Electrospinning-Based Biosensors for Health Monitoring
Abstract
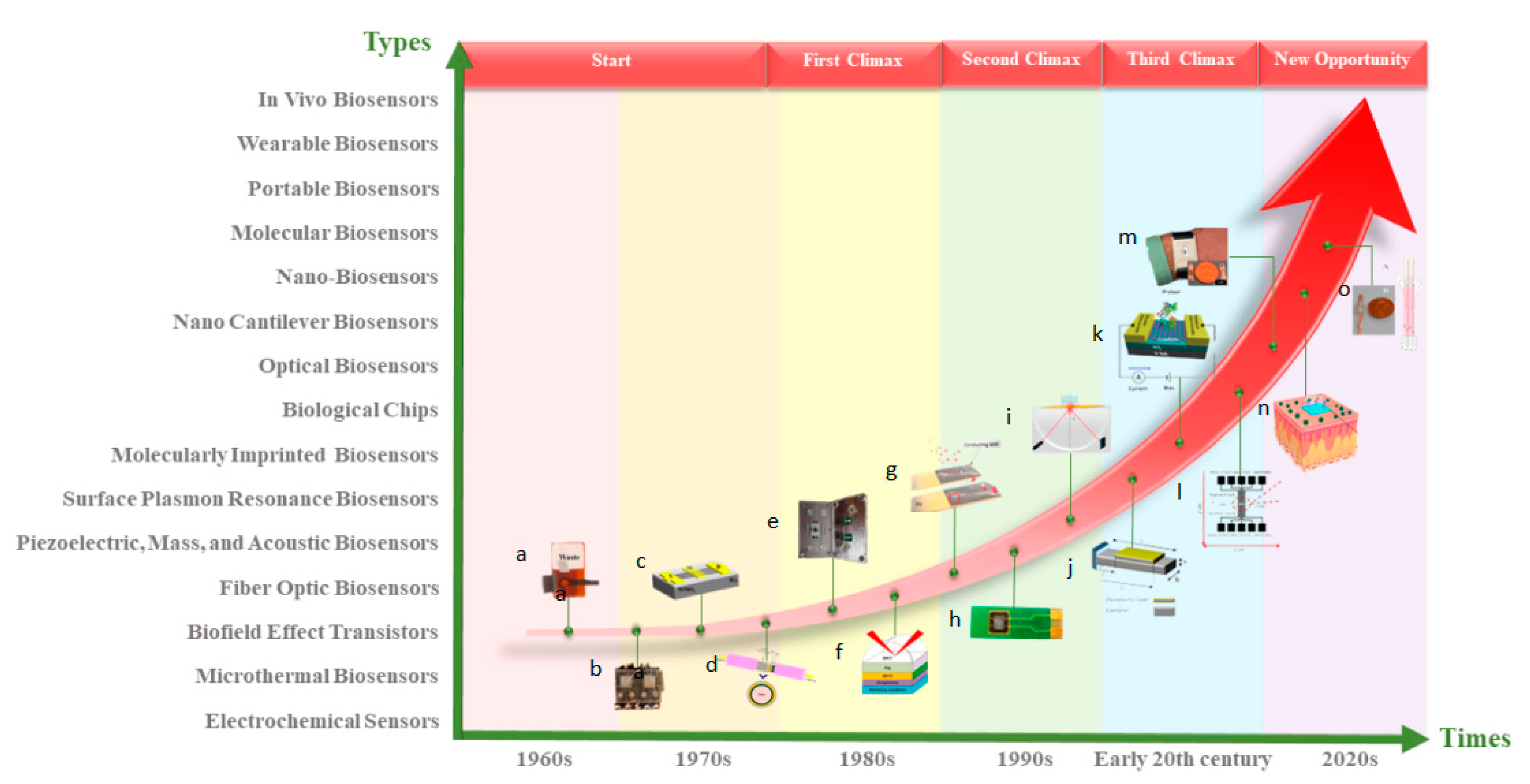
:1. Introduction
2. Electrospinning
2.1. Principle of Electrospinning
2.2. Types of Nanofibers
2.3. Preparation and Characteristics of Nanofibers
- Randomly distributed nanofibers: The preparation of such fibers is the simplest form by direct drawing of the instabilities of the jet. This results in a relatively small range of applications and poor mechanical properties for such fibers. However, the advantage is that the preparation is simple and there is no complicated process.
- Aligned nanofibers: This fiber is obtained by suppressing the instability of the jet on the basis of randomly distributed nanofibers. This makes the fibers have the advantages of high axial mechanical strength, good dimensional stability and high application value in tissue engineering, composite reinforcement, electrical and optical fields. However, due to the different methods used, the collection speed may be slow and the amount of fibers is relatively small.
- Core-shell nanofibers: Fibers of this structure are mostly obtained by coaxial electrospinning devices. Core-shell structured nanofibers solve the problem that some materials are not spinnable. Core-shell structured nanofibers solve the problem that some materials are not spinnable. This also makes this fiber widely used in biomedical fields such as drug release systems, tissue engineering scaffolds, drug-loaded medical dressings and sutures.
- Multispace nanofibers: The preparation of such fibers is mostly used to induce phase separation. For example, solvent evaporation and heating can induce phase separation to form multispace nanofibers. This fiber is characterized by a substantial increase in the specific surface area of the fiber.
- Pine-needle nanofibers: Such fibers are formed based on randomly distributed nanofibers. Then, other materials are generally grown on randomly distributed fibers by hydrothermal method.
- Patterned nanofibers: This fiber is obtained by changing the shape, movement mode and material of the collecting device. Its physical and chemical properties are basically the same as those of disordered fibers.
- Cobweb nanofibers: This fiber is a two-dimensional mesh fiber membrane material with ultrafine electrospun fibers as a scaffold. It has the advantages of large specific surface area, good adsorption and stable mechanical properties.
- Hollow nanofibers: This fiber is obtained on the basis of the core-shell structure fiber. Such fibers are generally obtained by coaxial electrospinning with a soluble or volatile substance as the core layer and a polymer solution as the shell layer and then removing the core layer by dissolving or heating. The disadvantage is that the production efficiency is relatively low.
