Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests
Abstract
:1. Introduction
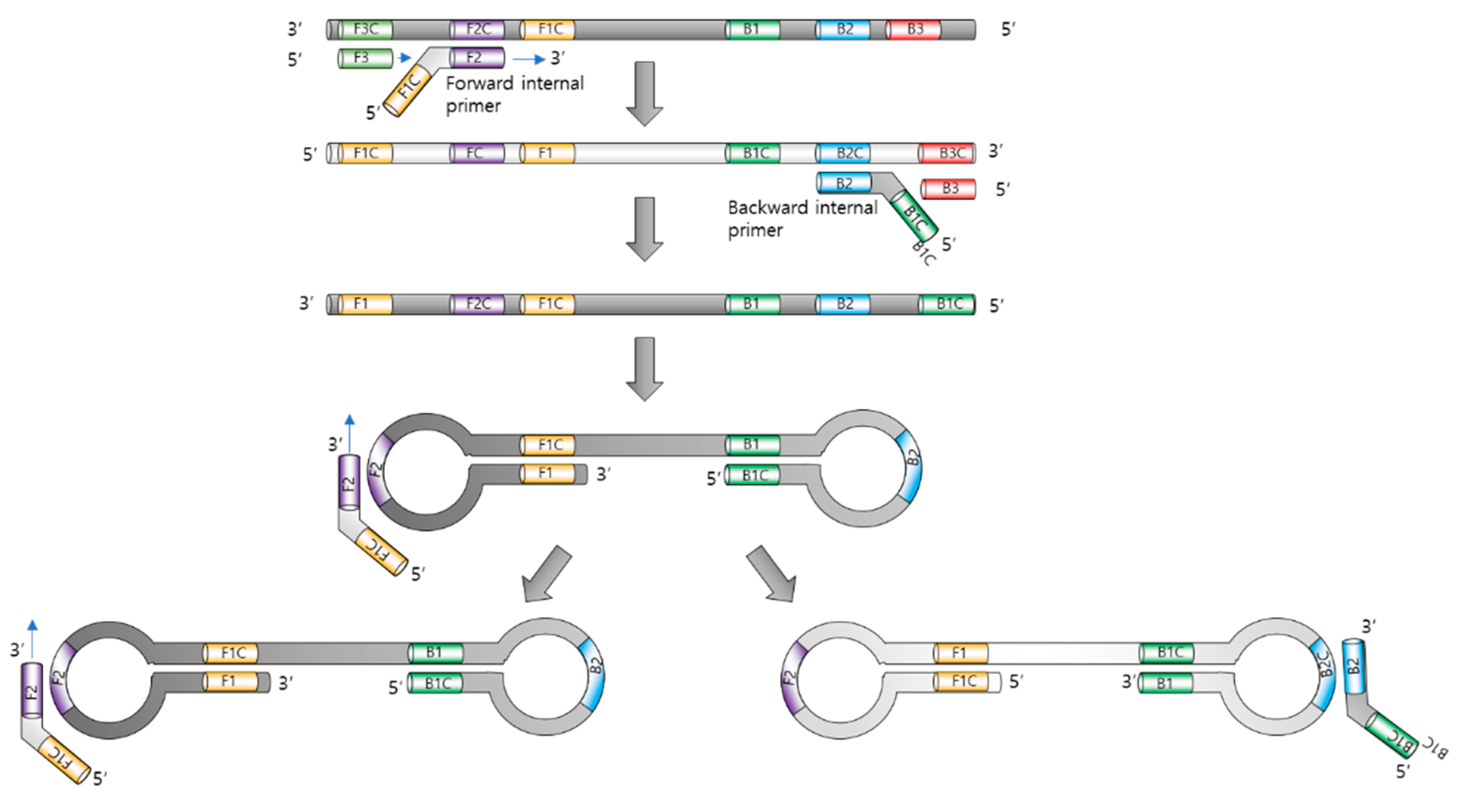
2. Principle
3. Monitoring Methods for LAMP
3.1. Gel Electrophoresis
3.2. Turbidity
3.3. Fluorescence
3.4. Naked-Eye Monitoring
3.4.1. Naked-Eye Monitoring with Intercalating Dyes
3.4.2. Naked-Eye-Monitoring Metal Indicator
3.4.3. Naked-Eye Monitoring with pH Indicator
4. Sensing Platform with LAMP
4.1. Electrochemical Sensing
4.2. Lateral-Flow-Assay-Based Sensing
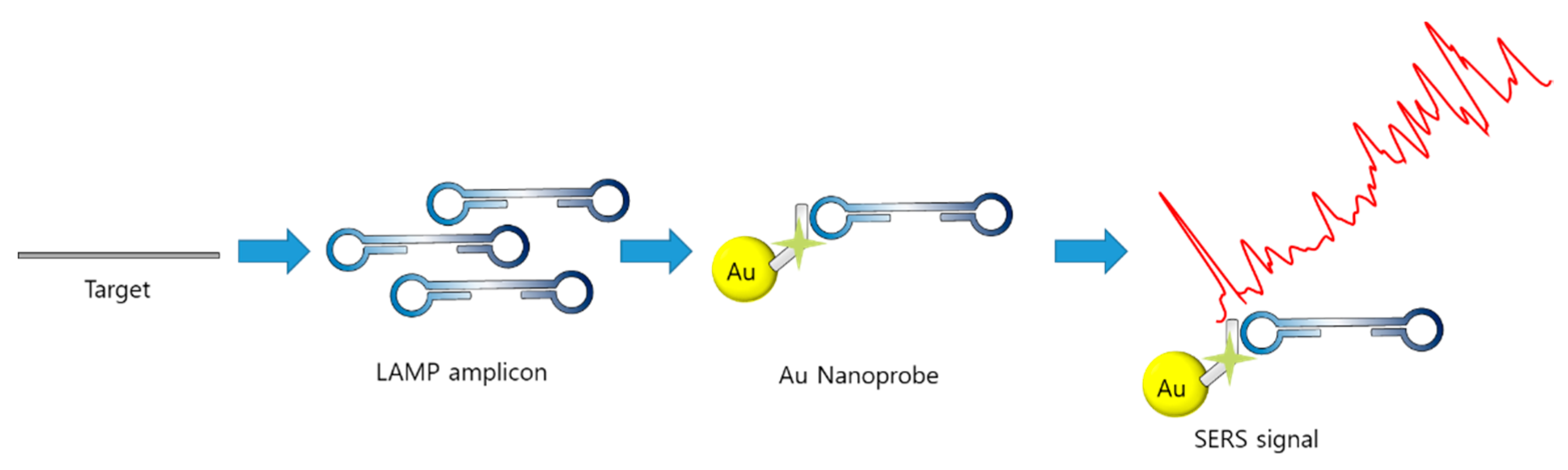
4.3. Optical Sensing with LAMP
5. Devices to Monitor LAMP
6. LAMP Assays for SARS-CoV-2
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Garzarelli, V.; Serena Chiriacò, M.; Cereda, M.; Autuori, I.; Ferrara, F. Miniaturized Real-Time PCR Systems for SARS-CoV-2 Detection at the Point-of-Care. Clin. Chim. Acta. 2022, 536, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-E.; Trick, A.Y.; Hasnain, A.C.; Hsieh, K.; Chen, L.; Shin, D.J.; Wang, T.-H. Ratiometric PCR in a Portable Sample-to-Result Device for Broad-Based Pathogen Identification. Anal. Chem. 2022, 94, 9372–9379. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Zubrzycki, A.; Henry, M.; Ranadheera, C.; Corbett, C.; Meyers, A.F.A.; Sandstrom, P.A.; Becker, M.G. Clinical Evaluation of the GeneXpert® Xpert® Xpress SARS-CoV-2/Flu/RSV Combination Test. J. Clin. Virol. Plus 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical Rt-Pcr Portable Device for Rapid Detection of Sars-Cov-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Zandotti, C.; Ninove, L.; Prudent, E.; Colson, P.; Gazin, C.; Million, M.; Tissot-Dupont, H.; Fenollar, F. Contribution of VitaPCR SARS-CoV-2 to the Emergency Diagnosis of COVID-19. J. Clin. Virol. 2020, 133, 104682. [Google Scholar] [CrossRef]
- Demeke, T.; Jenkins, G.R. Influence of DNA Extraction Methods, PCR Inhibitors and Quantification Methods on Real-Time PCR Assay of Biotechnology-Derived Traits. Anal. Bioanal. Chem. 2010, 396, 1977–1990. [Google Scholar] [CrossRef]
- Sy, R.; Rothman, R.E.; Yang, S. PCR-Based Diagnostics PCR-Based Diagnostics for Infectious Diseases: Uses, Limitations, and Future Applications in Acute-Care Settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar]
- Zou, Y.; Mason, M.G.; Botella, J.R. Evaluation and Improvement of Isothermal Amplification Methods for Point-of-Need Plant Disease Diagnostics. PLoS ONE 2020, 15, e0235216. [Google Scholar] [CrossRef]
- Keikha, M. LAMP Method as One of the Best Candidates for Replacing with PCR Method. Malays. J. Med. Sci. 2018, 25, 121–123. [Google Scholar] [CrossRef]
- Zanoli, L.M.; Spoto, G. Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors 2013, 3, 18–43. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA Tsugunori. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Denschlag, C.; Vogel, R.F.; Niessen, L. Hyd5 Gene Based Analysis of Cereals and Malt for Gushing-Inducing Fusarium Spp. by Real-Time LAMP Using Fluorescence and Turbidity Measurements. Int. J. Food Microbiol. 2013, 162, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Nzelu, C.O.; Gomez, E.A.; Cáceres, A.G.; Sakurai, T.; Martini-Robles, L.; Uezato, H.; Mimori, T.; Katakura, K.; Hashiguchi, Y.; Kato, H. Development of a Loop-Mediated Isothermal Amplification Method for Rapid Mass-Screening of Sand Flies for Leishmania Infection. Acta Trop. 2014, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP) of Gene Sequences and Simple Visual Detection of Products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Cruz, I.; Chicharro, C.; Sánchez, C.; Biéler, S.; Broger, T.; Moreno, J.; Carrillo, E. Evaluation of Fluorimetry and Direct Visualization to Interpret Results of a Loop-Mediated Isothermal Amplification Kit to Detect Leishmania DNA. Parasites Vectors 2018, 11, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Kitao, M.; Tomita, N.; Notomi, T. Real-Time Turbidimetry of LAMP Reaction for Quantifying Template DNA. J. Biochem. Biophys. Methods 2004, 59, 145–157. [Google Scholar] [CrossRef]
