2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements
Abstract
:1. Introduction
2. Black Phosphorus Platforms in Breast Cancer
3. General Methods of Synthesis
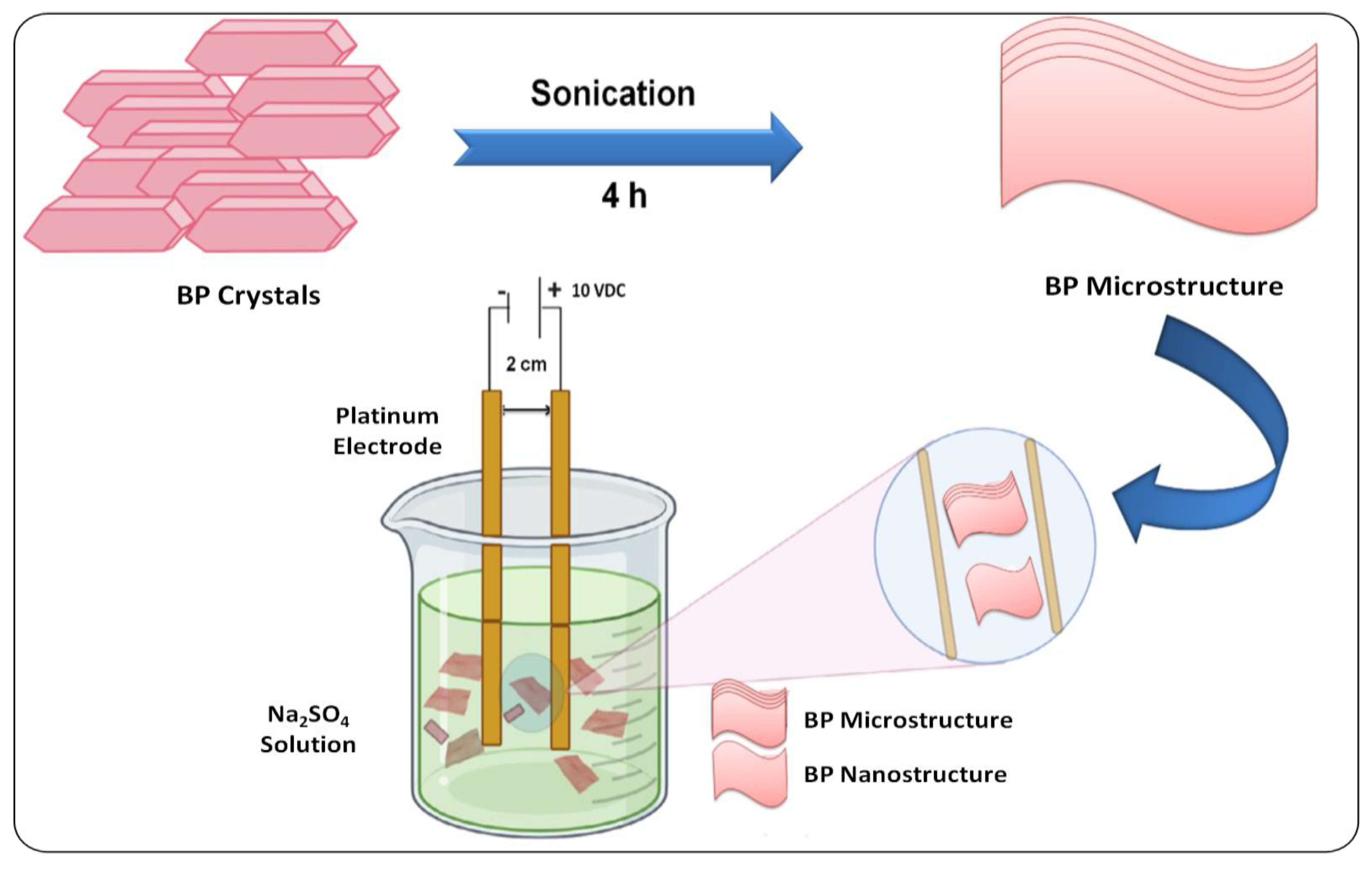
3.1. Top–Down Methods
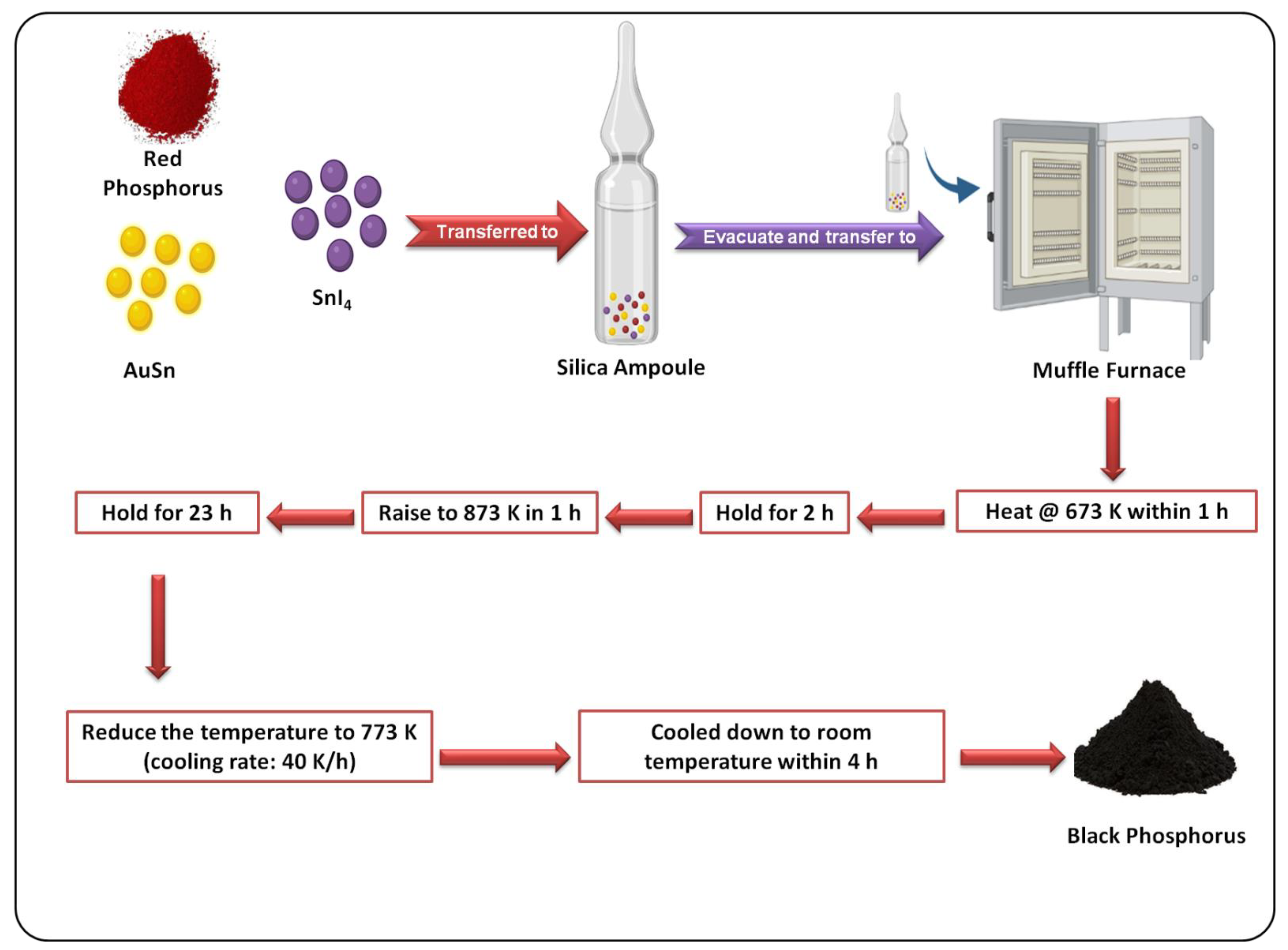
3.2. Bottom–Up Methods
4. Characteristics of BP Nanomaterials for Breast Cancer Theranostics
5. Surface Modification of BPNSs
5.1. Stimuli-Responsive Modifications
5.1.1. Functionalization for pH-Responsive Drug Release
5.1.2. Functionalization for Light-Responsive Drug Delivery
5.2. Cell-Organelle-Targeted Modifications
6. Modifications for Targeting
6.1. Active Targeting
6.1.1. Folic Acid (FA)
6.1.2. Hyaluronic Acid (HA)
6.1.3. Targeting with Cell Membrane Coating
Erythrocyte Membrane
Platelet Membrane
Neutrophil Membrane
6.1.4. Antibodies
6.1.5. Peptides
6.1.6. Proteins
6.2. Passive Targeting
6.2.1. pH-Sensitive Targeting
6.2.2. Hypoxia-Mediated Targeting
7. Imaging and Diagnosis
7.1. Fluorescence Imaging
7.2. Thermal Imaging
7.3. Photoacoustic (PA) Imaging
7.4. Imaging with Radionuclides
8. Strategies for Treating Breast Cancer Using BP Nanomaterials
8.1. Delivering Chemotherapeutic Agents
8.2. Gene-Therapy
8.3. Codelivery of Drugs
8.4. Photothermal Therapy (PTT)
8.5. Photodynamic Therapy (PDT)
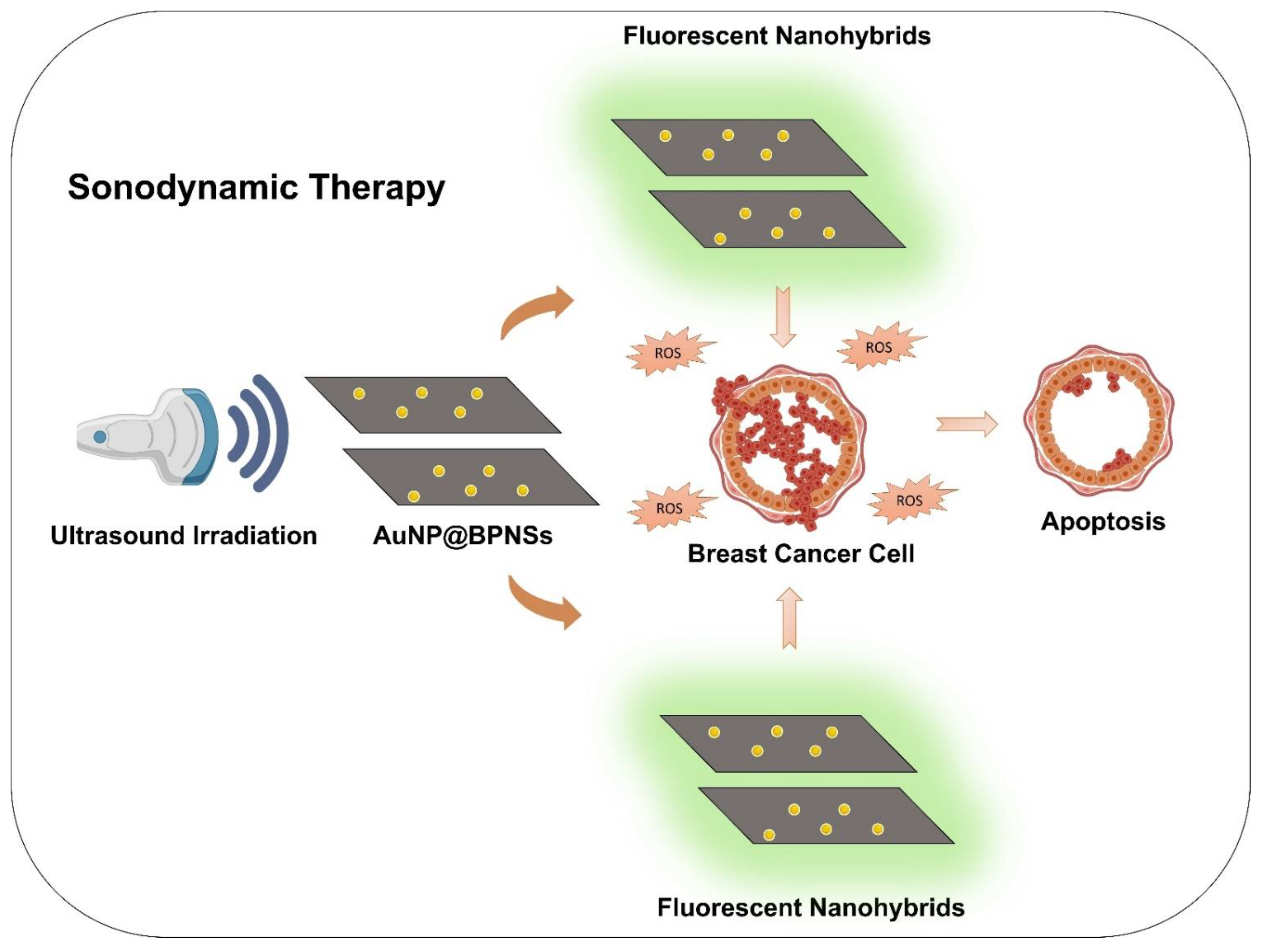
8.6. Sonodynamic Therapy
8.7. Combined Phototherapeutic Strategy
8.8. Reversing Drug Resistance
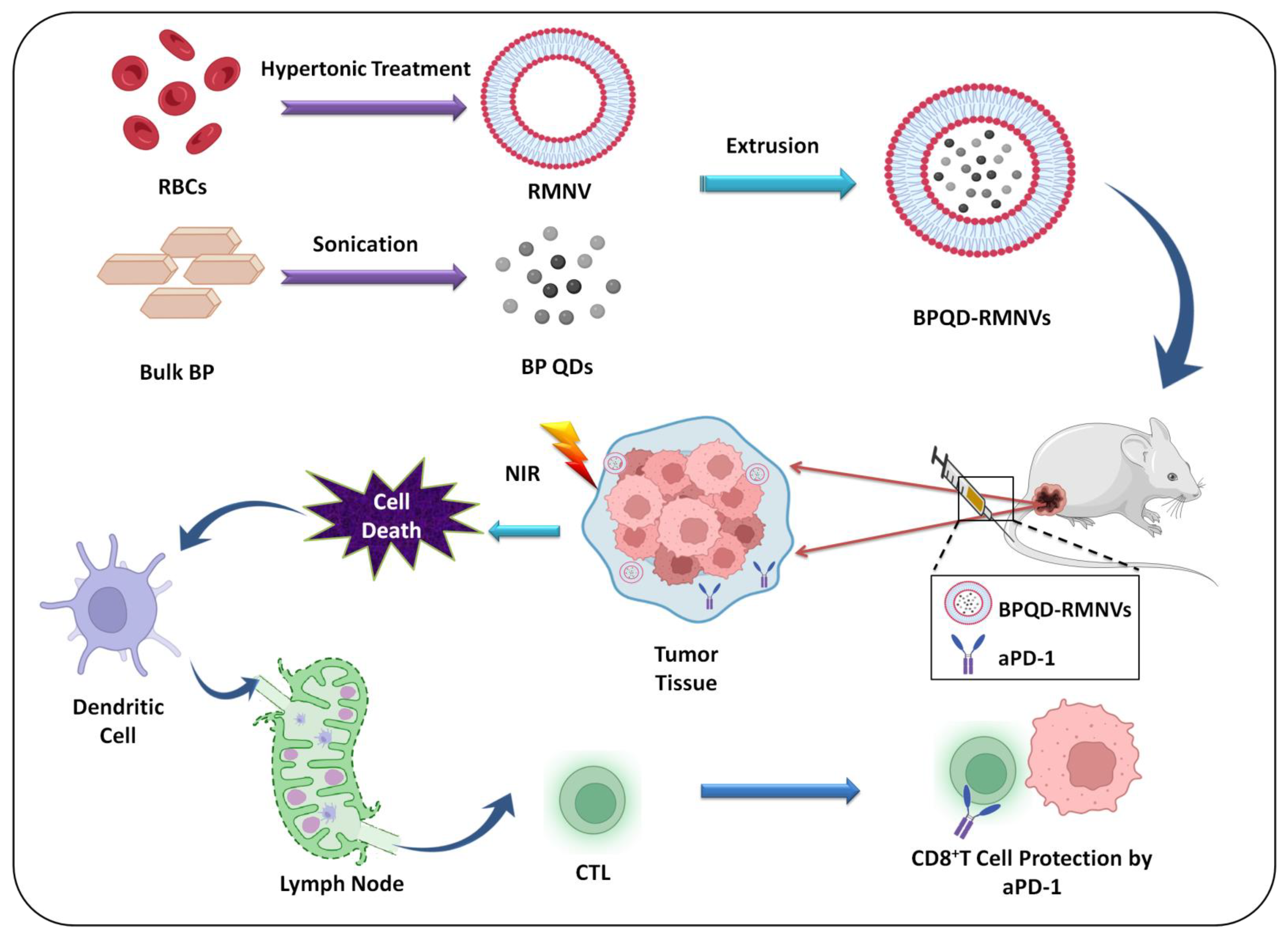
8.9. Immunotherapy
9. Degradability and Toxicity of BP Nanomaterials
10. Future Perspective
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hai, L.; Zhang, A.; Wu, X.; Cheng, H.; He, D.; Wang, T.; He, X.; Wang, K. Liposome-Stabilized Black Phosphorus for Photothermal Drug Delivery and Oxygen Self-Enriched Photodynamic Therapy. ACS Appl. Nano Mater. 2019, 3, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhang, Y.; Pan, J.; Lu, N.; Wang, S.; Lu, G. Gold Nanoparticles Increase PLK1-Specific Small Interfering RNA Transfection and Induce Apoptosis of Drug Resistance Breast Cancer Cells. