A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Preparation
2.2. Preparation and Activation of Biochar from Corn Stalk
2.3. Characterization of Activated Biochar
2.3.1. Raman Spectroscopy Analysis
2.3.2. Brunauer–Emmett–Teller (BET) Analysis
2.3.3. Apparatus and Electrode Measurement System
2.4. Optimization of ABC Concentration
2.5. Stepwise Fabrication of the Immunosensor
2.6. Antibody Conformity Test Using Dot-Blot Analysis
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results
3.1. Physical Properties of Activated Biochar Sample
3.2. The Optimum Concentration of Activated Biochar on Immunosensor Development
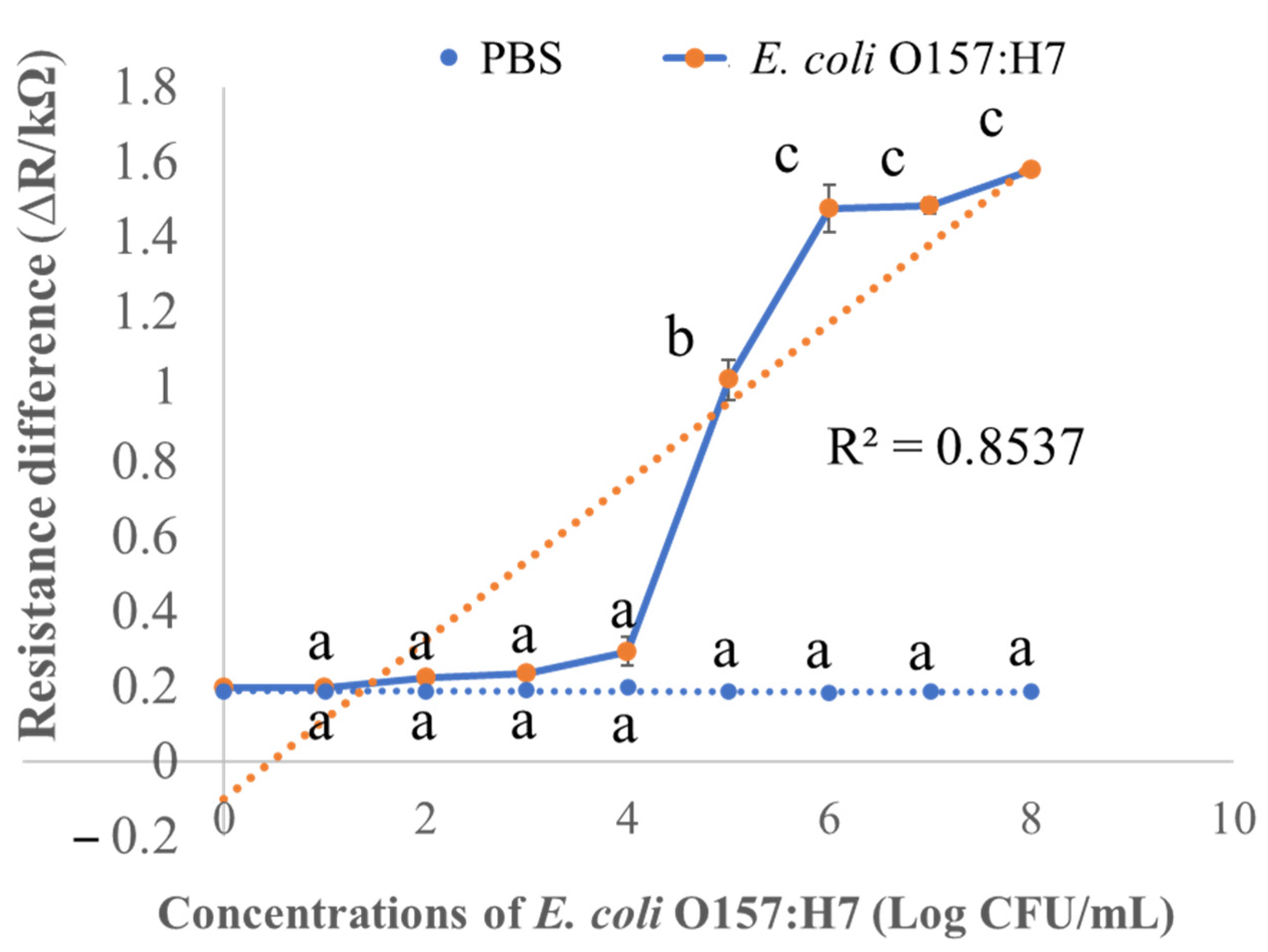
3.3. Detection Responses of Activated Biochar-Based Biosensor
3.4. Binding Specificity of pAbs toward E. coli O157:H7
3.5. Limit of Detection of the Activated Biochar-Based Immunosensor
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eom, K.H.; Hyun, K.H.; Lin, S.; Kim, J.W. The Meat Freshness Monitoring System Using the Smart RFID Tag. Int. J. Distrib. Sens. Netw. 2014, 10, 591812. [Google Scholar] [CrossRef] [Green Version]
- Schumann, B.; Schmid, M. Packaging Concepts for Fresh and Processed Meat–Recent Progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. An Electrochemical Biosensor for Rapid Detection of: E. Coli O157:H7 with Highly Efficient Bi-Functional Glucose Oxidase-Polydopamine Nanocomposites and Prussian Blue Modified Screen-Printed Interdigitated Electrodes. Analyst 2016, 141, 5441–5449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhan, A.; Lee, J.; Park, M.-K.; Oh, J.-H. Rapid Detection of Yersinia Enterocolitica Using a Single–Walled Carbon Nanotube-Based Biosensor for Kimchi Product. LWT 2019, 108, 48–54. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart Packaging: Sensors for Monitoring of Food Quality and Safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Cella, L.N.; Shirale, D.J.; Park, M.; Muñoz, F.J.; Yates, M.V.; Mulchandani, A. Carbon Nanotubes-Based Chemiresistive Biosensors for Detection of Microorganisms. Biosens. Bioelectron. 2010, 26, 1437–1441. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Kim, S.W.; Park, C.; Lee, J. Assessment of Peanut Allergen Ara H1 in Processed Foods Using a SWCNTs-Based Nanobiosensor. Biosci. Biotechnol. Biochem. 2018, 82, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Sobhan, A.; Oh, J.H.; Park, M.K.; Lee, J. Reusability of a Single-Walled Carbon Nanotube-Based Biosensor for Detecting Peanut Allergens and Y. Enterocolitica. Microelectron. Eng. 2020, 225, 111281. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the Food and Bioprocessing Industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Van Den Top, T.; Zhou, R. Development of an Activated Carbon-Based Nanocomposite Film with Antibacterial Property for Smart Food Packaging. Mater. Today Commun. 2020, 23, 101124. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The Use of Activated Biochar for Development of a Sensitive Electrochemical Sensor for Determination of Methyl Parathion. J. Electroanal. Chem. 2017, 799, 602–608. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Lamy-Mendes, A.C.; Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. Electrochemical Determination of Copper Ions in Spirit Drinks Using Carbon Paste Electrode Modified with Biochar. Food Chem. 2015, 171, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant’Anna, M.V.S.; Carvalho, S.W.M.M.; Gevaerd, A.; Silva, J.O.S.; Santos, E.; Carregosa, I.S.C.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Electrochemical Sensor Based on Biochar and Reduced Graphene Oxide Nanocomposite for Carbendazim Determination. Talanta 2020, 220, 121334. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, L.Y. Subtle Application of Electrical Field-Induced Lossy Mode Resonance to Enhance Performance of Optical Planar Waveguide Biosensor. Biosensors 2021, 11, 86. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Yang, Y.; Mu, B.; Nikitina, M.A.; Xiao, X. Biosensors and Bioelectronics: X Vis/NIR Optical Biosensors Applications for Fruit Monitoring. Biosens. Bioelectron. X 2022, 11, 100197. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen Detection with Electrochemical Biosensors: Advantages, Challenges and Future Perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-Printed Electrodes for Biosensing: A Review (2008-2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Kim, S.; Yu, G.; Kim, T.; Shin, K.; Yoon, J. Rapid Bacterial Detection with an Interdigitated Array Electrode by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2012, 82, 126–131. [Google Scholar] [CrossRef]
- Petrovszki, D.; Valkai, S.; Gora, E.; Tanner, M.; Bányai, A.; Fürjes, P.; Dér, A. An Integrated Electro-Optical Biosensor System for Rapid, Low-Cost Detection of Bacteria. Microelectron. Eng. 2021, 239, 111523. [Google Scholar] [CrossRef]
- Brunetti, G.; Conteduca, D.; Armenise, M.N.; Ciminelli, C. Novel Micro-Nano Optoelectronic Biosensor for Label-Free Real-Time Biofilm Monitoring. Biosensors 2021, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, D.; Brunetti, G.; Dell’Olio, F.; Armenise, M.N.; Krauss, T.F.; Ciminelli, C. Monitoring of Individual Bacteria Using Electro-Photonic Traps. Biomed. Opt. Express 2019, 10, 3463. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Qiao, Q.; Rahman, M.T.; Ghimire, N. Development and Characterization of a Novel Activated Biochar-Based Polymer Composite for Biosensors. Int. J. Polym. Anal. Charact. 