3. The Principle and Characteristics of Biosensors
3.1. Working Principle
3.2. Characteristics of Biosensors
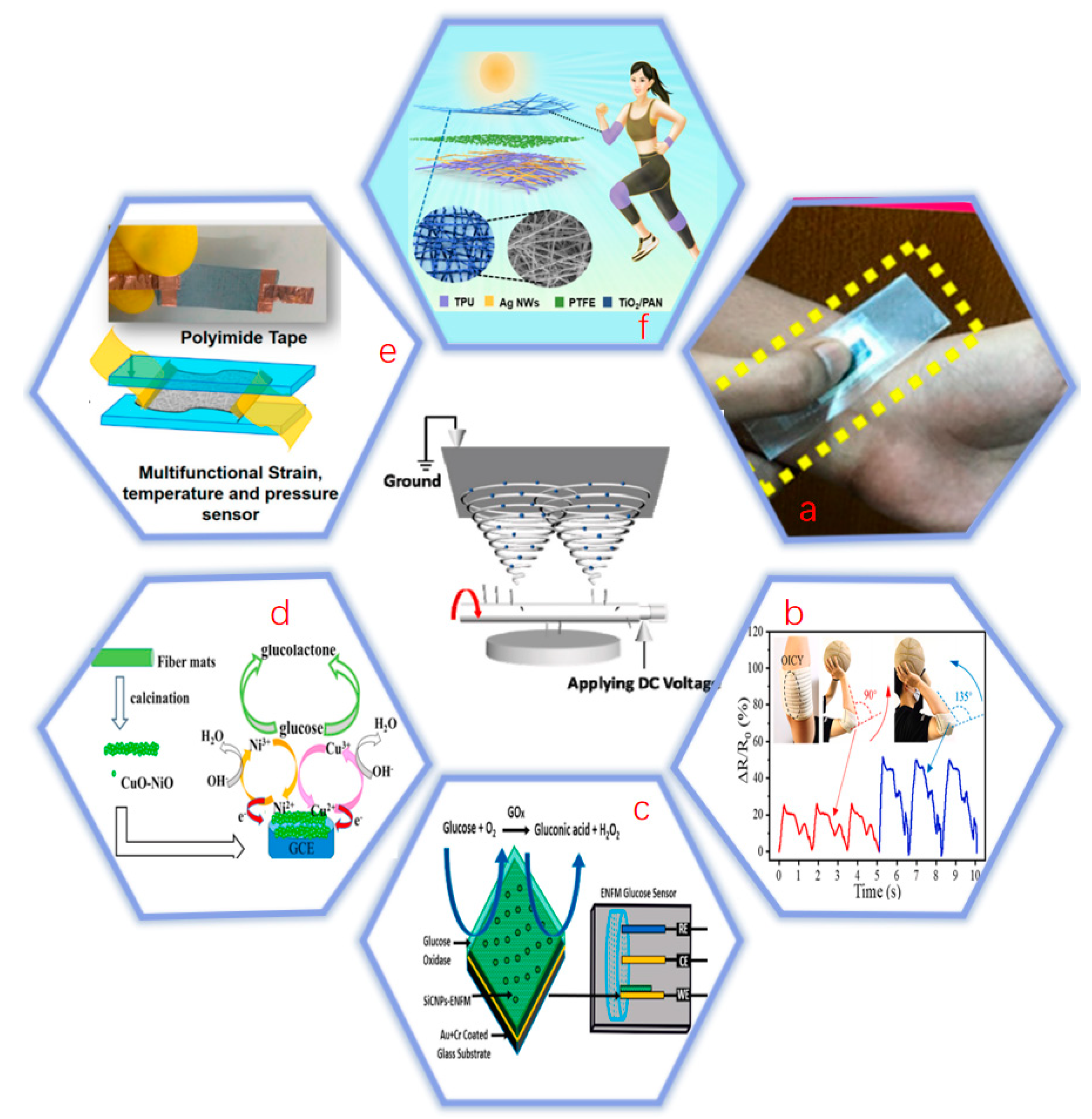
4. Application of Electrospinning in Biosensors
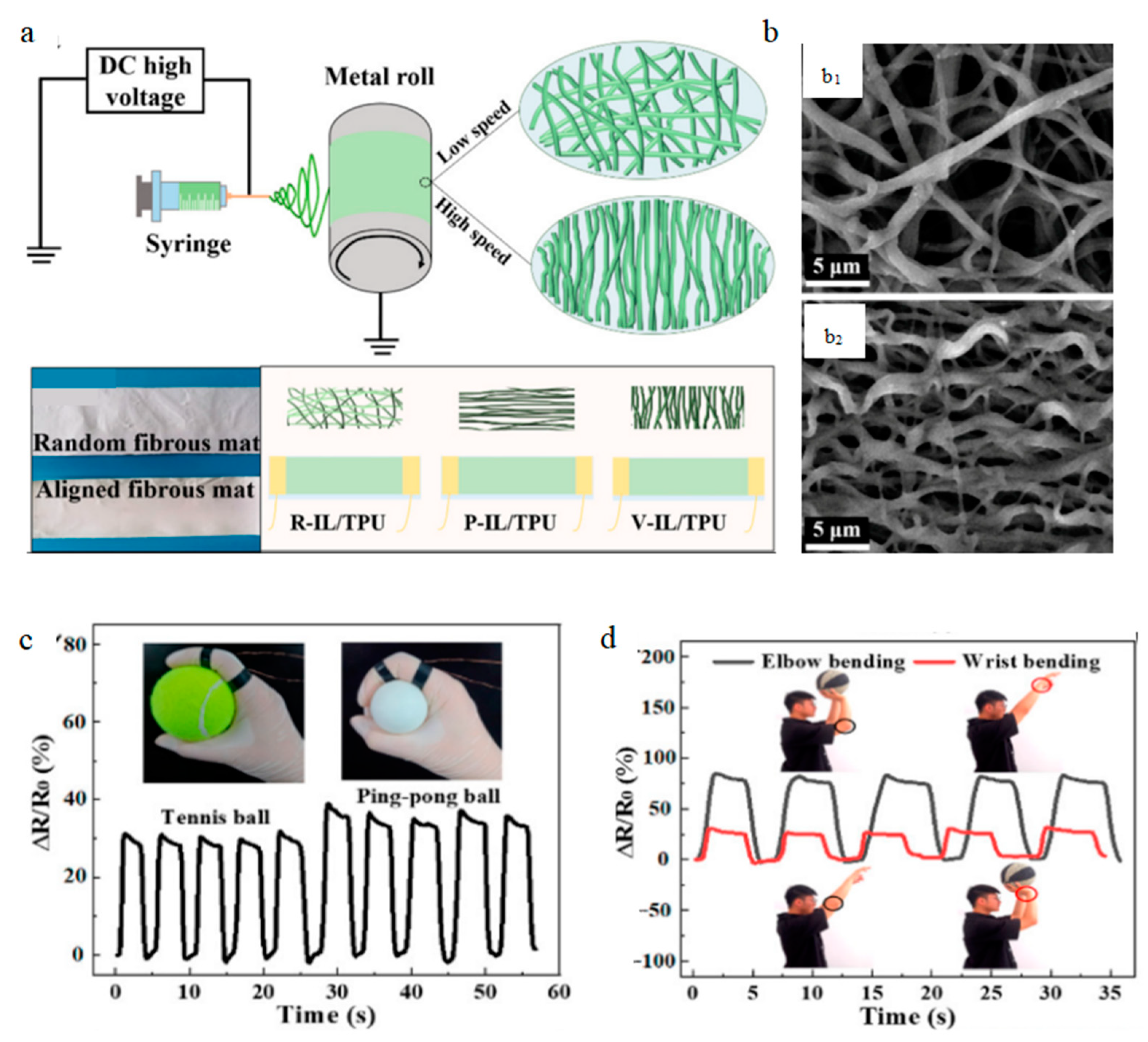
4.1. Piezoelectric Biosensor
4.1.1. Monitoring Heart Rate
4.1.2. Monitoring Body Movement
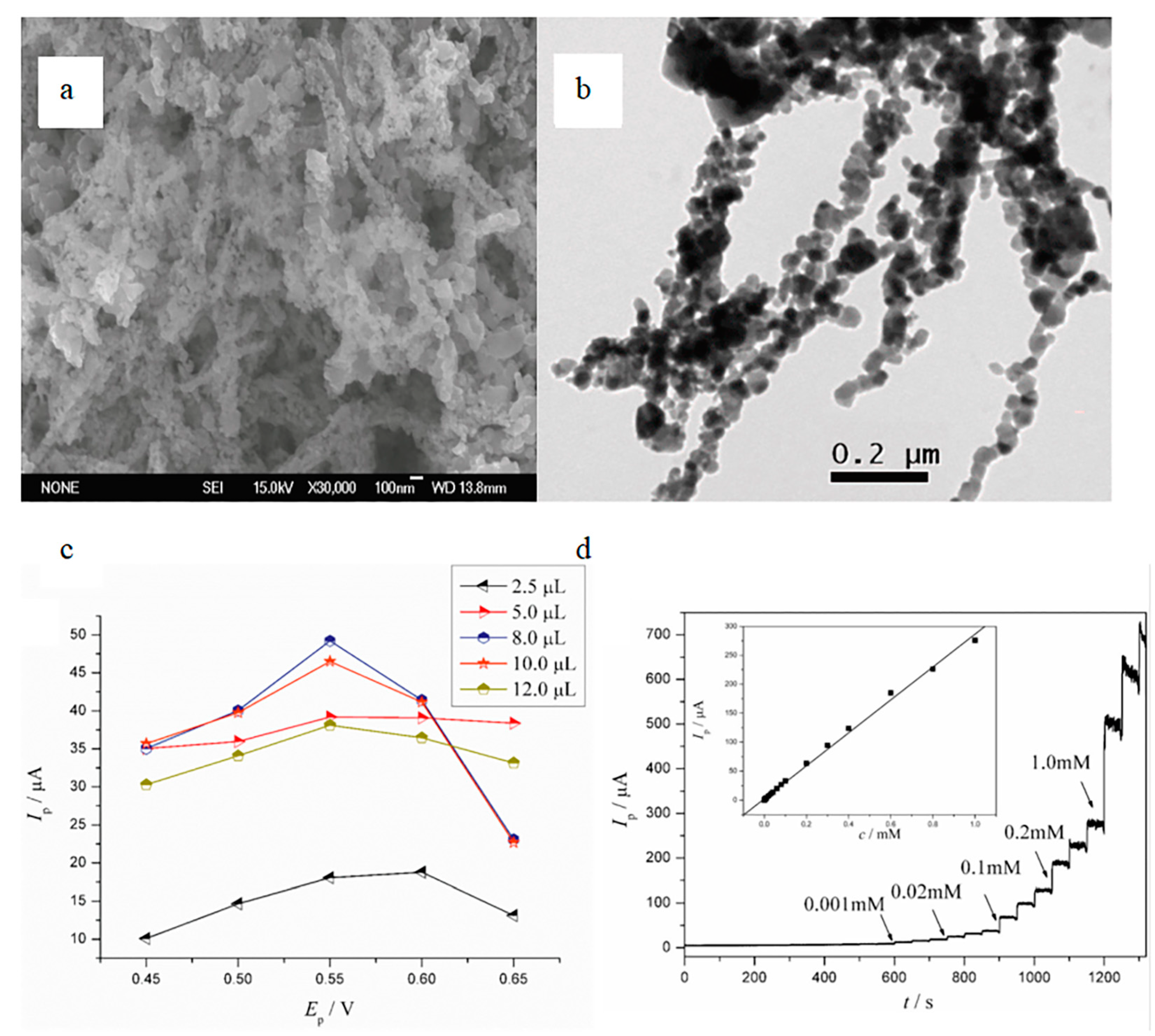
4.2. Electrochemical Biosensor
4.2.1. Enzyme Biosensors
4.2.2. Non-Enzymatic Biosensors
4.3. Thermosensitive Biosensors
4.4. Optical Biosensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshmanan, A.; Jin, Z.; Nety, S.P.; Sawyer, D.P.; Gosselin, A.L.; Malounda, D.; Swift, M.B.; Maresca, D.; Shapiro, M.G. Publisher Correction: Acoustic biosensors for ultrasound imaging of enzyme activity. Nat. Chem. Biol. 2020, 16, 1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun Doping of Carbon Nanotubes and Platinum Nanoparticles into the β-Phase Polyvinylidene Difluoride Nanofibrous Membrane for Biosensor and Catalysis Applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhan, L.; Huang, C.Z. Recent insights into functionalized electrospun nanofibrous films for chemo-/bio-sensors. TrAC Trends Anal. Chem. 2020, 124, 115813. [Google Scholar] [CrossRef]
- Arida, I.A.; Ali, I.H.; Nasr, M.; El-Sherbinyet, I.M. Electrospun polymer-based nanofiber scaffolds for skin regeneration. J. Drug Deliv. Sci. Technol. 2021, 64, 102623. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Recent progress on designing electrospun nanofibers for colorimetric biosensing applications. Curr. Opin. Biomed. Eng. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, H.; Zhao, Y.; Wang, Y.; Li, F. Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens. Bioelectron. 2022, 199, 113906. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sipe, D.M.; Xu, Y.; Lin, Q. A MEMS Thermal Biosensor for Metabolic Monitoring Applications. J. Microelectromech. Syst. 2008, 17, 318–327. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, G.; Chang, Y.W.; Kim, H.J.; Jose, J.; Kim, E.; Pyun, J.; Yoo, K.H. A carbon nanotube metal semiconductor field effect transistor-based biosensor for detection of amyloid-beta in human serum. Biosens. Bioelectron. 2013, 50, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Paliwal, A.; Gupta, V.; Tomar, M. Smartphone integrated handheld Long Range Surface Plasmon Resonance based fiber-optic biosensor with tunable SiO2 sensing matrix. Biosens. Bioelectron. 2022, 201, 113919. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.T.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Suo, S.; Li, X.; Cheng, Q.; Meng, S. Sensitivity enhancement by employing BiFeO3 and graphene hybrid structure in surface plasmon resonance biosensors. Opt. Mater. 2021, 121, 111618. [Google Scholar] [CrossRef]
- Hong, C.-C.; Lin, C.C.; Hong, C.L.; Lin, Z.X.; Chung, M.H.; Hsieh, P.W. Handheld analyzer with on-chip molecularly-imprinted biosensors for electrical detection of propofol in plasma samples. Biosens. Bioelectron. 2016, 86, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Rawley, O.; Wood, T.; Galvin, P. Monitoring of cell growth in vitro using biochips packaged with indium tin oxide sensors. Sens. Actuators B Chem. 2009, 139, 187–193. [Google Scholar] [CrossRef]
- Carrascosa, L.G.; Huertas, C.S.; Lechuga, L.M. Prospects of optical biosensors for emerging label-free RNA analysis. TrAC Trends Anal. Chem. 2016, 80, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Jabbari Behrouz, S.; Rahmani, O.; Hosseini, S.A. On nonlinear forced vibration of nano cantilever-based biosensor via couple stress theory. Mech. Syst. Signal Process. 2019, 128, 19–36. [Google Scholar] [CrossRef]
- Farmani, H.; Farmani, A.; Nguyen, T.A. 12-Graphene-based field effect transistor (GFET) as nanobiosensors. In Silicon-Based Hybrid Nanoparticles; Thomas, S., Ahmadi, M., Joshi, N., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–275. [Google Scholar]