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Dhas, N.; Longhi, M.; García, M.C. Overcoming Biological Barriers With Block Copolymers-Based Self-Assembled Nanocarriers. Recent Advances in Delivery of Anticancer Therapeutics. Front. Pharmacol. 2020, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.R.; Parmar, C.; Liu, Y.; Coroller, T.P.; Cruz, G.; Stringfield, O.; Ye, Z.; Makrigiorgos, M.; Fennessy, F.; Mak, R.H.; et al. Somatic Mutations Drive Distinct Imaging Phenotypes in Lung Cancer. Cancer Res. 2017, 77, 3922–3930. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, D.B.; Patel, V.; Sawant, K.K. Design and characterization of dual responsive mesoporous silica nanoparticles for breast cancer targeted therapy. Eur. J. Pharm. Sci. 2020, 152, 105428. [Google Scholar] [CrossRef]
- Bhavsar, D.; Gajjar, J.; Sawant, K. Formulation and development of smart pH responsive mesoporous silica nanoparticles for breast cancer targeted delivery of anastrozole: In vitro and in vivo characterizations. Microporous Mesoporous Mater. 2018, 279, 107–116. [Google Scholar] [CrossRef]
- DeSantis, C.; Siegel, R.; Bandi, P.; Jemal, A. Breast cancer statistics, 2011. CA A Cancer J. Clin. 2011, 61, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Bleyer, A.; Welch, H.G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, G.S.; Doval, D.C.; Desai, C.J.; Chaturvedi, H.; Sharma, S.; Somashekhar, S. Overview of Breast Cancer and Implications of Overtreatment of Early-Stage Breast Cancer: An Indian Perspective. JCO Glob. Oncol. 2020, 6, 789–798. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Hegde, A.R.; Padya, B.S.; Soman, S.; Mutalik, S. A simple, precise, and sensitive HPLC method for quantification of letrozole in rat plasma: Development, validation, and preclinical pharmacokinetics. J. Anal. Sci. Technol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M. Nanotechnology in Cancer Drug Delivery and Selective Targeting. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, R. Nanomedicine-The Future of Cancer Treatment: A Review. J. Cancer Prev. Curr. Res. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Baji, S.; Hegde, A.R.; Kulkarni, M.; Raut, S.Y.; Manikkath, J.; Reddy, M.S.; Mutalik, S. Skin permeation of gemcitabine hydrochloride by passive diffusion, iontophoresis and sonophoresis: In vitro and in vivo evaluations. J. Drug Deliv. Sci. Technol. 2018, 47, 49–54. [Google Scholar] [CrossRef]
