Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters
Abstract
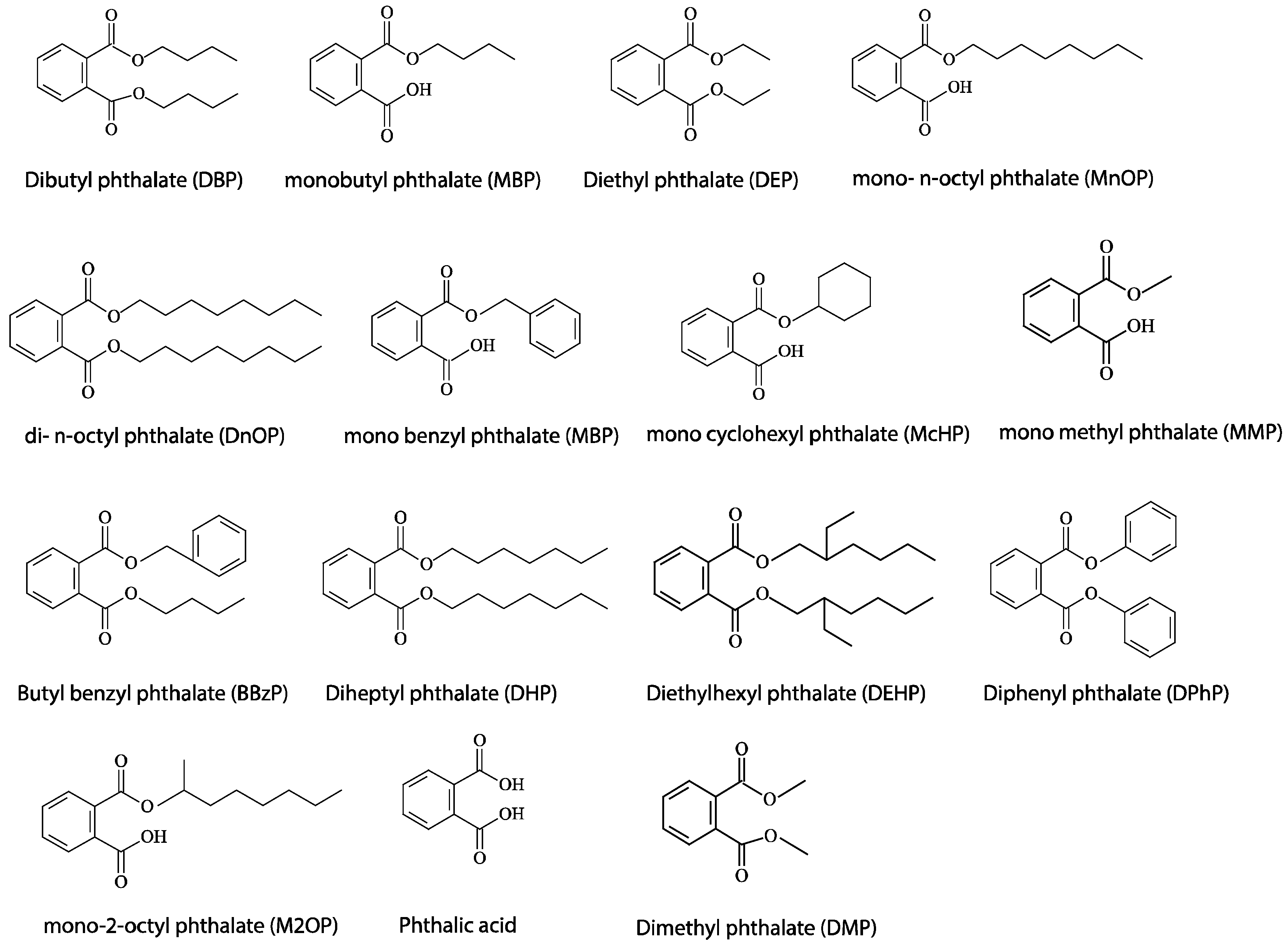
1. Introduction
2. Materials and Methods
2.1. Materials and Components
2.2. Methods
2.2.1. Synthesis of Hapten-Protein Conjugates
2.2.2. Synthesis of Gold Nanoparticles and Their Conjugation with Antibody
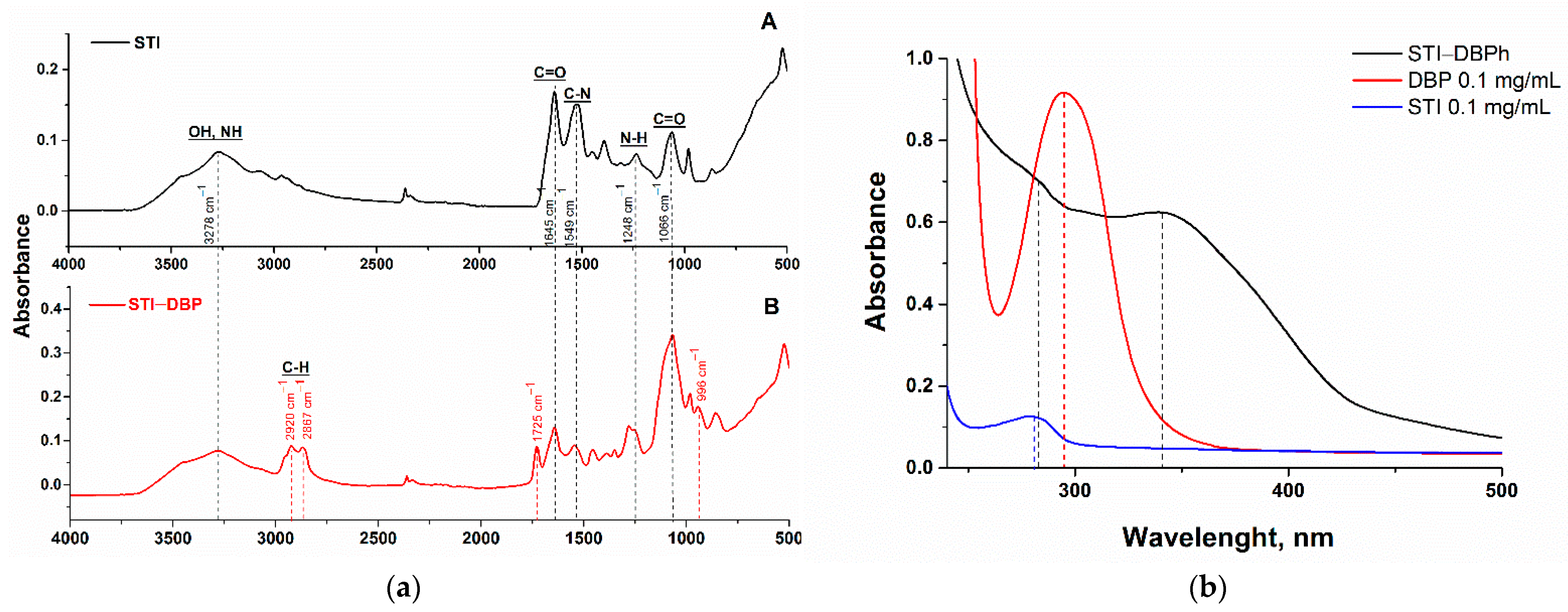
2.2.3. Characterization of the Obtained Conjugates
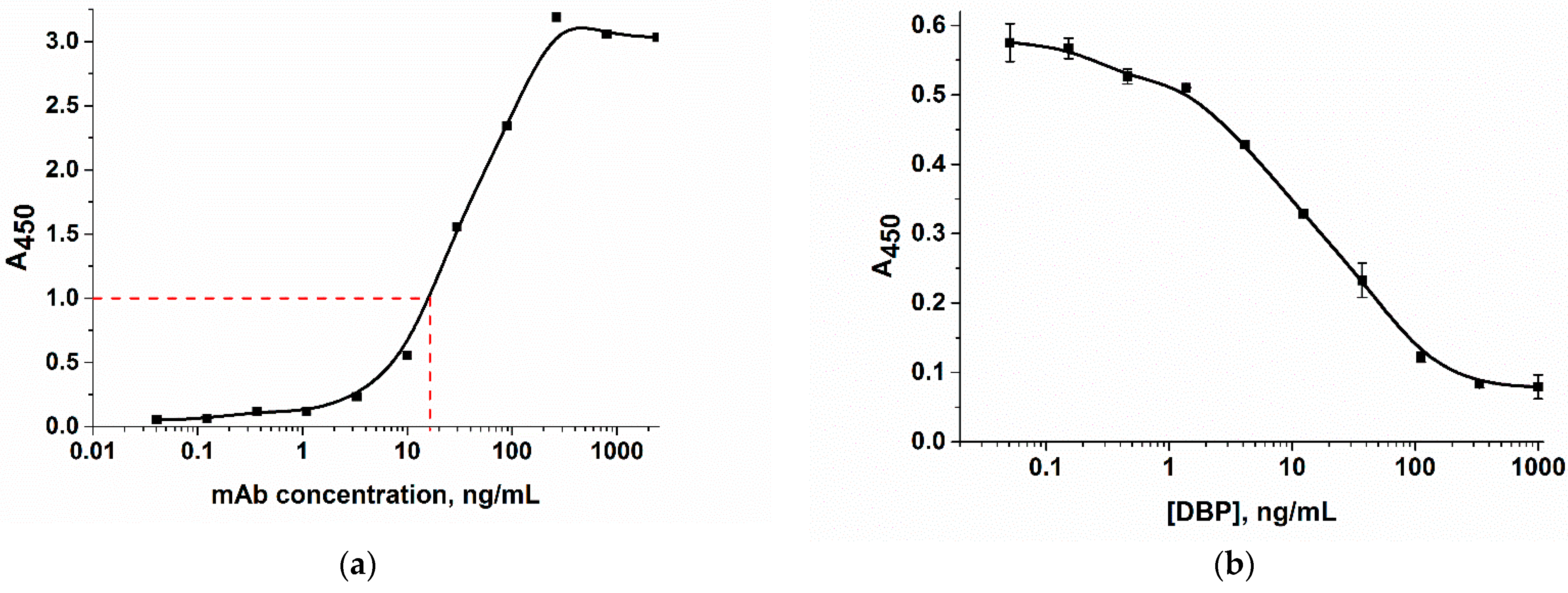
2.2.4. Characterization of Antibody by ELISA Technique
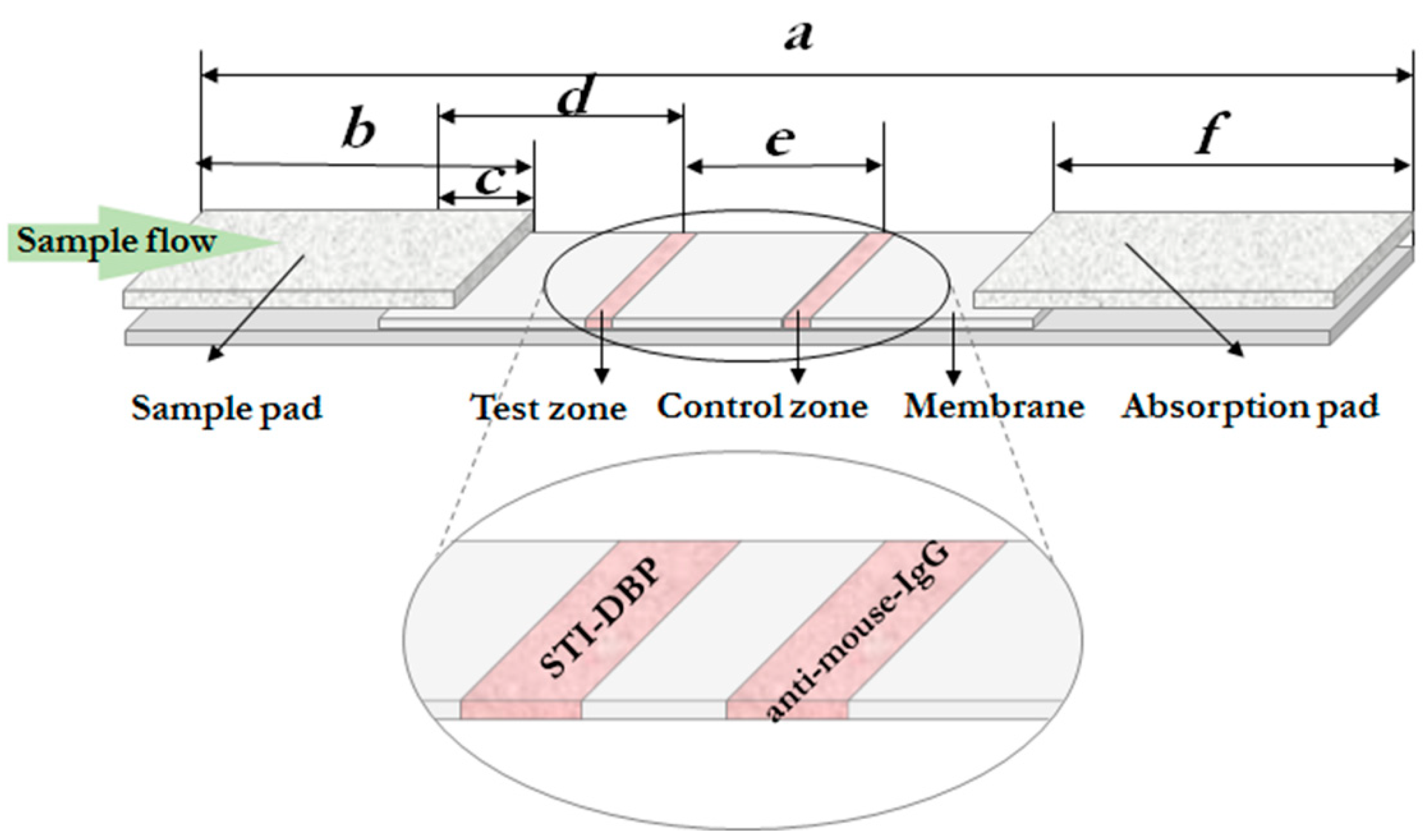
2.2.5. Composition of Lateral Flow Tests
2.2.6. Collection and Preparation of Water Samples
3. Results and Discussion
3.1. Synthesis and Characterization of DBP–Protein Conjugate
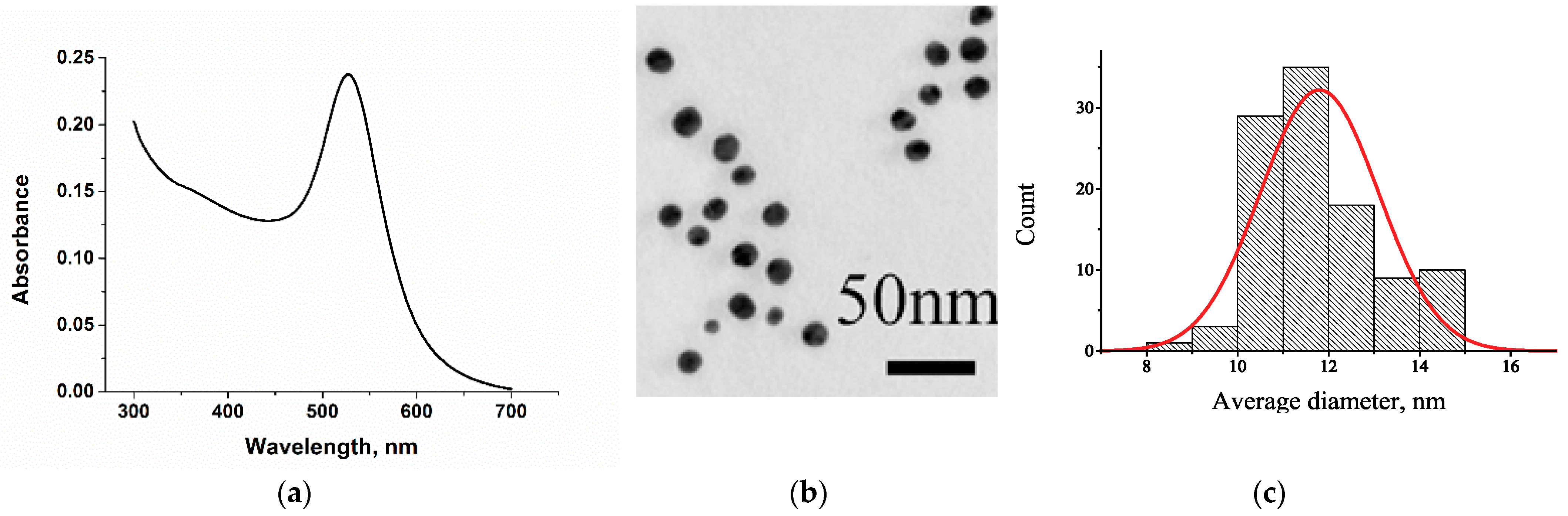
3.2. Synthesis and Characterization of Gold Nanoparticles Conjugated with the Anti-DBP Antibody
3.3. The LFIA Format
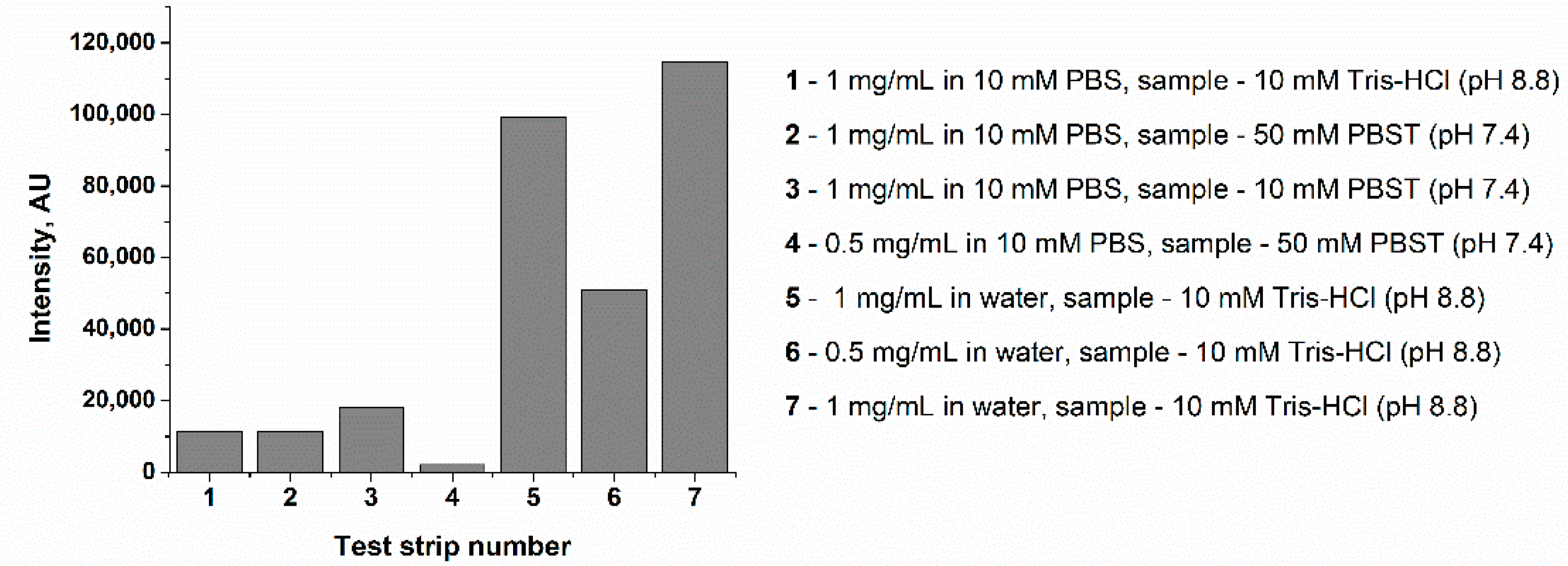
