Numerical Study on a Bio-Inspired Micropillar Array Electrode in a Microfluidic Device
Abstract
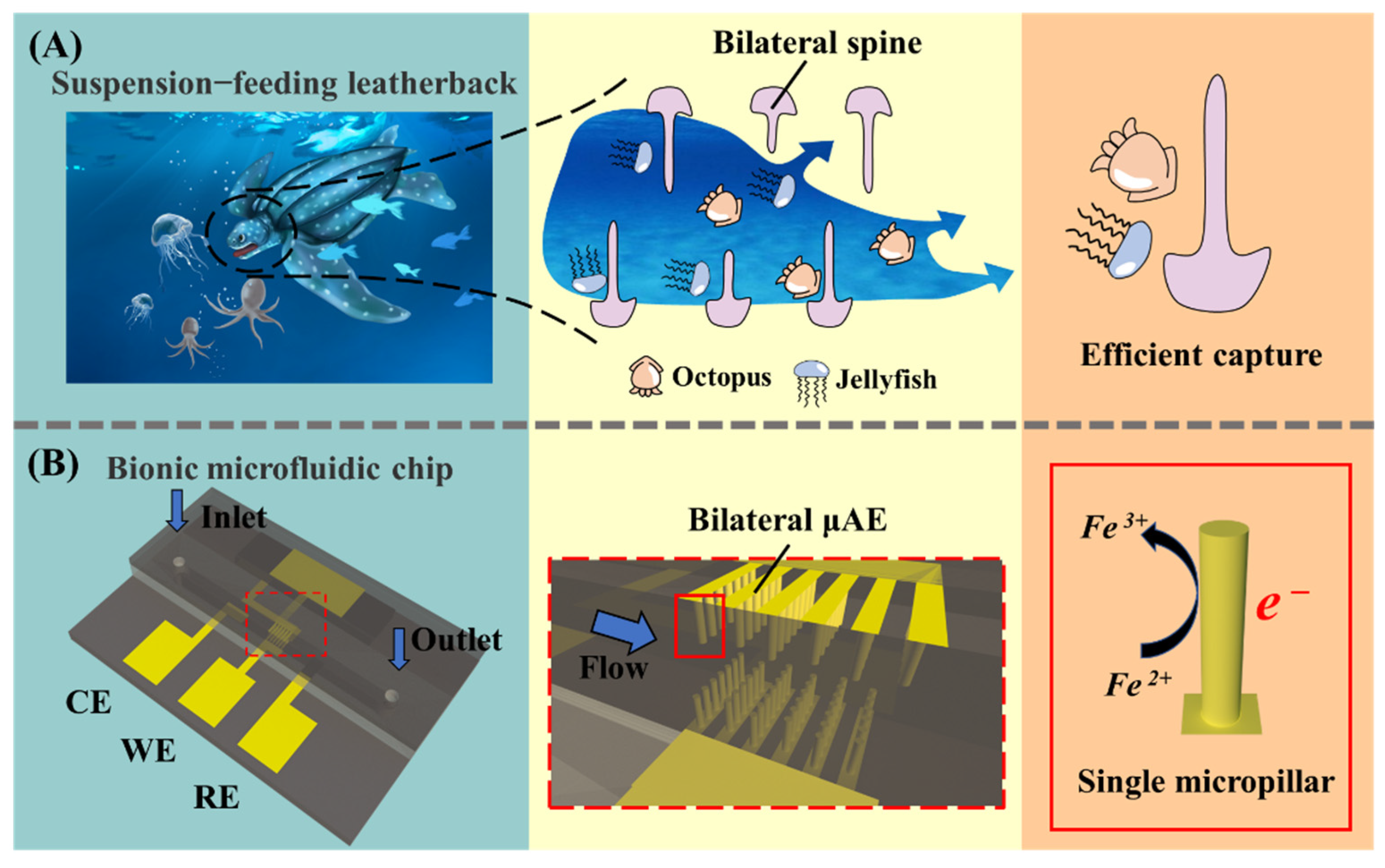
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
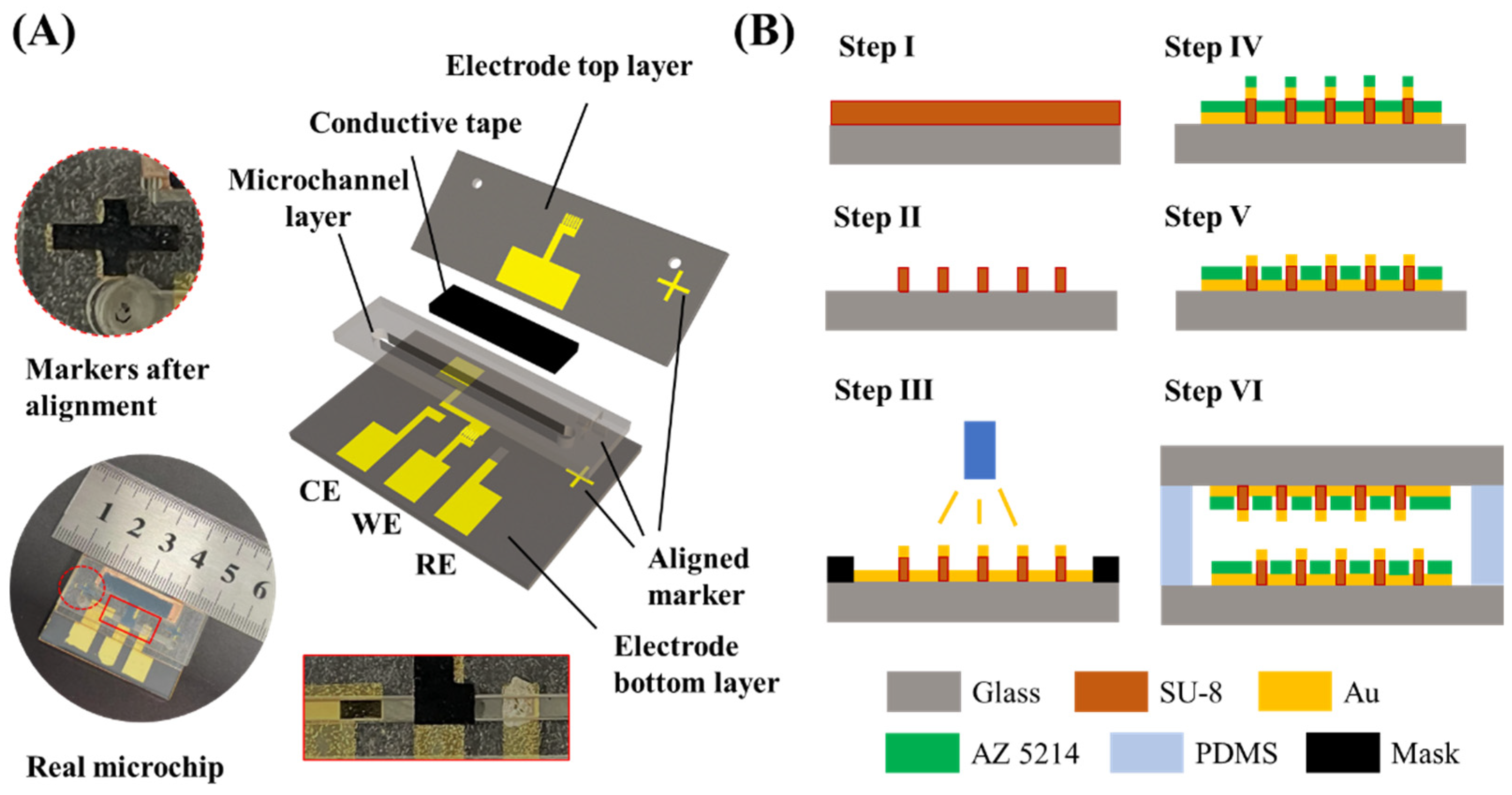
2.2. Configuration of Bionic Microchip
2.3. Simulation Method of BμAE
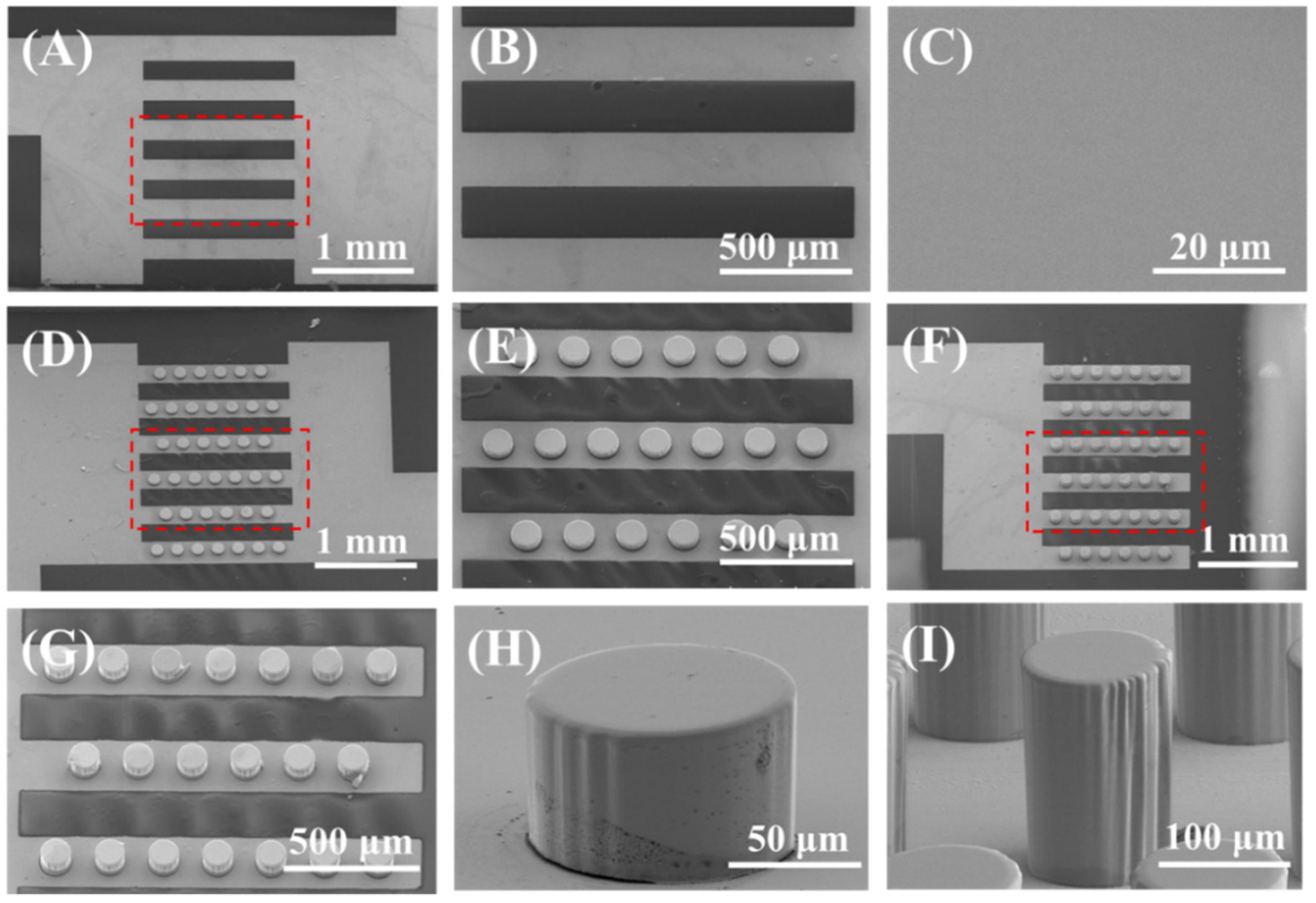
2.4. Fabrication of Bionic Microchip
2.5. Experiments of Electrochemical Detection
3. Results and Discussion
3.1. Effect of Flow Rate and Spacing
3.2. Effect of Micropillar Height
3.3. Effect of Micropillar Layout
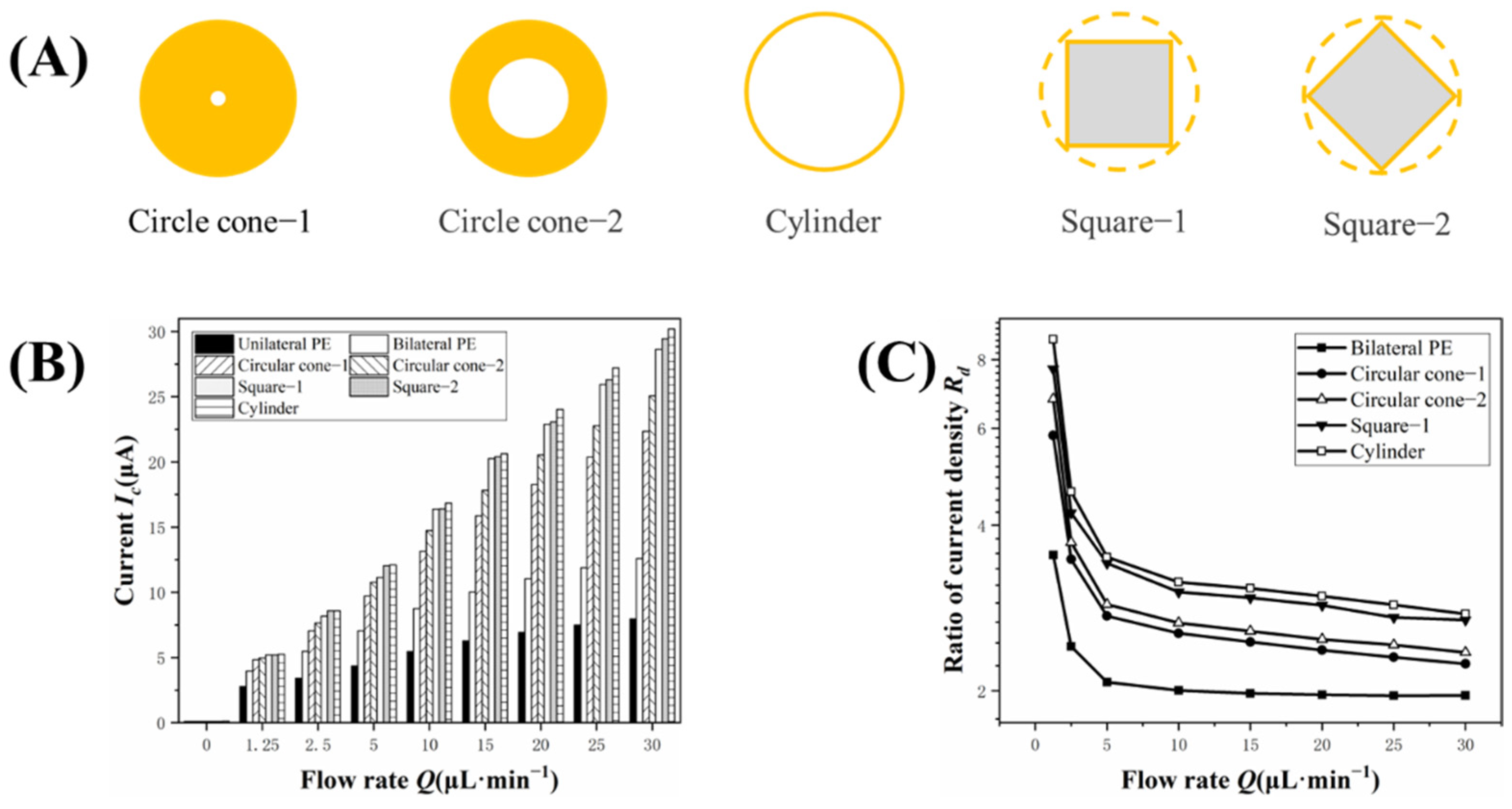
3.4. Effect of Micropillar Shape
3.5. Experimental Verification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wang, Z.; Cui, X.; Jiang, L.; Zhi, Y.; Ding, X.; Nie, Z.; Zhou, P.; Cui, D. Microfluidic Device Directly Fabricated on Screen-Printed Electrodes for Ultrasensitive Electrochemical Sensing of PSA. Nanoscale Res. Lett. 2019, 14, 71. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Pakchin, P.S.; Shamloo, A.; Mota, A.; de la Guardia, M.; Omidian, H.; Omidi, Y. Label-free electrochemical microfluidic biosensors: Futuristic point-of-care analytical devices for monitoring diseases. Mikrochim. Acta 2022, 189, 252. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Su, J.; Xu, Y.; He, G.; Wang, C.; Wang, X.; Pan, T.; Ding, X.; Mi, X. DNA tetrahedron-mediated immune-sandwich assay for rapid and sensitive detection of PSA through a microfluidic electrochemical detection system. Microsyst. Nanoeng. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tong, L.; Tong, W.; Chen, H.; Lan, M.; Sun, X.; Zhu, Y. Current progress in long-term and continuous cell metabolite detection using microfluidics. TrAC Trends Anal. Chem. 2019, 117, 263–279. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Shui, L.; Chen, H.; Zhu, Y. Recent advances in microdroplet techniques for single-cell protein analysis. TrAC Trends Anal. Chem. 2021, 143, 116411. [Google Scholar] [CrossRef]