- Tanner, N.A.; Evans, T.C. Loop-Mediated Isothermal Amplification for Detection of Nucleic Acids. Curr. Protoc. Mol. Biol. 2013. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.I. Colorimetric Detection of Loop-Mediated Isothermal Amplification Reaction by Using Hydroxy Naphthol Blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, B.H.; Jung, J.H.; Choi, G.; Lee, D.C.; Kim, D.H.; Seo, T.S. Centrifugal Loop-Mediated Isothermal Amplification Microdevice for Rapid, Multiplex and Colorimetric Foodborne Pathogen Detection. Biosens. Bioelectron. 2016, 75, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, B.; Zhao, C.; Liu, H. Visualized Quantitation of Trace Nucleic Acids Based on the Coffee-Ring Effect on Colloid-Crystal Substrates. Langmuir 2019, 35, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rahman, I.A.; Ahmed, M.U. Paper-Based Rapid Detection of Pork and Chicken Using LAMP-Magnetic Bead Aggregates. Anal. Methods 2016, 8, 2391–2399. [Google Scholar] [CrossRef]
- Iwamoto, T.; Sonobe, T.; Hayashi, K. Loop-Mediated Isothermal Amplification for Direct Detection of Mycobacterium Tuberculosis Complex, M. Avium, and M. Intracellulare in Sputum Samples. J. Clin. Microbiol. 2003, 41, 2616–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardinge, P.; Kiddle, G.; Tisi, L.; Murray, J.A.H. Optimised LAMP Allows Single Copy Detection of 35Sp and NOSt in Transgenic Maize Using Bioluminescent Assay in Real Time (BART). Sci. Rep. 2018, 8, 17590. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Wei, S.X.; Ying, J.L.Z.; Safavieh, M.; Ahmed, M.U. A Novel, Sensitive and Label-Free Loop-Mediated Isothermal Amplification Detection Method for Nucleic Acids Using Luminophore Dyes. Biosens. Bioelectron. 2016, 86, 346–352. [Google Scholar] [CrossRef]
- Parida, M.M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.L.; Morita, K. Loop Mediated Isothermal Amplification (LAMP): A New Generation of Innovative Gene Amplification Technique; Perspectives in Clinical Diagnosis of Infectious Diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Accurate, and Cost-Effective Diagnostic Method for Infectious Diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Le, T.H.; Nguyen, N.T.B.; Truong, N.H.; Van De, N. Development of Mitochondrial Loop-Mediated Isothermal Amplification for Detection of the Small Liver Fluke Opisthorchis Viverrini (Opisthorchiidae; Trematoda; Platyhelminthes). J. Clin. Microbiol. 2012, 50, 1178–1184. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Kuo, S.T.; Renault, T.; Chang, P.H. The Development of a Loop-Mediated Isothermal Amplification Assay for Rapid and Sensitive Detection of Abalone Herpesvirus DNA. J. Virol. Methods 2014, 196, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Hataoka, Y.; Zhang, L.; Mori, Y.; Tomita, N.; Notomi, T.; Baba, Y. Analysis of Specific Gene by Integration of Isothermal Amplification and Electrophoresis on Poly(Methyl Methacrylate) Microchips. Anal. Chem. 2004, 76, 3689–3693. [Google Scholar] [CrossRef]
- Compton, J. Nucleic Acid Sequence-Based Amplification. Nature 1991, 354, 737–740. [Google Scholar]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic Amplification of β-Globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, N.; Zheng, L.; Cai, G.; Lin, J. A Lab-on-Chip Device for the Sample-in-Result-out Detection of Viable: Salmonella Using Loop-Mediated Isothermal Amplification and Real-Time Turbidity Monitoring. Lab Chip 2020, 20, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Wachiralurpan, S.; Sriyapai, T.; Areekit, S.; Sriyapai, P.; Thongphueak, D.; Santiwatanakul, S.; Chansiri, K. A One-Step Rapid Screening Test of Listeria Monocytogenes in Food Samples Using a Real-Time Loop-Mediated Isothermal Amplification Turbidity Assay. Anal. Methods 2017, 9, 6403–6410. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Liu, Y.; Kong, J.; Jiang, X. Loop-Mediated Isothermal Amplification Integrated on Microfluidic Chips for Point-of-Care Quantitative Detection of Pathogens. Anal. Chem. 2010, 82, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Amin Almasi, M. Development of Colorimetric Loop-Mediated Isothermal Amplification Assay for Rapid Detection of the Tomato Yellow Leaf Curl Virus. J. Plant Pathol. Microbiol. 