- Nuzaihan, M.N.M.; Hashim, U.; Md Arshad, M.K.; Kasjoo, S.R.; Rahman, S.F.A.; Ruslinda, A.R.; Fathil, M.F.M.; Adzhri, R.; Shahimin, M.M. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens. Bioelectron. 2016, 83, 106–114. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Gao, W. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef]
- Cordeiro, C.A.; Vries, M.D.; Ngabi, W.; Oomen, P.E.; Gremers, T.I.; Westerink, B.H.C. In vivo continuous and simultaneous monitoring of brain energy substrates with a multiplex amperometric enzyme-based biosensor device. Biosens. Bioelectron. 2015, 67, 677–686. [Google Scholar] [CrossRef]
- Smoak, M.M.; Hogan, K.J.; Grande-Allen, K.J.; Mikos, A.G. Bioinspired electrospun dECM scaffolds guide cell growth and control the formation of myotubes. Sci. Adv. 2021, 7, eabg4123. [Google Scholar] [CrossRef]
- Guo, J.; Fu, S.; Deng, Y.; Xu, X.; Laima, S.; Liu, D.; Zhang, P.; Zhou, J.; Zhao, H.; Yu, H.; et al. Hypocrystalline ceramic aerogels for thermal insulation at extreme conditions. Nature 2022, 606, 909–916. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Si, Y.; Yu, J.; Ding, B. Direct synthesis of highly stretchable ceramic nanofibrous aerogels via 3D reaction electrospinning. Nat. Commun. 2022, 13, 2637. [Google Scholar] [CrossRef]
- Singh, R.K.; Lye, S.W.; Miao, J. Holistic investigation of the electrospinning parameters for high percentage of β-phase in PVDF nanofibers. Polymer 2021, 214, 123366. [Google Scholar]
- Gade, H.; Nikam, S.; Chase, G.G.; Reneker, D.H. Effect of electrospinning conditions on β-phase and surface charge potential of PVDF fibers. Polymer 2021, 228, 123902. [Google Scholar] [CrossRef]
- Raksa, A.; Numpaisal, P.O.; Ruksakulpiwat, Y. The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Mater. Today: Proc. 2021, 47, 3458–3461. [Google Scholar] [CrossRef]
- Raman, A.; Jayan, J.S.; Bds, D.; Appukuttan, S.; Joseph, K. Electrospun Nanofibers as Effective Superhydrophobic Surfaces: A Brief review. Surf. Interfaces 2021, 24, 101140. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Bayan, M.A.H.; Taromi, F.A.; Lanzi, M.; Pierini, F. Enhanced efficiency in hollow core electrospun nanofiber-based organic solar cells. Sci. Rep. 2021, 11, 21144. [Google Scholar]
- Yang, G.; Tang, X.; Zhao, G.; Li, Y.; Ma, C.; Zhuang, X.; Yan, J. Highly sensitive, direction-aware, and transparent strain sensor based on oriented electrospun nanofibers for wearable electronic applications. Chem. Eng. J. 2022, 435, 135004. [Google Scholar] [CrossRef]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Li, J. Porous poly(L–lactic acid)/chitosan nanofibres for copper ion adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Chen, C.; Li, D.; Zhang, W. Study on preparation process and enhanced piezoelectric performance of pine-needle-like ZnO@PVDF composite nanofibers. Polym. Test. 2022, 108, 107513. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, W.; Liu, Q.; Yu, M. A new magnetic melt spinning device for patterned nanofiber. Sci. Rep. 2021, 11, 8895. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, X.; Miao, C.; He, Y.B.; Liang, G.; Zhou, D.; Liu, M.; Han, C.; Li, B.; Kang, F. A honeycomb-cobweb inspired hierarchical core–shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon 2016, 98, 582–591. [Google Scholar] [CrossRef]
- Zou, D.; Wang, W.; Liu, J.; Weng, J.; Duan, J.; Zhou, J.; Zhou, P. Insights into the storage mechanism of novel mesoporous hollow TiO2-x/C nanofibers as a high-performance anode material for sodium-ion batteries. Carbon 2022, 194, 248–256. [Google Scholar] [CrossRef]
- Kadadou, D.; Tizani, L.; SWadi, V.; Banat, F.; Alsafar, H.; FYousef, A.; Barcelo, D.; WHasan, S. Recent advances in the biosensors application for the detection of bacteria and viruses in wastewater. J. Environ. Chem. Eng. 2022, 10, 107070. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, M.G.; Silva-Lance, F.; Parra-Arroyo, L.; Medina-Salazar, D.A.; Martínez-Ruiz, M.; Melchor-Martínez, E.M.; Martínez-Prado, M.A.; Iqbal, H.; Parra-Saldívar, R.; Barceló, D. Biosensors for the detection of disease outbreaks through wastewater-based epidemiology. TrAC Trends Anal. Chem. 2022, 155, 116585. [Google Scholar] [CrossRef]