- Liu, B.; Ran, B.; Chen, C.; Shi, L.; Liu, Y.; Chen, H.; Zhu, Y. A low-cost and high-performance 3D micromixer over a wide working range and its application for high-sensitivity biomarker detection. React. Chem. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; He, Z.; Hou, L.; Ge, C.; Wang, L.; Li, S.; Xu, Y. Detection of prostate specific antigen in whole blood by microfluidic chip integrated with dielectrophoretic separation and electrochemical sensing. Biosens. Bioelectron. 2022, 204, 114057. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, M.; Wang, Z.; Chen, K.; Li, X.; Ni, Z.H. Layer-by-Layer Self-Assembly of Mos2/Pdda Hybrid Film in Microfluidic Chips for Ultrasensitive Electrochemical Immunosensing of Alpha-Fetoprotein. Microchem. J. 2020, 158, 105209. [Google Scholar] [CrossRef]
- Ran, B.; Chen, C.; Liu, B.; Lan, M.; Chen, H.; Zhu, Y. A Ti3 C2 Tx /Pt-Pd Based Amperometric Biosensor for Sensitive Cancer Biomarker Detection. Electrophoresis 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.; Yang, J.; He, Y.; Li, Y. Application of Multiplex Microfluidic Electrochemical Sensors in Monitoring Hematological Tumor Biomarkers. Anal. Chem. 2020, 92, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Numthuam, S.; Kakegawa, T.; Anada, T.; Khademhosseini, A.; Suzuki, H.; Fukuda, J. Synergistic effects of micro/nano modifications on electrodes for microfluidic electrochemical ELISA. Sens. Actuators B Chem. 2011, 156, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.R.; Alam, M.A. A