3.4. The Choice of STI−DBP Immobilization Medium
3.5. Selection of the Working Membrane
3.6. Analytical Characteristics in Optimized Conditions
3.7. Analysis of Natural Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berenstein, G.; Hughes, E.A.; Zalts, A.; Basack, S.; Bonesi, S.M.; Montserrat, J.M. Environmental fate of dibutylphthalate in agricultural plastics: Photodegradation, migration and ecotoxicological impact on soil. Chemosphere 2022, 290, 133221. [Google Scholar] [CrossRef] [PubMed]
- Erna, M.; Harazono, A.; Miyawaki, E.; Ogawa, Y. Developmental toxicity of mono-n-benzyl phthalate, one of the major metabolites of the plasticizer n-butyl benzyl phthalate, in rats. Toxicol. Lett. 1996, 86, 19–25. [Google Scholar] [CrossRef]
- Martino-Andrade, A.J.; Chahoud, I. Reproductive toxicity of phthalate esters. Mol. Nutr. Food Res. 2010, 54, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.; Lake, B.; Thomas, L.V.; Cook, M.; Gangolli, S. Studies on the testicular effects and zinc excretion produced by various isomers of monobutyl-o-phthalate in the rat. Chem. Biol. Interact. 1981, 34, 233–238. [Google Scholar] [CrossRef]
- Sedha, S.; Lee, H.; Singh, S.; Kumar, S.; Jain, S.; Ahmad, A.; Bin Jardan, Y.A.; Sonwal, S.; Shukla, S.; Simal-Gandara, J.; et al. Reproductive toxic potential of phthalate compounds—State of art review. Pharmacol. Res. 2021, 167, 105536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef] [PubMed]
- Benson, R. Hazard to the developing male reproductive system from cumulative exposure to phthalate esters—Dibutyl phthalate, diisobutyl phthalate, butylbenzyl phthalate, diethylhexyl phthalate, dipentyl phthalate, and diisononyl phthalate. Regul. Toxicol. Pharm. 2009, 53, 90–101. [Google Scholar] [CrossRef]