- Wang, F.R.; Wang, R. Modification of polyacrylonitrile-derived carbon nanofibers and bacteriophages on screen-printed electrodes: A portable electrochemical biosensor for rapid detection of Escherichia coli. Bioelectrochemistry 2022, 148, 108229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Q.; Lu, K.; Huang, J.; Zhang, Y.; Hou, Y.; Qiao, H.; Li, D.; Wei, Q. Encapsulating enzyme into metal-organic framework during in-situ growth on cellulose acetate nanofibers as self-powered glucose biosensor. Biosens. Bioelectron. 2021, 171, 112690. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H. Design and testing of an electrospun nanofiber mat as a pH biosensor and monitor the pH associated quality in fresh date fruit (Rutab). Polym. Test. 2019, 75, 76–84. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Shan, Y.; Jiang, M.; Jin, X.; Gong, M.; Xu, J. A novel and sensitive electrogenerated chemiluminescence biosensor for detection of p16INK4a gene based on the functional paste-like nanofibers composites-modified screen-printed carbon electrode. J. Electroanal. Chem. 2018, 823, 368–377. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Facure Murilo, H.M.; Pena, R.B.; Sanfelice, R.C.; Luiz, H.C.; Correa, D.S. Ultrasensitive biosensor based on polyvinylpyrrolidone/chitosan/reduced graphene oxide electrospun nanofibers for 17α—Ethinylestradiol electrochemical detection. Appl. Surf. Sci. 2018, 458, 431–437. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; Banavoth, M. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Zhu, P.; Peng, H.; Rwei, A.Y. Flexible, wearable biosensors for digital health. Med. Nov. Technol. Devices 2022, 14, 100118. [Google Scholar] [CrossRef]
- Wu, J.; Yin, F. Sensitive enzymatic glucose biosensor fabricated by electrospinning composite nanofibers and electrodepositing Prussian blue film. J. Electroanal. Chem. 2013, 694, 1–5. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.; Li, J.; Liu, L.; Ding, B. Wearable biosensor for sensitive detection of uric acid in artificial sweat enabled by a fiber structured sensing interface. Nano Energy 2021, 85, 106031. [Google Scholar] [CrossRef]
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Ding, B. In-situ electrospinning of thymol-loaded polyurethane fibrous membranes for waterproof, breathable, and antibacterial wound dressing application. J. Colloid Interface Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Maity, K.; Garain, S.; Henkel, K.; Schmeier, D.; Mandal, D. Self-Powered Human-Health Monitoring through Aligned PVDF Nanofibers Interfaced Skin-Interactive Piezoelectric Sensor. ACS Appl. Polym. Mater. 2020, 2, 862–878. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.; Ma, S.; Yan, T.; Pan, Z. Flexible strain sensor based on CNT/TPU composite nanofiber yarn for smart sports bandage. Compos. Part B Eng. 2022, 232, 109605. [Google Scholar] [CrossRef]
- Puttananjegowda, K.; Takshi, A.; Thomas, S. Silicon carbide nanoparticles electrospun nanofibrous enzymatic glucose sensor. Biosens. Bioelectron. 2021, 186, 113285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, Y.; Zhang, L.; Zhang, X. Highly sensitive enzyme-free glucose sensor based on CuO–NiO nanocomposites by electrospinning. Compos. Commun. 2021, 25, 100687. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Bi2S3/PVDF/Ppy-Based Freestanding, Wearable, Transient Nanomembrane for Ultrasensitive Pressure, Strain, and Temperature Sensing. ACS Appl. Bio Mater. 2021, 4, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dong, K.; An, J.; Liang, F.; Yi, J.; Peng, X.; Ning, C.; Ye, C.; Wang, Z. UV-Protective, Self-Cleaning, and Antibacterial Nanofiber-Based Triboelectric Nanogenerators for Self-Powered Human Motion Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 11205–11214. [Google Scholar] [CrossRef]
- Xiao, N.; Yu, W.; Han, X. Wearable heart rate monitoring intelligent sports bracelet based on Internet of things. Measurement 2020, 164, 108102. [Google Scholar] [CrossRef]