2012, 04, 153. [Google Scholar] [CrossRef] [Green Version]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a Loop-Mediated Isothermal Amplification Reaction for Diagnostic Applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, A.; James, D. Detection and Differentiation of Plum Pox Virus Using Real-Time Multiplex PCR with SYBR Green and Melting Curve Analysis: A Rapid Method for Strain Typing. J. Virol. Methods 2005, 123, 213–220. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Tan, B.; Li, P.; Wang, F.X.; Guo, L.; Yang, Y.; Sun, N.; Zhu, H.W.; Wen, Y.J.; Cheng, S.P. Comparison of Conventional RT-PCR, Reverse-Transcription Loop-Mediated Isothermal Amplification, and SYBR Green I-Based Real-Time RT-PCR in the Rapid Detection of Bovine Viral Diarrhea Virus Nucleotide in Contaminated Commercial Bovine Sera Batches. J. Virol. Methods 2014, 207, 204–209. [Google Scholar] [CrossRef]
- Kamra, E.; Singh, N.; Khan, A.; Singh, J.; Chauhan, M.; Kamal, H.; Mehta, P.K. Diagnosis of Genitourinary Tuberculosis by Loop-Mediated Isothermal Amplification Based on SYBR Green I Dye Reaction. Biotechniques 2022, 73, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, N.W.; Demas, A.; Narayanan, J.; Sumari, D.; Kabanywanyi, A.; Patrick Kachur, S.; Barnwell, J.W.; Udhayakumar, V. Real-Time Fluorescence Loop Mediated Isothermal Amplification for the Diagnosis of Malaria. PLoS ONE 2010, 5, e13733. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.P.; Chen, S.H.; Levin, R.E. Application of Ethidium Bromide Monoazide for Quantification of Viable and Dead Cells of Salmonella Enterica by Real-Time Loop-Mediated Isothermal Amplification. J. Microbiol. Methods 2015, 117, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nogva, H.K.; Drømtorp, S.M.; Nissen, H.; Rudi, K. Ethidium Monoazide for DNA-Based Differentiation of Viable and Dead Bacteria by 5′-Nuclease PCR. Biotechniques 2003, 34, 804–813. [Google Scholar] [CrossRef]
- Ahmad, F.; Seyrig, G.; Tourlousse, D.M.; Stedtfeld, R.D.; Tiedje, J.M.; Hashsham, S.A. A CCD-Based Fluorescence Imaging System for Real-Time Loop-Mediated Isothermal Amplification-Based Rapid and Sensitive Detection of Waterborne Pathogens on Microchips. Biomed. Microdevices 2011, 13, 929–937. [Google Scholar] [CrossRef]
- Ohtsuki, R.; Kawamoto, K.; Kato, Y.; Shah, M.M.; Ezaki, T.; Makino, S.I. Rapid Detection of Brucella Spp. by the Loop-Mediated Isothermal Amplification Method. J. Appl. Microbiol. 2008, 104, 1815–1823. [Google Scholar] [CrossRef]
- Stedtfeld, R.D.; Tourlousse, D.M.; Seyrig, G.; Stedtfeld, T.M.; Kronlein, M.; Price, S.; Ahmad, F.; Gulari, E.; Tiedje, J.M.; Hashsham, S.A. Gene-Z: A Device for Point of Care Genetic Testing Using a Smartphone. Lab Chip 2012, 12, 1454–1462. [Google Scholar] [CrossRef]
- Wang, S.; Qin, A.; Chau, L.Y.; Fok, E.W.T.; Choy, M.Y.; Brackman, C.J.; Siu, G.K.H.; Huang, C.-L.; Yip, S.P.; Lee, T.M.H. Amine-Functionalized Quantum Dots as a Universal Fluorescent Nanoprobe for a One-Step Loop-Mediated Isothermal Amplification Assay with Single-Copy Sensitivity. ACS Appl. Mater. Interfaces 2022, 14, 35299–35308. [Google Scholar] [CrossRef]
- Takayama, I.; Nakauchi, M.; Takahashi, H.; Oba, K.; Semba, S.; Kaida, A.; Kubo, H.; Saito, S.; Nagata, S.; Odagiri, T.; et al. Development of Real-Time Fluorescent Reverse Transcription Loop-Mediated Isothermal Amplification Assay with Quenching Primer for Influenza Virus and Respiratory Syncytial Virus. J. Virol. Methods 2019, 267, 53–58. [Google Scholar] [CrossRef]