compact analytical formalism for current transients in electrochemical systems. Analyst 2012, 138, 525–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poujouly, C.; Gonzalez-Losada, P.; Boukraa, R.; Freisa, M.; Le Gall, J.; Bouville, D.; Deslouis, C.; Gamby, J. Diffusion–convection impedance for a micro-band electrode under microfluidic conditions. Electrochem. Commun. 2022, 137, 107262. [Google Scholar] [CrossRef]
- Panini, N.V.; Messina, G.A.; Salinas, E.; Fernández, H.; Raba, J. Integrated microfluidic systems with an immunosensor modified with carbon nanotubes for detection of prostate specific antigen (PSA) in human serum samples. Biosens. Bioelectron. 2008, 23, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Hoshikawa, K.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. A Concentric Ring Electrode for a Wall-jet Cell in a Microfluidic Device. Electroanalysis 2019, 31, 1736–1743. [Google Scholar] [CrossRef]
- Chen, C.; Ran, B.; Wang, Z.; Zhao, H.; Lan, M.; Chen, H.; Zhu, Y. Development of micropillar array electrodes for highly sensitive detection of biomarkers. RSC Adv. 2020, 10, 41110–41119. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, C.; Chen, C.; Ran, B.; Lan, M.; Chen, H.; Zhu, Y. Electrochemical Performance of Micropillar Array Electrodes in Microflows. Micromachines 2020, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Piao, Y.; Choi, J.S.; Seo, T.S. Three-dimensional graphene micropillar based electrochemical sensor for phenol detection. Biosens. Bioelectron. 2013, 50, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ran, B.; Liu, B.; Liu, X.; Liu, Y.; Lan, M.; Manasseh, R.; Zhu, Y. Development of a novel microfluidic biosensing platform integrating micropillar array electrode and acoustic microstreaming techniques. Biosens. Bioelectron. 2022, 114703, in press. [Google Scholar] [CrossRef]