- Latini, G.; Del Vecchio, A.; Massaro, M.; Verrotti, A.; De Felice, C. Phthalate exposure and male infertility. Toxicology 2006, 226, 90–98. [Google Scholar] [CrossRef]
- Giribabu, N.; Reddy, P.S. Protection of male reproductive toxicity in rats exposed to di-n-butyl phthalate during embryonic development by testosterone. Biomed. Pharmacother. 2017, 87, 355–365. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, S.S.W.; Wu, D.C.; Lin, Y.C.; Ku, C.C.; Wu, C.C.; Chai, C.Y.; Lee, J.N.; Tsai, E.M.; Lin, C.L.; et al. Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis. 2013, 4, e907. [Google Scholar] [CrossRef]
- He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: A review. Agron. Sustain. Dev. 2015, 35, 519–534. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, F.; Zhu, C.; Chen, Z.; Liu, S.; Wang, C.; Gu, C. Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors. Water Res. 2021, 204, 117597. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, P.; Wu, Y.; Zhou, Y.; Sheng, Y.; Lao, K. Microplastic acts as a vector for contaminants: The release behavior of dibutyl phthalate from polyvinyl chloride pipe fragments in water phase. Environ. Sci. Pollut. Res. 2020, 27, 42082–42091. [Google Scholar] [CrossRef] [PubMed]
- SANPIN 1.2.3685-21; Hygienic Standards and Requirements for Ensuring the Safety and (or) Harmlessness of Environmental Factors for Humans, Federal Service for Supervision of Consumer Rights Protection and Human Welfare. Ministry of Justice of Russia: Moscow, Russia, 2021.
- Dada, E.O.; Ikeh, R.K. Phthalate and Metal Concentrations in Drinking Water in Lagos, Nigeria. J. Health Pollut. 2018, 8, 180603. [Google Scholar] [CrossRef] [PubMed]
- US EPA United States Environmental Protection Agency (US). National Primary Drinking Water Regulations; Federal Register: Washington, DC, USA, 1991; Volume 56, pp. 30266–30281.
