mg BOD/L) | [131], 2008 |
| Magnetically functionalized B. subtilis bacteria on a matrix of ultramicroelectrodes. Amperometric determination of oxygen using palladium nanoparticles and reduced carboxyl graphene | Water samples | Range of measured BOD5 values, 2–15 mg O2/dm3; measurement time, 5 min; sensitivity, 2.093 nA/(mg O2/L) | [89], 2017 |
| Yeast S. cerevisiae. Automated microfluidic chemiluminescent system with ferricyanide and quinone mediators | GGA/water samples | Range of measured BOD5 values, 10–315 mg O2/dm3 | [132], 2018 |
| A real-time respirometric system with photosensors and sensors for counting bubbles of released gas | GGA, municipal wastewaters | Range of measured BOD5 values, 0–420 mg O2/dm3; R = 0.99 | [39], 2021 |
| Model | Manufacturer, Country | Type | BOD Detection Range, mg/dm3 | Assay Time, Min | Assay Error |
|---|---|---|---|---|---|
| Biox-1010 | Endress and Hauser, Switzerland | Bioreactor | 5–100,000 | 3–15 | 3% |
| BioMonitor | LAR, USA | Bioreactor | 1–200,000 | 3–4 | <5% |
| BOD-3000 | Central Kagaku Corp., Japan | Bioreactor | 0–1000 | 30–60 | – |
| DKK BOD sensor 7842 | DKK Corporation, Japan | Biosensor | 0–60 | 5 | 10% |
| HABS-2000 | KORBI, South Korea | Biofuel cell | 0–200 | 10 | 5% |
| MB-DBO | Biosensores, Spain | Bioreactor | 10–1000 | 30 | <3% |
| Ra-BOD | AppliTek, Belgium | Bioreactor | 20–100,000 | 30 | <5% |
| QuickBOD α1000 | Central Kagaku Corp., Japan | Biosensor | 5–50 | 60 | 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. https://doi.org/10.3390/bios12100842
Arlyapov VA, Plekhanova YV, Kamanina OA, Nakamura H, Reshetilov AN. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors. 2022; 12(10):842. https://doi.org/10.3390/bios12100842
Chicago/Turabian StyleArlyapov, Vyacheslav A., Yulia V. Plekhanova, Olga A. Kamanina, Hideaki Nakamura, and Anatoly N. Reshetilov. 2022. "Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects" Biosensors 12, no. 10: 842. https://doi.org/10.3390/bios12100842