- Sánchez-Molas, D.; Esquivel, J.P.; Sabaté, N.; Muñoz, F.X.; del Campo, F.J. High Aspect-Ratio, Fully Conducting Gold Micropillar Array Electrodes: Silicon Micromachining and Electrochemical Characterization. J. Phys. Chem. C 2012, 116, 18831–18846. [Google Scholar] [CrossRef]
- Prehn, R.; Abad, L.; Molas, D.S.; Duch, M.; Sabaté, N.; del Campo, F.J.; Muñoz, L.A.; Compton, R.G. Microfabrication and characterization of cylinder micropillar array electrodes. J. Electroanal. Chem. 2011, 662, 361–370. [Google Scholar] [CrossRef]
- Pai, R.S.; Walsh, K.M.; Crain, M.M.; Roussel, J.T.J.; Jackson, D.J.; Baldwin, R.P.; Keynton, R.S.; Naber, J.F. Fully Integrated Three-Dimensional Electrodes for Electrochemical Detection in Microchips: Fabrication, Characterization, and Applications. Anal. Chem. 2009, 81, 4762–4769. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.J.F.; Streeter, I.; Compton, R.G. Chronoamperometry and Cyclic Voltammetry at Conical Electrodes, Microelectrodes, and Electrode Arrays: Theory. J. Phys. Chem. B 2007, 112, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, S.; Jiang, N.; Li, X.; Liu, C.; Li, J.; Liu, Y. Novel sinuous band microelectrode array for electrochemical amperometric sensing. Electrochem. Commun. 2021, 133, 107159. [Google Scholar] [CrossRef]
- Bonin, F.; Devaux, B.; Dupré, A. Turtles of the World, A Guide to Every Family; Chelonian Research Foundation and Turtle Conservancy: Lunenburg, MA, USA, 2021. [Google Scholar]