- Kaisti, M.; Panula, T.; Leppanen, J.; Punkkinen, R.; Tadi, M.J.; Vasankari, T.; Jaakkola, S.; Kivinieimi, T.; Airaksinen, J.; Kostiainen, P.; et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. Npj Digit. Med. 2019, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrestani, S.; Chou, T.C.; Shang, K.M.; Zada, G.; Borok, Z.; PRao, A.; Tai, Y.C. A wearable eddy current based pulmonary function sensor for continuous non-contact point-of-care monitoring during the COVID-19 pandemic. Sci. Rep. 2021, 11, 20144. [Google Scholar] [CrossRef]
- Lin, J.; Fu, R.; Zhong, X.; Yu, P.; Tan, G.; Li, W. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2021, 2, 100541. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, X.; Li, J.; Liu, H.; Han, N. Poly-l-Lactic Acid/Graphene Electrospun Composite Nanofibers for Wearable Sensors. Energy Technol. 2020, 8, 1901252. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Qi, K.; He, J.; Wang, H.; Zhou, Y.; Cui, S. A Highly Stretchable Nanofiber-Based Electronic Skin with Pressure-, Strain-, and Flexion-Sensitive Properties for Health and Motion Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 42951–42960. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Zhu, G.; Wang, C.; Cui, X.; Xi, M.; Chang, X.; Zhu, Y. Breathable Strain/Temperature Sensor Based on Fibrous Networks of Ionogels Capable of Monitoring Human Motion, Respiration, and Proximity. ACS Appl. Mater. Interfaces 2021, 13, 51567–51577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, J.; Wang, H.; Qi, K.; Nan, N.; You, X.; Shao, W.; Wang, L.; Ding, B.; Cui, S. Highly sensitive, self-powered and wearable electronic skin based on pressure-sensitive nanofiber woven fabric sensor. Sci. Rep. 2017, 7, 12949. [Google Scholar]
- Su, Y.; Li, W.; Yuan, L.; Chen, C.; Pan, H.; Xie, G.; Conta, G.; Ferrier, S.; Zhao, X.; Chen, G. Piezoelectric fiber composites with polydopamine interfacial layer for self-powered wearable biomonitoring. Nano Energy 2021, 89, 106321. [Google Scholar] [CrossRef]
- Yang, C.-R.; Lin, M.F.; Huang, C.K.; Huang, W.C.; Tseng, S.F.; Chiang, H.H. Highly sensitive and wearable capacitive pressure sensors based on PVDF/BaTiO3 composite fibers on PDMS microcylindrical structures. Measurement 2022, 202, 111817. [Google Scholar] [CrossRef]
- Qin, Z.; Lv, Y.; Fang, X.; Zhao, B.; Niu, F.; Min, L.; Pan, K. Ultralight polypyrrole crosslinked nanofiber aerogel for highly sensitive piezoresistive sensor. Chem. Eng. J. 2022, 427, 131650. [Google Scholar]
- Kang, S.; Kim, S.H.; Lee, H.B.; Mhin, S.; Ryu, J.H.; Kim, Y.W.; LJones, J.; Son, Y.; Lee, N.K.; Lee, K. High-power energy harvesting and imperceptible pulse sensing through peapod-inspired hierarchically designed piezoelectric nanofibers. Nano Energy 2022, 99, 107386. [Google Scholar] [CrossRef]
- Yhuwana, Y.G.Y.; Apsari, R.; Yasin, M. Fiber optic sensor for heart rate detection. Optik 2017, 134, 28–32. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, L.; Huang, J.; Li, S.; Chen, Z.; Zhang, W.; Lai, Y. Rational designed microstructure pressure sensors with highly sensitive and wide detection range performance. J. Mater. Sci. Technol. 2022, 130, 184–192. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Xu, H.; Jin, Y.; Chen, G.; Zheng, W.; Wang, W.; Wang, Y.; Gao, L. Highly sensitive and wearable bionic piezoelectric sensor for human respiratory monitoring. Sens. Actuators A Phys. 2022, 345, 113818. [Google Scholar] [CrossRef]
- Zhu, G.; Ren, P.G.; Wang, J.; Duan, Q.; Yan, D.X. A Highly Sensitive and Broad-Range Pressure Sensor Based on Polyurethane Mesodome Arrays Embedded with Silver Nanowires. ACS Appl. Mater. Interfaces 2020, 12, 19988–19999. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.M.; Li, H.Y.; Xu, J.; Zhang, C.; Zhu, G. Facile Fabrication of Flexible Pressure Sensor with Programmable Lattice Structure. ACS Appl. Mater. Interfaces 2021, 13, 10388–10396. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Zhou, J.; Men, D.; Zhang, X.-E. Progress in wearable sweat sensors and their applications. Chin. J. Anal. Chem. 2022, 50, 87–96. [Google Scholar] [CrossRef]