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef]
- Song, P.; Jiang, N.; Zhang, K.; Li, X.; Li, N.; Zhang, Y.; Wang, Q.; Wang, J. Ecotoxicological evaluation of zebrafish liver (Danio rerio) induced by dibutyl phthalate. J. Hazard. Mater. 2022, 425, 128027. [Google Scholar] [CrossRef]
- Xu, Y.; Weng, R.; Lu, Y.; Wang, X.; Zhang, D.; Li, Y.; Qiu, J.; Qian, Y. Evaluation of Phthalic Acid Esters in Fish Samples Using Gas Chromatography Tandem Mass Spectrometry with Simplified QuEChERS Technique. Food Anal. Methods 2018, 11, 3293–3303. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J. Analytical methods for the determination of phthalates in food. Curr. Opin. Food Sci. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Phthalates in Beverages and Plastic Bottles: Sample Preparation and Determination. Food Anal. Methods 2018, 11, 48–61. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Wei, C.; Ding, S.; You, H.; Zhang, Y.; Wang, Y.; Yang, X.; Yuan, J. An Immunoassay for Dibutyl Phthalate Based on Direct Hapten Linkage to the Polystyrene Surface of Microtiter Plates. PLoS ONE 2011, 6, e29196. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wei, J.; Du, H.; Zhang, J.; Yang, D.; Peng, Y. Synthesis of an artificial antigen and preparation of a polyclonal antibody for the sensitive determination of phthalate esters by enzyme-linked immunoassay. Anal. Methods 2015, 7, 3402–3410. [Google Scholar] [CrossRef]
- Zeng, Q.; Wei, C.; Wu, Y.; Li, K.; Ding, S.; Yuan, J.; Yang, X.; Chen, M. Approach to distribution and accumulation of dibutyl phthalate in rats by immunoassay. Food Chem. Toxicol. 2013, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Baranovskaya, V.; Berlina, A.; Eremin, S. A Fluorescence Polarization Immunoassay Procedure for Determining Dibutyl Phthalate in Water. J. Anal. Chem. 2022, 77, 466–472. [Google Scholar] [CrossRef]
- Li, J.; Jin, H.; Wei, M.; Ren, W.; Wang, J.; Zhang, Y.; Wu, L.; He, B. Dual mode competitive electrochemical immunoassay for dibutyl phthalate detection based on PEI functionalized nitrogen doped graphene-CoSe2/gold nanowires and thionine-Au@Pt core-shell. Sens. Actuators B 2021, 331, 129401. [Google Scholar] [CrossRef]
- Zhang, M.-C.; Wang, Q.-E.; Zhuang, H.-S. A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate. Anal. Bioanal. Chem. 2006, 386, 1401–1406. [Google Scholar] [CrossRef]
- Zhang, M.C.; Wang, Q.E.; Zhuang, H.S. Determination of dibutyl o-phthalate by antigen-coated competitive fluorescence immunoassay. Anal. Lett. 2007, 40, 127–137. [Google Scholar] [CrossRef]
- Tian, X.; Dong, Y.; Wang, Y.; Song, Z.; Meng, M.; Eremin, S.A.; Deng, C.; Yin, Y.; Xi, R. Quantification of Diethyl Phthalate by a Rapid and Homogenous Fluorescence Polarization Immunoassay. Anal. Lett. 2015, 48, 2843–2855. [Google Scholar] [CrossRef]
- Hermanson, G. Bioconjugate Techniques, 2nd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Wang, Y.; He, C.-H.; Zheng, H.; Zhang, H.-B. Characterization and Comparison of Fumonisin B1-Protein Conjugates by Six Methods. Int. J. Mol. Sci. 2012, 13, 84. [Google Scholar] [CrossRef]
- Chalmers, J.M.; Griffiths, P.R. Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2002; Volume 4. [Google Scholar]
- Ranaldi, S.; Belle, V.; Woudstra, M.; Rodriguez, J.; Guigliarelli, B.; Sturgis, J.; Carriere, F.; Fournel, A. Lid opening and unfolding in human pancreatic lipase at low pH revealed by site-directed spin labeling EPR and FTIR spectroscopy. Biochemistry 2009, 48, 630–638. [Google Scholar] [CrossRef]
- Ramesh, S.; Yin, T.S.; Liew, C.-W. Effect of dibutyl phthalate as plasticizer on high-molecular weight poly(vinyl chloride)–lithium tetraborate-based solid polymer electrolytes. Ionics 2011, 17, 705. [Google Scholar] [CrossRef]
- Rajamanikyam, M.; Vadlapudi, V.; Parvathaneni, S.P.; Koude, D.; Sripadi, P.; Misra, S.; Amanchy, R.; Upadhyayula, S.M. Isolation and characterization of phthalates from Brevibacterium mcbrellneri that cause cytotoxicity and cell cycle arrest. EXCLI J. 2017, 16, 375. [Google Scholar] [PubMed]