- Shirato, K.; Semba, S.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Takayama, I.; Kageyama, T.; Notomi, T.; Kamitani, W.; Matsuyama, S.; et al. Development of Fluorescent Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Using Quenching Probes for the Detection of the Middle East Respiratory Syndrome Coronavirus. J. Virol. Methods 2018, 258, 41–48. [Google Scholar] [CrossRef]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification Using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Hirano, T.; Notomi, T. Sequence Specific Visual Detection of LAMP Reactions by Addition Og Cationic Polymers. BMC Biotechnol. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.; Ros, R.; Ros, A.; Wilking, S.D.; Sewald, N.; Anselmetti, D. Identification of Binding Mechanisms in Single Molecule-DNA Complexes. Biophys. J. 2003, 85, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Sano, S.; Takahashi, K.; Jikihara, T. Method for Colorimetric Detection of Double-Stranded Nucleic Acid Using Leuco Triphenylmethane Dyes. Anal. Biochem. 2015, 473, 28–33. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Márquez-Sánchez, S.; Santos Santos, D.; Febrer-Sendra, B.; Crego-Vicente, B.; Muñoz-Bellido, J.L.; Belhassen-García, M.; Corchado Rodríguez, J.M.; Muro, A. SMART-LAMP: A Smartphone-Operated Handheld Device for Real-Time Colorimetric Point-of-Care Diagnosis of Infectious Diseases via Loop-Mediated Isothermal Amplification. Biosensors 2022, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J.F. Shining a Light on LAMP Assays’ A Comparison of LAMP Visualization Methods Including the Novel Use of Berberine. Biotechniques 2015, 58, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Wastling, S.L.; Picozzi, K.; Kakembo, A.S.L.; Welburn, S.C. LAMP for Human African Trypanosomiasis: A Comparative Study of Detection Formats. Int. Cent. Insect Physiol. Ecol. 2010, 4, e865. [Google Scholar] [CrossRef] [Green Version]
- Lucchi, N.W.; Ljolje, D.; Silva-Flannery, L.; Udhayakumar, V. Use of Malachite Green-Loop Mediated Isothermal Amplification for Detection of Plasmodium Spp. Parasites. PLoS ONE 2016, 11, e0151437. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Zha, L.; Fu, W.; Zou, M.; Li, W.; Xu, D. A Modified Visual Loop-Mediated Isothermal Amplification Method for Diagnosis and Differentiation of Main Pathogens from Mycobacterium Tuberculosis Complex. World J. Microbiol. Biotechnol. 2012, 28, 523–531. [Google Scholar] [CrossRef]
- Ma, X.; Shu, Y.; Nie, K.; Qin, M.; Wang, D.; Gao, R.; Wang, M.; Wen, L.; Han, F.; Zhou, S. Visual Detection of Pandemic Influenza A H1N1 Virus 2009 by Reverse-Transcription Loop-Mediated Isothermal Amplification with Hydroxynaphthol Blue Dye. J. Virol. Methods 2010, 167, 214–217. [Google Scholar] [CrossRef]
- Choopara, I.; Arunrut, N.; Kiatpathomchai, W.; Dean, D.; Somboonna, N. Rapid and Visual Chlamydia Trachomatis Detection Using Loop-Mediated Isothermal Amplification and Hydroxynaphthol Blue. Lett. Appl. Microbiol. 2017, 64, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Almasi, M.A.; Fatehi, F.; Struik, P.C.; Moradi, A. Visual Detection of Potato Leafroll Virus by One-step Reverse Transcription Loop-mediated Isothermal Amplification of DNA with Hydroxynaphthol Blue Dye. J. Phytopathol. 2013, 161, 120–124. [Google Scholar] [CrossRef]
- Cardoso, T.C.; Ferrari, H.F.; Bregano, L.C.; Silva-Frade, C.; Rosa, A.C.G.; Andrade, A.L. Visual Detection of Turkey Coronavirus RNA in Tissues and Feces by Reverse-Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) with Hydroxynaphthol Blue Dye. Mol. Cell. Probes 2010, 24, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Ma, C.; Wu, W.; Dong, M.; You, J.; Liu, J.; Yun, S. An Optimized Visual Loop Mediated Isothermal Amplification Assay for Efficient Detection of Minute Virus of Mice with Hydroxynaphthol Blue Dye. J. Virol. Methods 2022, 308, 114575. [Google Scholar] [CrossRef] [PubMed]
- Hongwarittorrn, I.; Chaichanawongsaroj, N.; Laiwattanapaisal, W. Semi-Quantitative Visual Detection of Loop Mediated Isothermal Amplification (LAMP)-Generated DNA by Distance-Based Measurement on a Paper Device. Talanta 2017, 175, 135–142. [Google Scholar] [CrossRef]