- Nyein, H.; Bariya, M.; Kivimki, L.; Uusitalo, S.; Javey, A. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.J.; Kim, K.O. Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Sci. Rep. 2020, 10, 18858. [Google Scholar] [CrossRef] [PubMed]
- Taşaltın, C. Glucose sensing performance of PAN: β-rhombohedral borophene based non-enzymatic electrochemical biosensor. Inorg. Chem. Commun. 2021, 133, 108973. [Google Scholar] [CrossRef]
- Rani, S.D.; Ramachandran, R.; Sheet, S.; Aziz, M.A.; Kumar, G.G. NiMoO4 nanoparticles decorated carbon nanofiber membranes for the flexible and high performance glucose sensors. Sens. Actuators B Chem. 2020, 312, 127886. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Yang, J.; Liu, G.; Li, J.; Guo, L.; Shen, S.; Guo, Q. NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sens. Actuators B Chem. 2018, 258, 920–928. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, L.Y.; Wang, B.C.; Huang, T.H.; Shih, M.J.; Hung, W.S.; Lai, J.Y.; Ho, K.C.; Yeh, M.H. Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat. Nano Energy 2022, 100, 107464. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B Chem. 2021, 343, 130131. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully integrated flexible biosensor for wearable continuous glucose monitoring. Biosens. Bioelectron. 2022, 196, 113760. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT:PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Guo, Y.; Lv, T.; Chen, T. Integration of interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors. Biosens. Bioelectron. 2021, 179, 113078. [Google Scholar] [CrossRef] [PubMed]
- Toi, P.T.; Trung, T.Q.; Dang, T.; Bae, C.W.; Lee, N.E. Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-Body Sweat Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, Y.; Yang, J.; Xue, Y. Core-sheath fibers composed of F-doped nickel hydroxide nanorods and graphene fibers for effective fiber-shaped nonenzymatic glucose sensors. J. Alloys Compd. 2021, 889, 161608. [Google Scholar] [CrossRef]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.E. Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Montazer, M.; Mazinani, S. Synthesis of wearable and flexible NiP0.1-SnOx/PANI/CuO/cotton towards a non-enzymatic glucose sensor. Biosens. Bioelectron. 2019, 135, 192–199. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Han, S.; Kim, J.; Won, S.M.; Ma, Y.; Kang, D.; Xie, Z.; Lee, K.; Chung, H.U.; Banks, A.; Min, S. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 2018, 10, eaan4950. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.N.; Lv, R.Q.; Zhao, Y.; Qian, J.K. A Mach-Zehnder interferometer-based High Sensitivity Temperature sensor for human body monitoring. Opt. Fiber Technol. 2018, 45, 93–97. [Google Scholar] [CrossRef]
- Jiang, N.; Li, H.; Hu, D.; Xu, Y.; Hu, Y.; Zhu, Y.; Han, X.; Zhao, G.; Chen, J.; Chang, X. Stretchable strain and temperature sensor based on fibrous polyurethane film saturated with ionic liquid. Compos. Commun. 2021, 27, 100845. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Liang, H.; Zhou, H.; Wang, X.; Heng, X.; Zhang, Z.; Gan, J.; Yang, Z. Stretchable and Strain-Decoupled Fluorescent Optical Fiber Sensor for Body Temperature and Movement Monitoring. ACS Photonics 2022, 9, 1415–1424. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, T.; Cao, P.; Cui, Y.; Nie, J.; Chen, T.; Yang, H.; Wang, F.; Sun, L. Carbonized Silk Nanofibers in Biodegradable, Flexible Temperature Sensors for Extracellular Environments. ACS Appl. Mater. Interfaces 2022, 14, 18110–18119. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Dang, T.; Ramasundaram, S.; Toi, P.T.; Lee, N.E. A Stretchable Strain-Insensitive Temperature Sensor Based on Free-Standing Elastomeric Composite Fibers for On-Body Monitoring of Skin Temperature. ACS Appl. Mater. Interfaces 2019, 11, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Poblete, F.R.; Zhu, Y. Tailoring the Temperature Coefficient of Resistance of Silver Nanowire Nanocomposites and their Application as Stretchable Temperature Sensors. ACS Appl. Mater. Interfaces 2019, 11, 17836–17842. [Google Scholar] [CrossRef]