- Bhatnagar, B.S.; Bogner, R.H.; Pikal, M.J. Protein Stability During Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Technol. 2007, 12, 505–523. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Islam, R.; Ahmed, M. Applications of gold nanoparticles in ELISA, PCR, and immuno-PCR assays: A review. Anal. Chim. Acta 2021, 1143, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.; Xu, F.; Jian, M.; Yao, C.; Zhang, H.; Wang, Z. Lateral flow immunoassay with peptide-functionalized gold nanoparticles for rapid detection of protein tyrosine phosphatase 1B. Anal. Biochem. 2022, 648, 114671. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, W.; Xie, R.; Yang, S.; Chen, A. A strip of lateral flow gene assay using gold nanoparticles for point-of-care diagnosis of African swine fever virus in limited environment. Anal. Bioanal. Chem. 2021, 413, 4665–4672. [Google Scholar] [CrossRef]
- Lu, X.; Mei, T.; Guo, Q.; Zhou, W.; Li, X.; Chen, J.; Zhou, X.; Sun, N.; Fang, Z. Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Microchim. Acta 2018, 186, 2. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Post-assay growth of gold nanoparticles as a tool for highly sensitive lateral flow immunoassay. Application to the detection of potato virus X. Microchim. Acta 2018, 185, 506. [Google Scholar] [CrossRef]
- Urusov, A.E.; Petrakova, A.V.; Zherdev, A.V.; Zvereva, E.A. Indirect labeling of antibodies as a universal approach to increase sensitivity of lateral flow tests: A case study for mycotoxins detection. Open Biotechnol. J. 2019, 13, 113–121. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Xu, C.; Eremin, S.A.; Dzantiev, B.B. Development of lateral flow immunoassay for rapid control and quantification of the presence of the colorant Sudan I in spices and seafood. Food Control 2017, 73, 247–253. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J. Food Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.A.; Byzova, N.A.; Hendrickson, O.D.; Popravko, D.S.; Belichenko, K.A.; Dzantiev, B.B.; Zherdev, A.V. Immunochromatographic Detection of Myoglobin as a Specific Biomarker of Porcine Muscle Tissues in Meat Products. Appl. Sci. 2020, 10, 7437. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Chapter 20—Antibody Modification and Conjugation. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 867–920. [Google Scholar] [CrossRef]
- Charbaji, A.; Smith, W.; Anagnostopoulos, C.; Faghri, M. Zinculose: A new fibrous material with embedded zinc particles. Eng. Sci. Technol. Int. J. 2021, 24, 571–578. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.-J.; Ahn, Y.-J.; Kim, M.; Lee, G.-J. In situ detection of hydrogen sulfide in 3D-cultured, live prostate cancer cells using a paper-integrated analytical device. Chemosensors 2022, 10, 27. [Google Scholar] [CrossRef]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef]
- Berlina, A.N.; Bartosh, A.V.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. Development of Immunochromatographic assay for determination of tetracycline in human serum. Antibiotics 2018, 7, 99. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead (ii) by lateral flow test strip. Anal. Chim. Acta 2021, 1155, 338318. [Google Scholar] [CrossRef]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Influence of the compounds used in immunochromatographic test-system on its analytical characteristics: Multi-factor optimization of the system for aflatoxin B1. Iran. (Iran.) J. Energy Environ. 2015, 6, 63–71. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, H.; Qiu, M.; Wu, N.; Zeng, K.; Du, D. Dual-label time-resolved fluoroimmunoassay as an advantageous approach for investigation of diethyl phthalate & dibutyl phthalate in surface water. Sci. Total Environ. 2019, 695, 133793. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.A.; Okedeyi, O.O.; Yusuf, K.A. Flame ionization gas chromatographic determination of phthalate esters in water, surface sediments and fish species in the Ogun river catchments, Ketu, Lagos, Nigeria. Environ. Monit. Assess. 2011, 172, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zhu, N.; Zhu, F.; Yakubu, S.; Lv, J.; Liu, J.; Zhang, Z. Investigation and risk assessment of dibutyl phthalate in a typical region by a high-throughput dual-signal immunoassay. J. Hazard. Mater. 2022, 425, 127991. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yao, N.; Zeng, K.; Zhu, N.; Wang, Y.; Zhao, B.; Zhang, Z. A Novel Enzyme-Free Ratiometric Fluorescence Immunoassay Based on Silver Nanoparticles for the Detection of Dibutyl Phthalate from Environmental Waters. Biosensors 2022, 12, 125. [Google Scholar] [CrossRef]