- Lin, M.; Li, Z.; Lin, Q.; Wang, P.; Liu, W.; Yuan, J.; Hong, Z.; Chen, Y. Development and Clinical Application of a Rapid and Visual Loop-Mediated Isothermal Amplification Test for TetM Gene in Clostridioides Difficile Strains Cultured from Feces. Int. J. Infect. Dis. 2022, 122, 676–684. [Google Scholar] [CrossRef]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.-J.A.; Ou, C.-P.; Amin-Desai, K.; Athanasiou, P.; et al. Simultaneous DNA Amplification and Detection Using a PH-Sensing Semiconductor System. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef]
- Tanner, N.A.; Zhang, Y.H.; Evans, T.C. Visual Detection of Isothermal Nucleic Acid Amplification Using PH-Sensitive Dyes. Biotechniques 2015, 58. [Google Scholar] [CrossRef] [Green Version]
- Urrutia-Cabrera, D.; Liou, R.H.C.; Wang, J.H.; Chan, J.; Hung, S.S.C.; Hewitt, A.W.; Martin, K.R.; Edwards, T.L.; Kwan, P.; Wong, R.C.B. Comparative Analysis of Loop-Mediated Isothermal Amplification (LAMP)-Based Assays for Rapid Detection of SARS-CoV-2 Genes. Sci. Rep. 2021, 11, 22493. [Google Scholar] [CrossRef]
- Jomoui, W.; Srivorakun, H.; Chansai, S.; Fucharoen, S. Loop-Mediated Isothermal Amplification (LAMP) Colorimetric Phenol Red Assay for Rapid Identification of A0-Thalassemia: Application to Population Screening and Prenatal Diagnosis. PLoS ONE 2022, 17, e0267832. [Google Scholar] [CrossRef]
- Chahar, M.; Anvikar, A.; Dixit, R.; Valecha, N. Evaluation of Four Novel Isothermal Amplification Assays towards Simple and Rapid Genotyping of Chloroquine Resistant Plasmodium Falciparum. Exp. Parasitol. 2018, 190, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Layne, T.R.; O’Connell, K.C.; Tanner, N.A.; Landers, J.P. Comparative Evaluation and Quantitative Analysis of Loop-Mediated Isothermal Amplification Indicators. Anal. Chem. 2020, 92, 13343–13353. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Pantazis, A.K.; Fikas, N.; Chatziioannidou, S.; Tsiakalou, V.; Michaelidou, K.; Pogka, V.; Megariti, M.; Vardaki, M.; Giarentis, K.; et al. Portable Real-Time Colorimetric LAMP-Device for Rapid Quantitative Detection of Nucleic Acids in Crude Samples. Sci. Rep. 2022, 12, 3775. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; de la Escosura-Muñiz, A. Electrochemical Biosensors Based on Nanomaterials for Aflatoxins Detection: A Review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef] [PubMed]
- Kampeera, J.; Pasakon, P.; Karuwan, C.; Arunrut, N.; Sappat, A.; Sirithammajak, S.; Dechokiattawan, N.; Sumranwanich, T.; Chaivisuthangkura, P.; Ounjai, P.; et al. Point-of-Care Rapid Detection of Vibrio Parahaemolyticus in Seafood Using Loop-Mediated Isothermal Amplification and Graphene-Based Screen-Printed Electrochemical Sensor. Biosens. Bioelectron. 2019, 132, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Saito, M.; Hossain, M.M.; Rao, S.R.; Furui, S.; Hino, A.; Takamura, Y.; Takagi, M.; Tamiya, E. Electrochemical Genosensor for the Rapid Detection of GMO Using Loop-Mediated Isothermal Amplification. Analyst 2009, 134, 966. [Google Scholar] [CrossRef]
- Jaroenram, W.; Kampeera, J.; Arunrut, N.; Karuwan, C.; Sappat, A.; Khumwan, P.; Jaitrong, S.; Boonnak, K.; Prammananan, T.; Chaiprasert, A.; et al. Graphene-Based Electrochemical Genosensor Incorporated Loop-Mediated Isothermal Amplification for Rapid on-Site Detection of Mycobacterium Tuberculosis. J. Pharm. Biomed. Anal. 2020, 186, 113333. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Tolba, M.; Zourob, M. Microfluidic Electrochemical Assay for Rapid Detection and Quantification of Escherichia Coli. Biosens. Bioelectron. 2012, 31, 523–528. [Google Scholar] [CrossRef]
- Olabarria, G.; Eletxigerra, U.; Rodriguez, I.; Bilbao, A.; Berganza, J.; Merino, S. Highly Sensitive and Fast Legionella Spp. in Situ Detection Based on a Loop Mediated Isothermal Amplification Technique Combined to an Electrochemical Transduction System. Talanta 2020, 217, 121061. [Google Scholar] [CrossRef]
- Luo, J.; Fang, X.; Ye, D.; Li, H.; Chen, H.; Zhang, S.; Kong, J. A Real-Time Microfluidic Multiplex Electrochemical Loop-Mediated Isothermal Amplification Chip for Differentiating Bacteria. Biosens. Bioelectron. 2014, 60, 84–91. [Google Scholar] [CrossRef]