- Pradhan, S.; Yadavalli, V.K. Photolithographically Printed Flexible Silk/PEDOT:PSS Temperature Sensors. ACS Appl. Electron. Mater. 2021, 3, 21–29. [Google Scholar] [CrossRef]
- Pang, Q.; Hu, H.; Zhang, H.; Qiao, B.; Ma, L. Temperature-Responsive Ionic Conductive Hydrogel for Strain and Temperature Sensors. ACS Appl. Mater. Interfaces 2022, 14, 26536–26547. [Google Scholar] [CrossRef]
- Li, F.; Xue, H.; Lin, X.; Zhao, H.; Zhang, T. Wearable Temperature Sensor with High Resolution for Skin Temperature Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 43844–43852. [Google Scholar] [CrossRef]
- Song, J.; Wei, Y.; Xu, M.; Gao, J.; Luo, L.; Wu, H.; Li, X.; Li, Y.; Wang, X. Highly Sensitive Flexible Temperature Sensor Made Using PEDOT:PSS/PANI. ACS Appl. Polym. Mater. 2022, 4, 766–772. [Google Scholar] [CrossRef]
- Pielak, R.; Wei, P.; Peyret, H.; Balooch, G.; Rogers, J. 14188 Wearable UV/HEV light sensor and smartphone application for personal monitoring and personalized recommendations. J. Am. Acad. Dermatol. 2020, 83, AB133. [Google Scholar] [CrossRef]
- Araki, H.; Kim, J.; Zhang, S.; Banks, A.; Crawford, K.E.; Sheng, X.; Gutruf, P.; Shi, Y.; Pielak, R.M.; Rogers, J.A. Materials and Device Designs for an Epidermal UV Colorimetric Dosimeter with Near Field Communication Capabilities. Adv. Funct. Mater. 2017, 27, 1604465. [Google Scholar] [CrossRef]
- Veeralingam, S.; Priya, S.; Badhulika, S. NiO nanofibers interspersed sponge based low cost, multifunctional platform for broadband UV protection, ultrasensitive strain and robust finger-tip skin inspired pressure sensor. Chem. Eng. J. 2020, 389, 124415. [Google Scholar] [CrossRef]
- Cui, X.; Chen, J.; Wu, W.; Liu, Y.; Li, H.; Xu, Z.; Zhu, Y. Flexible and breathable all-nanofiber iontronic pressure sensors with ultraviolet shielding and antibacterial performances for wearable electronics. Nano Energy 2022, 95, 107022. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, T.; Li, X.; Wei, X. ZnO-nanorod–fiber UV sensor based on evanescent field principle. Optik 2020, 202, 163672. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, J.; Gong, H. Bioinspired Self-Powered Piezoresistive Sensors for Simultaneous Monitoring of Human Health and Outdoor UV Light Intensity. ACS Appl. Mater. Interfaces 2022, 14, 5101–5111. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lou, J.; Zhang, R.; Ma, B.; Qin, Y. Self-Cleaning and Self-Powered UV Sensors for Highly Reliable Outdoor UV Detection. ACS Appl. Electron. Mater. 2020, 2, 1628–1634. [Google Scholar] [CrossRef]
- Sahare, P.D.; Srivastava, S.K.; Surender, K.; Fouran, S. n-ZnO/p-Si heterojunction nanodiodes based sensor for monitoring UV radiation. Sens. Actuators A Phys. 2018, 279, 351–360. [Google Scholar] [CrossRef]
- Yin, J.; Lu, C.; Li, C.; Yu, Z.; Shen, C.; Yang, Y.; Jiang, X.; Zhang, Y. A UV-filtering, environmentally stable, healable and recyclable ionic hydrogel towards multifunctional flexible strain sensor. Compos. Part B Eng. 2022, 230, 109528. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, D.I.; Seol, M.L.; Kim, B.; Han, J.W.; Meyyappan, M. Wearable UV Sensor Based on Carbon Nanotube-Coated Cotton Thread. ACS Appl. Mater. Interfaces 2018, 10, 40198–40202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yu, Q.W.; Chen, C.; Zhang, W.W.; Yu, C.; Yang, C.H.; Jin, S.X.; Xin, B.J. Research of Polysulfone Amide Micronanofiber Forming Technology. In Proceedings of the Textile Bioengineering and Informatics Symposium Proceedings (TBIS 2017), Wuhan, China, 16–19 May 2017; pp. 1205–1212. [Google Scholar]





| Biological Elements | Loading Methods | Purpose | Refs. |
|---|---|---|---|
| scherichia coli bacteriophage | electrostatic interaction | rapid detection of Escherichia coli | [39] |
| GOx | encapsulation of enzymes into metal frameworks for in situ growth | detect glucose | [40] |
| red cabbage extract (RCE) | doping in PVA solution | check pH | [41] |
| DNA oligonucleotides | soak the fiber membrane in the DNA solution and stir | detect p16INK4a gene | [42] |