- Pandey, A.; Kulkarni, S.; Vincent, A.P.; Nannuri, S.H.; George, S.D.; Mutalik, S. Hyaluronic acid-drug conjugate modified core-shell MOFs as pH responsive nanoplatform for multimodal therapy of glioblastoma. Int. J. Pharm. 2020, 588, 119735. [Google Scholar] [CrossRef]
- Kulkarni, S.; Pandey, A.; Mutalik, S. Liquid metal based theranostic nanoplatforms: Application in cancer therapy, imaging and biosensing. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102175. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2018, 48, 2053–2108. [Google Scholar] [CrossRef]
- Gao, N.; Xing, C.; Wang, H.; Feng, L.; Zeng, X.; Mei, L.; Peng, Z. PH-Responsive Dual Drug-Loaded Nanocarriers Based on Poly (2-Ethyl-2-Oxazoline) Modified Black Phosphorus Nanosheets for Cancer Chemo/Photothermal Therapy. Front. Pharmacol. 2019, 10, 270–284. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Tang, W.; Hu, L.; Chen, X.; Su, Y.; Zou, C.; Wang, J.; Lu, W.W.; Zhen, W.; et al. Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small 2019, 15, e1901560. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Pan, T.; Zhou, W.; Cui, H.; Wu, L.; Li, Z.; Chu, P.K.; Yu, X.-F. Bioactive phospho-therapy with black phosphorus for in vivo tumor suppression. Theranostics 2020, 10, 4720–4736. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Xiao, X.; Liang, D.; Liang, X.; Mi, L.; Wang, J.; Liu, J. Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors. Acta Biomater. 2020, 107, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-functionalized black phosphorus quantum dots for cancer theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Li, Y.; Feng, P.; Wang, C.; Miao, W.; Huang, H. Black phosphorus nanophototherapeutics with enhanced stability and safety for breast cancer treatment. Chem. Eng. J. 2020, 400, 125851. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef]