- Ramírez-Chavarría, R.G.; Castillo-Villanueva, E.; Alvarez-Serna, B.E.; Carrillo-Reyes, J.; Ramírez-Zamora, R.M.; Buitrón, G.; Alvarez-Icaza, L. Loop-Mediated Isothermal Amplification-Based Electrochemical Sensor for Detecting SARS-CoV-2 in Wastewater Samples. J. Environ. Chem. Eng. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chai, Y.; Yuan, Y.; Bai, L.; Yuan, R. Development of an Electrochemical Method for Ochratoxin A Detection Based on Aptamer and Loop-Mediated Isothermal Amplification. Biosens. Bioelectron. 2014, 55, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Inada, M.; Ito, K. Multiplex Real-Time Loop-Mediated Isothermal Amplification Using an Electrochemical DNA Chip Consisting of a Single Liquid-Flow Channel. Anal. Chem. 2019, 91, 3227–3232. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Tang, Y.; Tang, D. Converting Pyrophosphate Generated during Loop Mediated Isothermal Amplification to ATP: Application to Electrochemical Detection of Nosema Bombycis Genomic DNA PTP1. Biosens. Bioelectron. 2018, 102, 518–524. [Google Scholar] [CrossRef]
- Xie, S.; Yuan, Y.; Chai, Y.; Yuan, R. Tracing Phosphate Ions Generated during Loop-Mediated Isothermal Amplification for Electrochemical Detection of Nosema Bombycis Genomic DNA PTP1. Anal. Chem. 2015, 87, 10268–10274. [Google Scholar] [CrossRef] [PubMed]
- Thayanukul, P.; Lertanantawong, B.; Sirawaraporn, W.; Charasmongkolcharoen, S.; Chaibun, T.; Jittungdee, R.; Kittayapong, P. Simple, Sensitive, and Cost-Effective Detection of WAlbB Wolbachia in Aedes Mosquitoes, Using Loop Mediated Isothermal Amplification Combined with the Electrochemical Biosensing Method. PLoS Negl. Trop. Dis. 2022, 16, e0009600. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Qiu, Z.; Ma, J.; Zardán Gómez de la Torre, T.; Johansson, C.; Svedlindh, P.; Strömberg, M. Attomolar Zika Virus Oligonucleotide Detection Based on Loop-Mediated Isothermal Amplification and AC Susceptometry. Biosens. Bioelectron. 2016, 86, 420–425. [Google Scholar] [CrossRef]
- Martin, A.; Bouffier, L.; Grant, K.B.; Limoges, B.; Marchal, D. Real-Time Electrochemical LAMP: A Rational Comparative Study of Different DNA Intercalating and Non-Intercalating Redox Probes. Analyst 2016, 141, 4196–4203. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.L.; Zhu, P.; Zhou, C.X.; He, S.; Yan, X.J. The Development of Loop-Mediated Isothermal Amplification Combined with Lateral Flow Dipstick for Detection of Karlodinium Veneficum. Harmful Algae 2017, 62, 20–29. [Google Scholar] [CrossRef]
- Huang, H.L.; Zhu, P.; Zhou, C.X.; Yan, X.J.; Zou, Y.X.; Lv, P.W. Detection of Skeletonema Costatum Based on Loop-Mediated Isothermal Amplification Combined with Lateral Flow Dipstick. Mol. Cell. Probes 2017, 36, 36–42. [Google Scholar] [CrossRef]
- Mei, X.; Zhai, X.; Lei, C.; Ye, X.; Kang, Z.; Wu, X.; Xiang, R.; Wang, Y.; Wang, H. Development and Application of a Visual Loop-Mediated Isothermal Amplification Combined with Lateral Flow Dipstick (LAMP-LFD)Method for Rapid Detection of Salmonella Strains in Food Samples. Food Control 2019, 104, 9–19. [Google Scholar] [CrossRef]
- Park, B.H.; Oh, S.J.; Jung, J.H.; Choi, G.; Seo, J.H.; Kim, D.H.; Lee, E.Y.; Seo, T.S. An Integrated Rotary Microfluidic System with DNA Extraction, Loop-Mediated Isothermal Amplification, and Lateral Flow Strip Based Detection for Point-of-Care Pathogen Diagnostics. Biosens. Bioelectron. 2017, 91, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Combined with Nanoparticle-Based Lateral Flow Biosensor for the Diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef] [PubMed]
- Nurul Najian, A.B.; Engku Nur Syafirah, E.A.R.; Ismail, N.; Mohamed, M.; Yean, C.Y. Development of Multiplex Loop Mediated Isothermal Amplification (m-LAMP) Label-Based Gold Nanoparticles Lateral Flow Dipstick Biosensor for Detection of Pathogenic Leptospira. Anal. Chim. Acta 2016, 903, 142–148. [Google Scholar] [CrossRef]