| burkholderia cepacia lipase (BCL) | crosslinking with nanofibers | Detect 17α- ethinylestradiol (EE2) | [43] |
| Methods | Main Material | Sensitivity | Linear Range | Stability | Refs. |
|---|---|---|---|---|---|
| Electrospinning | PVDF | 18.376 kPa−1 | 0.002–10 kPa | 7500 | [64] |
| PVDF | 0.38 V/N | — — | 6834 | [65] | |
| PVDF | 5 kPa−1 | 0–5 kPa | — — | [66] | |
| CA | 60.28 kPa−1 | 0–24 kPa | 13,000 | [67] | |
| P(VDF-TrFE) | 437.5 mV/μm 41.7 mV/μm | 0–2 μm 2–10 μm | 2000 | [68] | |
| Fiber Optic | POF | 0.002 mV/μm 0.0004 mV/μm | 150–650 μm 1400–3450 μm | — — | [69] |
| Sacrifice template and sandpaper-treated | PDMS | 39.077 kPa−1 | 0.0009–160 kPa | 1400 | [70] |
| Laser etching | PVDF | 0.24 V/N | — — | 4000 | [71] |
| Coating | PU | 4.169 kPa−1 | 0.02–10.3 kPa | 2300 | [72] |
| TPU | 1.02 kPa−1 | 0.0007–160 kPa | 60,000 | [73] |
| Methods | Main Material | Sensitivity | Linear Range | Stability | Refs. |
|---|---|---|---|---|---|
| Electrospinning | PAN | 301.77 μAmM−1 cm−2 | 0.0003–4.5 mM | 80 days | [79] |
| PAN | 1947.2 μAmM−1 cm−2 | 0.005–19.175 mM | — — | [80] | |
| Coating | PVDF | 5.18 μAmM−1 cm−2 | — — | — — | [81] |
| fabric | 105.93 μAmM−1 cm−2 | 0.05–1 mM | — — | [82] | |
| PU | 12.69 μAmM−1 cm−2 | 1–30 mM | 16 days | [83] | |
| Hydrogel | PEDOT:PSS | 0.875 µAµM−1 cm−2 | 2.0–250 μmolL–1 | 25 days | [84] |
| Chemical vapor deposition | nickel textile | 14.45 µAµM−1 cm−2 | — — | — — | [85] |
| Wet spinning | PU | 140 μAmM−1 cm−2 | — — | 10,000 | [86] |
| PU | 425.9 μAmM−1 cm−2 | 10 μM–0.66 mM | — — | [87] | |
| Ni(OH) | 595.3 μAmM−1 cm−2 | 0.01–7.66 mM | — — | [88] | |
| Deposition | PDMS | 253.4 μAmM−1 cm−2 | — — | 1000 | [89] |
| In-situ synthesized | fabric | 1625 μAmM−1 cm−2 | 0.001–1 mM | — — | [90] |
| 1325 μAmM−1 cm−2 | 1–10 mM | ||||
| Dry Spinning | SEBS | 11.7 μAmM−1 cm−2 | 0–500 μM | — — | [91] |
| Methods | Main Material | Sensitivity | Linear Range | Stability | Refs. |
|---|---|---|---|---|---|
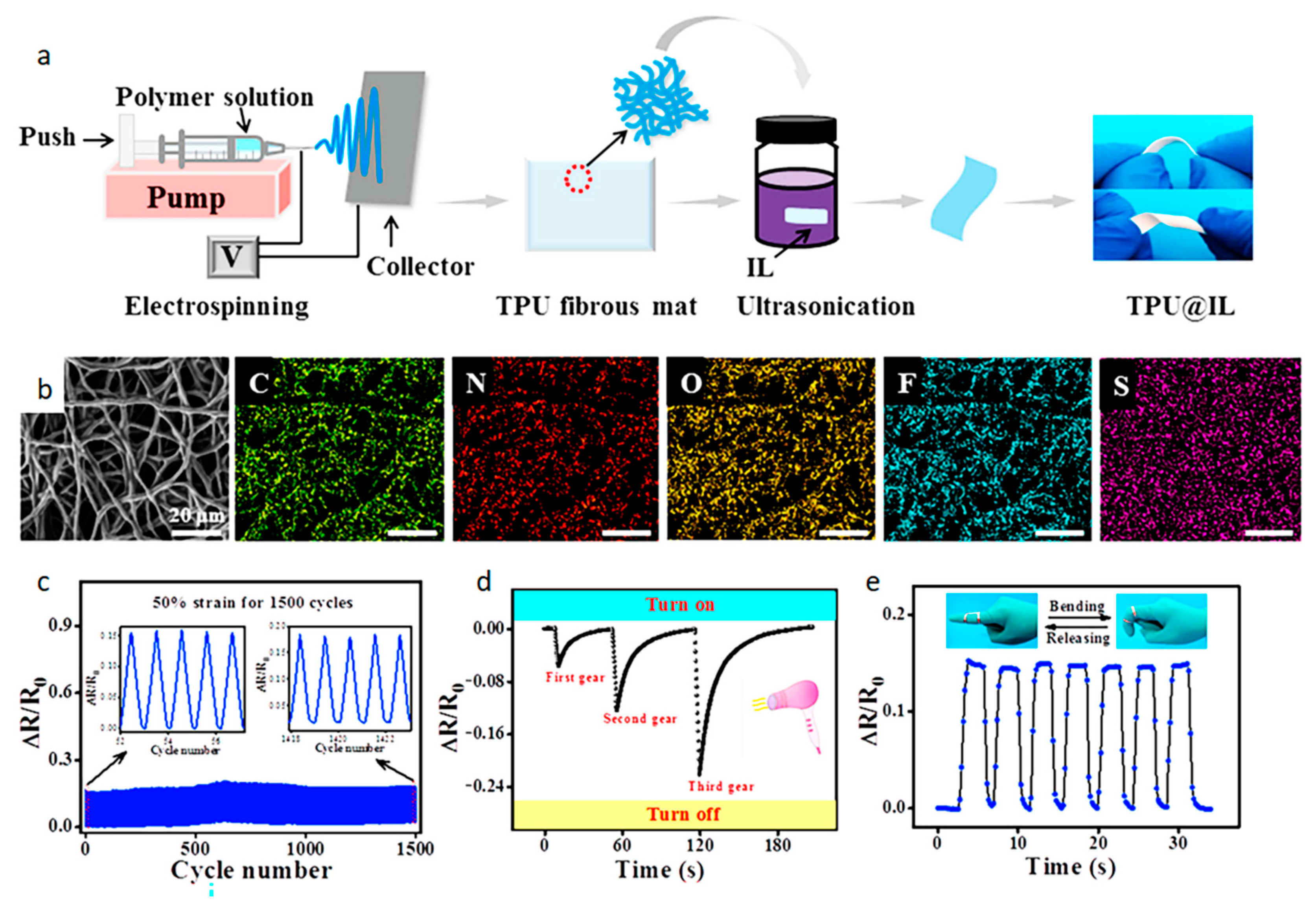
| Electrospinning | PVDF TPU | 57.76/°C 2.75%/°C | 24–48 °C — — | 5000 1000 | [54] [63] |
| Fiber Optic | PANI | 8.962 nm/°C | 33–43 °C | — — | [93] |
| PDMS | 1.3%/°C | 20–50 °C | — — | [95] | |
| Electrochemical Deposition | GuHCl | 1.75%/°C | 35–63 °C | — — | [96] |
| Wet-spinning | PU | 0.8%/°C | — — | — — | [97] |
| Spray-coated | AgNW | 0.47 Ω/°C | 25–60 °C | 1000 | [98] |
| PEDOT:PSS | −0.99%/°C | 20–50 °C | — — | [99] | |
| Free radical polymerization | NIPAAm | −1.39%/°C | 30–37 °C | — — | [100] |
| 0.37%/°C | 37–43 °C | ||||
| In situ synthesized | TPU | 0.95%/°C | 20–40 °C | — — | [101] |
| Drop coating | PEDOT:PSS | −0.803%/°C | 35–40 °C | — — | [102] |
| Methods | Main Material | Sensitivity | Linear Range | Stability | Refs. |
|---|---|---|---|---|---|
| Electrospinning | PAN | 0.0574% | — — | — — | [55] |
| TPU | 7.2% | — — | — — | [106] | |
| Fiber-Optic | ZnO ZnO | 7.096 W/(mWcm−2) — — | 1527–1534 nm 1.5–12.5 mWcm−2 | — — — — | [107] [108] |
| Spin-coated | BaTiO3 | 23.11% 46.85% 67.92% | 0.07 mWcm−2 0.3 mWcm−2 1.1 mWcm−2 | — — | [109] |
| Rf-sputtering | ZnO | 50 µW/cm2 | — — | 100 | [110] |
| Hydrogels | PVA | 10.88% | 365 nm | — — | [111] |
| 78.26% | 650 nm | ||||
| Ink-coating | Cellulose thread | 4.76 mW/cm2 | 254 nm | — — | [112] |
| 0.76% mW/cm2 | 365 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, G.; Chen, Z.; Li, H.; Awuye, D.E.; Guan, M.; Zhu, Y. Electrospinning-Based Biosensors for Health Monitoring. Biosensors 2022, 12, 876. https://doi.org/10.3390/bios12100876
Ji G, Chen Z, Li H, Awuye DE, Guan M, Zhu Y. Electrospinning-Based Biosensors for Health Monitoring. Biosensors. 2022; 12(10):876. https://doi.org/10.3390/bios12100876
Chicago/Turabian StyleJi, Guojing, Zhou Chen, Hui Li, Desire Emefa Awuye, Mengdi Guan, and Yingbao Zhu. 2022. "Electrospinning-Based Biosensors for Health Monitoring" Biosensors 12, no. 10: 876. https://doi.org/10.3390/bios12100876
APA StyleJi, G., Chen, Z., Li, H., Awuye, D. E., Guan, M., & Zhu, Y. (2022). Electrospinning-Based Biosensors for Health Monitoring. Biosensors, 12(10), 876. https://doi.org/10.3390/bios12100876