- Numthuam, S.; Ginoza, T.; Zhu, M.; Suzuki, H.; Fukuda, J. Gold-black micropillar electrodes for microfluidic ELISA of bone metabolic markers. Analyst 2010, 136, 456–458. [Google Scholar] [CrossRef] [Green Version]
- Kilchenmann, S.C.; Rollo, E.; Bianchi, E.; Guiducci, C. Metal-coated silicon micropillars for freestanding 3D-electrode arrays in microchannels. Sens. Actuators B Chem. 2013, 185, 713–719. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Miao, J.; Triantafyllou, M.S. Miniaturized chemical sensor with bio-inspired micropillar working electrode array for lead detection. Sens. Actuators B Chem. 2016, 233, 249–256. [Google Scholar] [CrossRef]
- Kimura, S.; Fukuda, J.; Tajima, A.; Suzuki, H. On-chip diagnosis of subclinical mastitis in cows by electrochemical measurement of neutrophil activity in milk. Lab a Chip 2012, 12, 1309–1315. [Google Scholar] [CrossRef]
- Sen, D.; Isaac, K.; Leventis, N.; Fritsch, I. Investigation of transient redox electrochemical MHD using numerical simulations. Int. J. Heat Mass Transf. 2011, 54, 5368–5378. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Streeter, I.; Compton, R.G. Theory of Chronoamperometry at Cylindrical Microelectrodes and Their Arrays. J. Phys. Chem. C 2008, 112, 11637–11644. [Google Scholar] [CrossRef]
- Lee, G.; Park, I.; Kwon, K.; Kwon, T.; Seo, J.; Chang, W.-J.; Nam, H.; Cha, G.S.; Choi, M.H.; Yoon, D.S.; et al. Electrochemical detection of high-sensitivity CRP inside a microfluidic device by numerical and experimental studies. Biomed. Microdevices 2011, 14, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, M.; Bellini, M.; Pogni, R.; Giaccherini, A.; Innocenti, M.; Vizza, F.; Lavacchi, A. Effect of Electrode Shape and Flow Conditions on the Electrochemical Detection with Band Microelectrodes. Sensors 2018, 18, 3196. [Google Scholar] [CrossRef] [Green Version]