- Liu, X.; Gaihre, B.; George, M.N.; Li, Y.; Tilton, M.; Yaszemski, M.J.; Lu, L. 2D phosphorene nanosheets, quantum dots, nanoribbons: Synthesis and biomedical applications. Biomater. Sci. 2021, 9, 2768–2803. [Google Scholar] [CrossRef]
- Dinh, K.N.; Zhang, Y.; Sun, W. The synthesis of black phosphorus: From zero- to three-dimensional nanostructures. J. Phys. Energy 2021, 3, 032007. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Dhanabalan, S.C.; Ponraj, J.S.; Zhang, H.; Bao, Q. Present perspectives of broadband photodetectors based on nanobelts, nanoribbons, nanosheets and the emerging 2D materials. Nanoscale 2016, 8, 6410–6434. [Google Scholar] [CrossRef]
- Qu, G.; Xia, T.; Zhou, W.; Zhang, X.; Zhang, H.; Hu, L.; Shi, J.; Yu, X.-F.; Jiang, G. Property–Activity Relationship of Black Phosphorus at the Nano–Bio Interface: From Molecules to Organisms. Chem. Rev. 2020, 120, 2288–2346. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, T.; Hu, Y.; Qiu, Y.; Liu, Y.; Wang, H.; Li, Y.; Chen, X.; Song, J.; Yang, H. A Highly Effective π–π Stacking Strategy to Modify Black Phosphorus with Aromatic Molecules for Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 9860–9871. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Nikam, A.N.; Padya, B.S.; Kulkarni, S.; Fernandes, G.; Shreya, A.B.; García, M.C.; Caro, C.; Páez-Muñoz, J.M.; Dhas, N.; et al. Surface architectured black phosphorous nanoconstructs based smart and versatile platform for cancer theranostics. Coord. Chem. Rev. 2021, 435, 213826. [Google Scholar] [CrossRef]
- Tumbapo, M.; Henry, M.B.; Jha, S.K.; Tayo, B.O. Physisorption of DNA Bases on Finite-Size Nanoribbons from Graphene, Phosphorene, and Silicene: Insights from Density Functional Theory. arXiv 2021, arXiv:211200048. [Google Scholar]
- Kumawat, R.L.; Pathak, B. Individual Identification of DNA Nucleobases on Atomically Thin Black Phosphorene Nanoribbons: Van der Waals Corrected Density Functional Theory Calculations. J. Phys. Chem. C 2019, 123, 22377–22383. [Google Scholar] [CrossRef]