- Kuang, H.; Liu, L.; Xu, L.; Ma, W.; Guo, L.; Wang, L.; Xu, C. Development of an Enzyme-Linked Immunosorbent Assay for Dibutyl Phthalate in Liquor. Sensors 2013, 13, 8331–8339. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, N.; Zou, Y.; Wu, X.; Qu, G.; Shi, J. A novel, enzyme-linked immunosorbent assay based on the catalysis of AuNCs@BSA-induced signal amplification for the detection of dibutyl phthalate. Talanta 2018, 179, 64–69. [Google Scholar] [CrossRef]


| No. | Running Buffer | The Appearance of Test Strips | |
|---|---|---|---|
| Control Line | Test Line | ||
| 1 | 10 mM Tris-HCl (pH 8.8) |  | |
| 2 | 10 mM borate buffer (pH 9.0) |  | |
| 3 | spring water/10 mM Tris-HCl (pH 8.8) 1:1 |  | |
| 4 | spring water |  | |
| 5 | 10 mM carbonate buffer (pH 9.0) |  | |
| No. | Conditions, Sample Pad | The Appearance of Test Strip | |
|---|---|---|---|
| Control Line | Test Line | ||
| 1 | 10 mM borate buffer (pH 9.0), (OD527 nm mAb−GNPs = 0.06), glass-fiber |  | |
| 2 | 10 mM borate buffer (pH 9.0) OD527 nm mAb−GNPs = 0.12, glass-fiber |  | |
| 3 | 10 mM borate buffer (pH 9.0), OD527 = 0.06, R7 sample pad |  | |
| 4 | 10 mM borate buffer (pH 9.0), OD527 Ab−GNPs = 0.12, R7 sample pad |  | |
| Parameter | Variations | Signal Intensity, AU | Chosen Conditions |
|---|---|---|---|
| Concentration of GNPs−mAb | OD527 = 0.06 | 10,025 | OD527 = 0.12 |
| OD527 = 0.12 | 25,163 | ||
| OD527 = 0.24 | 42,618 | ||
| OD527 = 0.5 | 90,247 | ||
| Concentration of STI−DBP | 0.25 mg/mL | 13,247 | 0.5 mg/mL |
| 0.5 mg/mL | 25,163 | ||
| 1.0 mg/mL | 46,319 | ||
| 2.0 mg/mL | 94,567 | ||
| Media for STI−DBP immobilization | 10 mM PBS, pH 7.4 | 4685 | Deionized water |
| Deionized water | 25,163 | ||
| Working membrane | CNPC 15 | 8630 | Millipore 180 |
| Millipore 180 | 23,645 | ||
| Sample pad | Glass-fiber | 42,618 | Glass-fiber |
| PTR7 | 17,255 | ||
| Diluting medium | 10 mM borate buffer, pH 9.0 | 41,845 | 10 mM borate buffer, pH 9.0 |
| 10 mM carbonate buffer, pH 10.0 | 17,020 | ||
| 10 mM Tris-HCl buffer, pH 8.8 | 20,784 | ||
| 10 mM PBS, pH 7.4 | 10,940 | ||
| Conditions for GAMI immobilization | 0.25 mg/mL 10 mM PBS, pH 7.4 | 25,537 | Accords to earlier data |
| Added DBP | Found DBP/Recoveries | |||||
|---|---|---|---|---|---|---|
| Water 1 | Water 2 | Water 3 | ||||
| ng/mL | ng/mL | % | ng/mL | % | ng/mL | % |
| 50 | 43.7 | 87.4 ± 4.9 | 43.35 | 86.7 ± 5.8 | 39.4 | 78.5 ± 7.2 |
| 100 | 75.0 | 75.0 ± 5.9 | 110.4 | 110.4 ± 7.4 | 80.7 | 80.7 ± 11.2 |
| 200 | 230 | 115.0 ± 6.5 | 186.4 | 93.2 ± 9.4 | 230 | 115.0 ± 13.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlina, A.N.; Ragozina, M.Y.; Komova, N.S.; Serebrennikova, K.V.; Zherdev, A.V.; Dzantiev, B.B. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors 2022, 12, 1002. https://doi.org/10.3390/bios12111002
Berlina AN, Ragozina MY, Komova NS, Serebrennikova KV, Zherdev AV, Dzantiev BB. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors. 2022; 12(11):1002. https://doi.org/10.3390/bios12111002
Chicago/Turabian StyleBerlina, Anna N., Maria Y. Ragozina, Nadezhda S. Komova, Kseniya V. Serebrennikova, Anatoly V. Zherdev, and Boris B. Dzantiev. 2022. "Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters" Biosensors 12, no. 11: 1002. https://doi.org/10.3390/bios12111002
APA StyleBerlina, A. N., Ragozina, M. Y., Komova, N. S., Serebrennikova, K. V., Zherdev, A. V., & Dzantiev, B. B. (2022). Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors, 12(11), 1002. https://doi.org/10.3390/bios12111002