2021, 26, 544–560. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Zhou, R.; Ghimire, N. Development of a Biosensor with Electrically Conductive and Biodegradable Composite by Combinatory Use of Silver Nanoparticles, Novel Activated Biochar, and Polylactic Acid. J. Electrochem. Soc. 2021, 168, 107501. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Q.; Kou, H.; Wu, D.; Zhang, W.; Xiong, J. Highly Sensitive NH3 Wireless Sensor Based on Ag-RGO Composite Operated at Room-Temperature. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Silva, T.H.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Improving the Extraction of Ara h 6 (a Peanut Allergen) from a Chocolate-Based Matrix for Immunosensing Detection: Influence of Time, Temperature and Additives. Food Chem. 2017, 218, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Jinapon, C.; Wangman, P.; Pengsuk, C.; Chaivisuthangkura, P.; Sithigorngul, P.; Longyant, S. Development of Monoclonal Antibodies for the Rapid Detection and Identification of Salmonella Enterica Serovar Enteritidis in Food Sample Using Dot-Blot Assays. J. Food Saf. 2020, 40, e12841. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holm, N. High Capacitive Performance of Exfoliated Biochar Nanosheets from Biomass Waste Corn Cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Liu, Y.; Piao, Y. Preparation of Highly Conductive Biochar Nanoparticles for Rapid and Sensitive Detection of 17β-Estradiol in Water. Electrochim. Acta 2018, 292, 55–62. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Yamada, K.; Kim, C.-T.; Kim, J.-H.; Chung, J.-H.; Lee, H.G.; Jun, S. Single Walled Carbon Nanotube-Based Junction Biosensor for Detection of Escherichia Coli. PLoS ONE 2014, 9, e105767. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-K.; Lee, J.; Park, M.-K.; Oh, J.-H. Development of Single-Walled Carbon Nanotube-Based Biosensor for the Detection of Staphylococcus Aureus. J. Food Qual. 2017, 2017, 5239487. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, G.; Wang, B.; Shi, L.; Gao, T.; Li, G. Fabrication of Reusable Electrochemical Biosensor and Its Application for the Assay of α-Glucosidase Activity. Anal. Chim. Acta 2018, 1026, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Palmer, J.; Flint, S. A Rapid Method for the Nonselective Enumeration of Yersinia Enterocolitica, a Foodborne Pathogen Associated with Pork. MESC 2016, 113, 59–61. [Google Scholar] [CrossRef]