- Watts, M.C.; Picco, L.; Russell-Pavier, F.S.; Cullen, P.L.; Miller, T.S.; Bartuś, S.P.; Payton, O.D.; Skipper, N.T.; Tileli, V.; Howard, C.A. Production of phosphorene nanoribbons. Nature 2019, 568, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Alvarez, J.V.; et al. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.-H.; Liu, X.; Chen, K.-S.; Hersam, M.C. Solvent Exfoliation of Electronic-Grade, Two-Dimensional Black Phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-Y.; Gao, L.-F.; Hu, C.-X.; Zhu, Z.-Y.; Zhao, M.; Wang, Q.; Zhang, H.-L. Preparation of large size, few-layer black phosphorus nanosheets via phytic acid-assisted liquid exfoliation. Chem. Commun. 2016, 52, 8107–8110. [Google Scholar] [CrossRef] [PubMed]
- BHORIA, R.S.; Tripathi, C.C. A Comparative Study of Organic Solvents, Ionic Liquids, Surfactants and Acids for Liquid Phase Exfoliation of Graphene. IJPAP 2019, 57, 322–333. [Google Scholar]
- Ma, J.; Lu, S.; Guo, Z.; Xu, X.; Zhang, H.; Tang, D.; Fan, D. Few-layer black phosphorus based saturable absorber mirror for pulsed solid-state lasers. Opt. Express 2015, 23, 22643–22648. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, Z.; Guo, Z.; Huang, H.; Xiao, Q.; Zhang, H.; Yu, X.-F. Solvothermal Synthesis and Ultrafast Photonics of Black Phosphorus Quantum Dots. Adv. Opt. Mater. 2016, 4, 1223–1229. [Google Scholar] [CrossRef]
- Erande, M.B.; Pawar, M.S.; Late, D.J. Humidity Sensing and Photodetection Behavior of Electrochemically Exfoliated Atomically Thin-Layered Black Phosphorus Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.; Das, C.M.; Ma, M.; Qiao, N.; Fan, T.; Zhang, H.; Xu, G.; Yong, K.-T. Bioengineering applications of black phosphorus and their toxicity assessment. Environ. Sci. Nano 2021, 8, 3452–3477. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Latiff, N.M.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Black Phosphorus Nanoparticle Labels for Immunoassays via Hydrogen Evolution Reaction Mediation. Anal. Chem. 2016, 88, 10074–10079. [Google Scholar] [CrossRef]