- Ledden, B.; Bruton, J. Ring electrode geometry for microfluidic electrochemistry. Sens. Actuators B Chem. 2019, 297, 126735. [Google Scholar] [CrossRef]
- Tait, R.J.; Bury, P.C.; Finnin, B.C.; Reed, B.L.; Bond, A. An explicit finite difference simulation for chronoamperometry at a disk microelectrode in a channel flow solution. J. Electroanal. Chem. 1993, 356, 25–42. [Google Scholar] [CrossRef]
- Zoski, C.G. Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Baur, J.E.; Motsegood, P.N. Diffusional interactions at dual disk microelectrodes: Comparison of experiment with three-dimensional random walk simulations. J. Electroanal. Chem. 2004, 572, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Panat, R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef]
- Hu, X.; Guo, H.; Qi, T.; Fan, W.; Jia, C.; Xu, B.; Jin, Q.-H.; Offenhaeusser, A.; Zhao, J.-L. Miniaturized electrochemical sensor with micropillar array working electrode for trace lead online measurement in tap water. J. Micromech. Microeng. 2019, 29, 105005. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C. High-power biofuel cells based on three-dimensional reduced graphene oxide/carbon nanotube micro-arrays. Microsyst. Nanoeng. 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Choi, J.S.; An, J.H. Reliable and Robust Fabrication Rules for Springtail-Inspired Superomniphobic Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 21120–21126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Hu, H.; Chen, C.-Z. The Effect of Sensor Dimensions on the Performance of Flexible Hot Film Shear Stress Sensors. Micromachines 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Planar | μAE | bμAE | ||||
|---|---|---|---|---|---|---|---|