- Wangman, P.; Surasilp, T.; Pengsuk, C.; Sithigorngul, P.; Longyant, S. Development of a Species-Specific Monoclonal Antibody for Rapid Detection and Identification of Foodborne Pathogen Vibrio Vulnificus. J. Food Saf. 2021, 41, e12939. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X.; Inza, I.; Figueras, M.J. Fast Detection of Salmonella Infantis with Carbon Nanotube Field Effect Transistors. Biosens. Bioelectron. 2008, 24, 279–283. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, P.; Liu, H.; Teng, Y.; Liu, Y.; Wang, Q.; Zhang, W.; Jin, L.; Jiang, L. Development of an Electrochemical Immunoassay for Rapid Detection of E. Coli Using Anodic Stripping Voltammetry Based on Cu@Au Nanoparticles as Antibody Labels. Biosens. Bioelectron. 2009, 24, 2155–2159. [Google Scholar] [CrossRef]
- Gan, W.; Xu, Z.; Li, Y.; Bi, W.; Chu, L.; Qi, Q.; Yang, Y.; Zhang, P.; Gan, N.; Dai, S.; et al. Rapid and Sensitive Detection of Staphylococcus Aureus by Using a Long-Period Fiber Grating Immunosensor Coated with Egg Yolk Antibody. Biosens. Bioelectron. 2022, 199, 113860. [Google Scholar] [CrossRef]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in Electrochemical Aptasensors and Immunosensors for Detection of Bacterial Pathogens in Food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]







| Concentration of Activated Biochar (mg/mL) | Resistance (kΩ) | ||||
|---|---|---|---|---|---|
| 1st Washing | 2nd Washing | 3rd Washing | 4th Washing | 5th Washing | |
| 5 | NA | NA | NA | NA | NA |
| 10 | 2.16 ± 0.0045 a | 1.93 ± 0.0047 b | 1.87 ± 0.0015 c | 1.81 ± 0.0011 c | 1.74 ± 0.002 c |
| 20 | 4.86 ± 0.007 d | 3.41 ± 1.28 d | 3.83 ± 0.005 d | 3.97 ± 0.031 d | 3.82 ± 0.239 d |
| 30 | 0.41 ± 0.0015 q | 0.403 ± 0.002 q | 0.32 ± 0.002 q | 0.386 ± 0.002 q | 0.381 ± 0.001 q |
| 40 | 0.98 ± 0.002 q | 0.684 ± 0.0036 q | 0.865 ± 0.004 q | 0.962 ± 0.003 q | 0.989 ± 0.0083 q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobhan, A.; Jia, F.; Kelso, L.C.; Biswas, S.K.; Muthukumarappan, K.; Cao, C.; Wei, L.; Li, Y. A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7. Biosensors 2022, 12, 908. https://doi.org/10.3390/bios12100908
Sobhan A, Jia F, Kelso LC, Biswas SK, Muthukumarappan K, Cao C, Wei L, Li Y. A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7. Biosensors. 2022; 12(10):908. https://doi.org/10.3390/bios12100908
Chicago/Turabian StyleSobhan, Abdus, Fei Jia, Lisa Cooney Kelso, Sonatan Kumar Biswas, Kasiviswanathan Muthukumarappan, Changyong Cao, Lin Wei, and Yanbin Li. 2022. "A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7" Biosensors 12, no. 10: 908. https://doi.org/10.3390/bios12100908
APA StyleSobhan, A., Jia, F., Kelso, L. C., Biswas, S. K., Muthukumarappan, K., Cao, C., Wei, L., & Li, Y. (2022). A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7. Biosensors, 12(10), 908. https://doi.org/10.3390/bios12100908