- Witkowska McConnell, W.; Davis, C.; Sabir, S.R.; Garrett, A.; Bradley-Stewart, A.; Jajesniak, P.; Reboud, J.; Xu, G.; Yang, Z.; Gunson, R.; et al. Paper Microfluidic Implementation of Loop Mediated Isothermal Amplification for Early Diagnosis of Hepatitis C Virus. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- RAMAN, C.V.; KRISHNAN, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Teixeira, A.; Paris, J.L.; Roumani, F.; Diéguez, L.; Prado, M.; Espiña, B.; Abalde-Cela, S.; Garrido-Maestu, A.; Rodriguez-Lorenzo, L. Multifuntional Gold Nanoparticles for the SERS Detection of Pathogens Combined with a LAMP–in–Microdroplets Approach. Materials 2020, 13, 1934. [Google Scholar] [CrossRef] [Green Version]
- Draz, M.S.; Lu, X. Development of a Loop Mediated Isothermal Amplification (LAMP) - Surface Enhanced Raman Spectroscopy (SERS) Assay for the Detection of Salmonella Enterica Serotype Enteritidis. Theranostics 2016, 6, 522–532. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Why You Should Be Using More SPR Biosensor Technology. Drug Discov. Today Technol. 2004, 1, 301–308. [Google Scholar] [CrossRef]
- Nawattanapaiboon, K.; Kiatpathomchai, W.; Santanirand, P.; Vongsakulyanon, A.; Amarit, R.; Somboonkaew, A.; Sutapun, B.; Srikhirin, T. SPR-DNA Array for Detection of Methicillin-Resistant Staphylococcus Aureus (MRSA) in Combination with Loop-Mediated Isothermal Amplification. Biosens. Bioelectron. 2015, 74, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Donolato, M.; Antunes, P.; Bejhed, R.S.; Zardán Gómez De La Torre, T.; Østerberg, F.W.; Strömberg, M.; Nilsson, M.; Strømme, M.; Svedlindh, P.; Hansen, M.F.; et al. Novel Readout Method for Molecular Diagnostic Assays Based on Optical Measurements of Magnetic Nanobead Dynamics. Anal. Chem. 2015, 87, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Ma, J.; Zardán, T.; Gómezgómez De La Torre, Z.; Dámdám Baíint, A.; Donolato, M.; Hansen, M.F.; Svedlindh, P.; Strö, M. Rapid Newcastle Disease Virus Detection Based on Loop-Mediated Isothermal Amplification and Optomagnetic Readout. Acs Sens. 2016, 1, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Minero, G.A.S.; Nogueira, C.; Rizzi, G.; Tian, B.; Fock, J.; Donolato, M.; Strömberg, M.; Hansen, M.F. Sequence-Specific Validation of LAMP Amplicons in Real-Time Optomagnetic Detection of Dengue Serotype 2 Synthetic DNA. Analyst 2017, 142, 3441–3450. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Lin, C.W.; Kwon, J.S.; Chuang, H.S. Rotational Diffusometric Sensor with Isothermal Amplification for Ultra-Sensitive and Rapid Detection of SARS-CoV-2 Nsp2 CDNA. Biosens. Bioelectron. 2022, 210, 114293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, X.; Huang, Z.; Luo, Y.; Tang, L.; Jiang, J.H. BEAMing LAMP: Single-Molecule Capture and on-Bead Isothermal Amplification for Digital Detection of Hepatitis C Virus in Plasma. Chem. Commun. 2018, 54, 291–294. [Google Scholar] [CrossRef]
- Paul, R.; Ostermann, E.; Chen, Y.; Saville, A.C.; Yang, Y.; Gu, Z.; Whitfield, A.E.; Ristaino, J.B.; Wei, Q. Integrated Microneedle-Smartphone Nucleic Acid Amplification Platform for in-Field Diagnosis of Plant Diseases. Biosens. Bioelectron. 2021, 187, 113312. [Google Scholar] [CrossRef]
- Rodriguez-Manzano, J.; Karymov, M.A.; Begolo, S.; Selck, D.A.; Zhukov, D.V.; Jue, E.; Ismagilov, R.F. Reading Out Single-Molecule Digital RNA and DNA Isothermal Amplification in Nanoliter Volumes with Unmodified Camera Phones. ACS Nano 2016, 10, 3102–3113. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Chen, S.; Zhang, L.; He, X.; Ma, Y.; Wu, H.; Zou, B.; Zhou, G. Multiplex Detection of Blood-Borne Pathogens on a Self-Driven Microfluidic Chip Using Loop-Mediated Isothermal Amplification. Anal. Bioanal. Chem. 2021, 413, 2923–2931. [Google Scholar] [CrossRef]
- Kaarj, K.; Akarapipad, P.; Yoon, J.Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-Mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef]