- Zhao, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Large-Scale, Highly Efficient, and Green Liquid-Exfoliation of Black Phosphorus in Ionic Liquids. ACS Appl. Mater. Interfaces 2015, 7, 27608–27612. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Ponraj, J.S.; Guo, Z.; Li, S.; Bao, Q.; Zhang, H. Emerging Trends in Phosphorene Fabrication towards Next Generation Devices. Adv. Sci. 2017, 4, 1600305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Zhang, X.-Q.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J. Synthesis of Large-area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.B.; Hagaman, D.; Ji, H.-F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar] [CrossRef] [PubMed]
- Tiouitchi, G.; Ali, M.A.; Benyoussef, A.; Hamedoun, M.; Lachgar, A.; Benaissa, M.; Kara, A.; Ennaoui, A.; Mahmoud, A.; Boschini, F.; et al. An easy route to synthesize high-quality black phosphorus from amorphous red phosphorus. Mater. Lett. 2018, 236, 56–59. [Google Scholar] [CrossRef]
- Nilges, T.; Kersting, M.; Pfeifer, T. A fast low-pressure transport route to large black phosphorus single crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]
- Bao, T.N.; Tegus, O.; Jun, N. Preparation of Black Phosphorus by the Mechanical Ball Milling Method and Its Characterization. In Proceedings of the Solid State Phenomena. Trans Tech. Publ. 2018, 271, 18–22. [Google Scholar]
- He, N.; Zhang, Z.; Qu, J.; Yuan, X.; Guan, J. Facile One-Step Synthesis of Black Phosphorus via Microwave Irradiation with Excellent Photocatalytic Activity. Part. Part. Syst. Charact. 2018, 35, 1800306. [Google Scholar] [CrossRef]
- Boddula, R.; Asiri, A.M. Black Phosphorus: Synthesis, Properties and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2019; ISBN 3-030-29555-9. [Google Scholar]
- Zhang, W.; Yu, L.; Jiang, Y.; Guo, C. Phycocyanin-functionalized black phosphorus quantum dots enhance PDT/PTT therapy by inducing ROS and irreparable DNA damage. Biomater. Sci. 2021, 9, 5302–5318. [Google Scholar] [CrossRef]
- Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.K.; Yu, X. Black Phosphorus: Bioactive Nanomaterials with Inherent and Selective Chemotherapeutic Effects. Angew. Chem. 2019, 131, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, A.C.M.; Kubota, L.T. Recent Trends in Field-Effect Transistors-Based Immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Das, S.; Zhang, W.; Demarteau, M.; Hoffmann, A.; Dubey, M.; Roelofs, A. Tunable Transport Gap in Phosphorene. Nano Lett. 2014, 14, 5733–5739. [Google Scholar] [CrossRef]
- Liu, G.; Tsai, H.-I.; Zeng, X.; Qi, J.; Luo, M.; Wang, X.; Mei, L.; Deng, W. Black phosphorus nanosheets-based stable drug delivery system via drug-self-stabilization for combined photothermal and chemo cancer therapy. Chem. Eng. J. 2019, 375, 121917. [Google Scholar] [CrossRef]
- Qian, X.; Gu, Z.; Chen, Y. Two-dimensional black phosphorus nanosheets for theranostic nanomedicine. Mater. Horiz. 2017, 4, 800–816. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.-P.; Zhang, H.; Kim, J.S. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef]
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, D.; Yang, P.; Xu, J.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. Integration of Upconversion Nanoparticles and Ultrathin Black Phosphorus for Efficient Photodynamic Theranostics under 808 nm Near-Infrared Light Irradiation. Chem. Mater. 2016, 28, 4724–4734. [Google Scholar] [CrossRef]
- Dibaba, S.T.; Wei, R.; Xi, W.; Zhao, L.; Shi, L.; Ren, W.; Mayr, T.; Sun, L. Theranostic nanocomposite from upconversion luminescent nanoparticles and black phosphorus nanosheets. RSC Adv. 2018, 8, 35706–35718. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Zhu, J.; Xue, L.; Ou, C.; Wang, W.; Lu, M.; Song, X.; Dong, X. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem. Sci. 2019, 10, 3779–3785. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Nikam, A.N.; Fernandes, G.; Kulkarni, S.; Padya, B.S.; Prassl, R.; Das, S.; Joseph, A.; Deshmukh, P.K.; Patil, P.O.; et al. Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials 2021, 11, 13. [Google Scholar] [CrossRef]