| Projection area (mm2) | 1.5×2.5 | ||||||
| Radius r (μm) | - | 50 | 50 | ||||
| Height h (μm) | - | 150/250 | 50/100/150/200/250 | ||||
| Spacing 1 d (μm) | - | 200 | 200 | 200 | 150/200/250 | 200 | 200 |
| Number of pillars n | - | 78 | 78 | 78 | 136/78/46 | 78 | 78 |
| Surface area S (mm2) | 3.8 | 7.4/9.9 | 5.0 | 6.2 | 10.2/7.4/5.9 | 8.7 | 9.9 |
| Area ratio 2 | 1 | 1.9/2.6 | 1.3 | 1.6 | 2.7/1.9/1.6 | 2.3 | 2.6 |
| Parameters | Unit | Value |
|---|---|---|
| Diffusion coefficient D | cm2/s | 6.39 × 10−6 |
| Faraday’s constant F | C/mol | 96,485.33 |
| Standard heterogeneous rate constant k0 | m/s | 1 × 10−4 |
| Transfer coefficient α | - | 0.6 |
| Gas constant R | J/(mol·K) | 8.314 |
| Absolute temperature T | K | 298.15 |
| Formal potential E0′ | V | 0.15 |
| Applied potential E | V | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Ran, B.; Liu, B.; Liu, X.; Jin, J.; Zhu, Y. Numerical Study on a Bio-Inspired Micropillar Array Electrode in a Microfluidic Device. Biosensors 2022, 12, 878. https://doi.org/10.3390/bios12100878
Chen C, Ran B, Liu B, Liu X, Jin J, Zhu Y. Numerical Study on a Bio-Inspired Micropillar Array Electrode in a Microfluidic Device. Biosensors. 2022; 12(10):878. https://doi.org/10.3390/bios12100878
Chicago/Turabian StyleChen, Chaozhan, Bin Ran, Bo Liu, Xiaoxuan Liu, Jing Jin, and Yonggang Zhu. 2022. "Numerical Study on a Bio-Inspired Micropillar Array Electrode in a Microfluidic Device" Biosensors 12, no. 10: 878. https://doi.org/10.3390/bios12100878
APA StyleChen, C., Ran, B., Liu, B., Liu, X., Jin, J., & Zhu, Y. (2022). Numerical Study on a Bio-Inspired Micropillar Array Electrode in a Microfluidic Device. Biosensors, 12(10), 878. https://doi.org/10.3390/bios12100878