- Song, J.; Cha, B.; Moon, J.; Jang, H.; Kim, S.; Jang, J.; Yong, D.; Kwon, H.-J.; Lee, I.-C.; Lim, E.-K.; et al. Smartphone-Based SARS-CoV-2 and Variants Detection System Using Colorimetric DNAzyme Reaction Triggered by Loop-Mediated Isothermal Amplification (LAMP) with Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR). ACS Nano 2022, 16, 11300–11314. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manzano, J.; Malpartida-Cardenas, K.; Moser, N.; Pennisi, I.; Cavuto, M.; Miglietta, L.; Moniri, A.; Penn, R.; Satta, G.; Randell, P.; et al. Handheld Point-of-Care System for Rapid Detection of SARS-CoV-2 Extracted RNA in under 20 Min. ACS Cent. Sci. 2021, 7, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent On-Site Detection of Multiple Pathogens Using Smartphone-Based Portable Device with Paper-Based Isothermal Amplification Chip. Microchim. Acta 2022, 189, 333. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Bui, H.K.; Phan, V.M.; Seo, T.S. An Internet of Things-Based Point-of-Care Device for Direct Reverse-Transcription-Loop Mediated Isothermal Amplification to Identify SARS-CoV-2. Biosens. Bioelectron. 2022, 195, 113655. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.M.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A Colorimetric RT-LAMP Assay and LAMP-Sequencing for Detecting SARS-CoV-2 RNA in Clinical Samples. Sci. Transl. Med. 2020, 12, eabc7075. [Google Scholar] [CrossRef]
- Yu, L.; Wu, S.; Hao, X.; Dong, X.; Mao, L.; Pelechano, V.; Chen, W.-H.; Yin, X. Rapid Detection of COVID-19 Coronavirus Using a Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) Diagnostic Platform. Clin. Chem. 2020, 66, 975–977. [Google Scholar] [CrossRef]
- Ludwig, K.U.; Schmithausen, R.M.; Li, D.; Jacobs, M.L.; Hollstein, R.; Blumenstock, K.; Liebing, J.; Słabicki, M.; Ben-Shmuel, A.; Israeli, O.; et al. LAMP-Seq Enables Sensitive, Multiplexed COVID-19 Diagnostics Using Molecular Barcoding. Nat. Biotechnol. 2021, 39, 1556–1562. [Google Scholar] [CrossRef]
- Song, J.; El-Tholoth, M.; Li, Y.; Graham-Wooten, J.; Liang, Y.; Li, J.; Li, W.; Weiss, S.R.; Collman, R.G.; Bau, H.H. Single- and Two-Stage, Closed-Tube, Point-of-Care, Molecular Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 13063–13071. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-gonzalez, A.; et al. CRISPR – Cas12-Based Detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.S.; Lim, D.H.; Yoon, J.; Kim, A.; Lim, M.; Nam, J.; Yanagihara, R.; Ryu, S.W.; Jung, B.K.; Ryoo, N.H.; et al. Development of a Multiplex Loop-Mediated Isothermal Amplification (LAMP) Assay for Onsite Diagnosis of SARS CoV-2. PLoS ONE 2021, 16, e0248042. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Yokota, I.; Fukumoto, T.; Iwasaki, S.; Fujisawa, S.; Takahashi, M.; Negishi, S.; Hayasaka, K.; Sato, K.; Oguri, S.; et al. SARS-CoV-2 Detection by Fluorescence Loop-Mediated Isothermal Amplification with and without RNA Extraction. J. Infect. Chemother. 2021, 27, 410–412. [Google Scholar] [CrossRef] [PubMed]










| Indicator | Cross Contamination | Adding Moment | Detection Mechanism | |
|---|---|---|---|---|
| Magnesium pyrophosphate | Turbidity | No | Not adding | Insoluble precipitant |
| SYBR Green I | Color to green | Possible | After LAMP | Intercalation |
| Hydroxy Naphthol Blue (HNB) | Color to blue | No | Before LAMP | Metal binding |
| Calcein | fluorescent | No | Before LAMP | Metal binding |
| Malachite Green | Color to blue | No | Before LAMP | Metal binding |
| Phenol Red | Color to yellow | No | Before LAMP | pH indicator |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W. Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests. Biosensors 2022, 12, 857. https://doi.org/10.3390/bios12100857
Park J-W. Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests. Biosensors. 2022; 12(10):857. https://doi.org/10.3390/bios12100857
Chicago/Turabian StylePark, Jee-Woong. 2022. "Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests" Biosensors 12, no. 10: 857. https://doi.org/10.3390/bios12100857
APA StylePark, J.-W. (2022). Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests. Biosensors, 12(10), 857. https://doi.org/10.3390/bios12100857