- Bies, C.; Lehr, C.-M.; Woodley, J.F. Lectin-mediated drug targeting: History and applications. Adv. Drug Deliv. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Perfusion and Microenvironment of Human Tumors. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Luo, M.; Cheng, W.; Zeng, X.; Mei, L.; Liu, G.; Deng, W. Folic Acid-Functionalized Black Phosphorus Quantum Dots for Targeted Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2019, 11, 242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Peng, F.; Qin, L.; Yang, D.; Li, R.; Jiang, S.; He, H.; Zhang, P. pH/near infrared dual-triggered drug delivery system based black phosphorus nanosheets for targeted cancer chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2019, 180, 353–361. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Lei, Z.; Liu, L.; Zhang, J.; You, H.; Zhang, H.; Wang, Z. Facile Preparation of Doxorubicin-Loaded [Email Protected] Nanoplatforms for Simultaneous in Vivo Multimodality Imaging and Chemophotothermal Synergistic Therapy. Adv. Healthc. Mater. 2015, 4, 559–568. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.; Chu, X.; Zhang, Q.; Su, Y.; Sun, B.; Lu, T.; Zhou, N.; Zhang, J.; Wang, J.; et al. Black phosphorus nanosheets-based nanocarriers for enhancing chemotherapy drug sensitiveness via depleting mutant p53 and resistant cancer multimodal therapy. Chem. Eng. J. 2019, 370, 387–399. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Choi, H.W.; Lim, J.H.; Kim, J.W.; Chung, B.G. Near-Infrared Light-Triggered Thermo-responsive Poly(N-Isopropylacrylamide)-Pyrrole Nanocomposites for Chemo-photothermal Cancer Therapy. Nanoscale Res. Lett. 2020, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shamul, J.G.; Kwizera, E.A.; He, X. Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials 2022, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm. Sin. B 2018, 8, 862–880. [Google Scholar] [CrossRef]
- Qi, J.; Xiong, Y.; Cheng, K.; Huang, Q.; Cao, J.; He, F.; Mei, L.; Liu, G.; Deng, W. Heterobifunctional PEG-grafted black phosphorus quantum dots: “Three-in-One” nano-platforms for mitochondria-targeted photothermal cancer therapy. Asian J. Pharm. Sci. 2020, 16, 222–235. [Google Scholar] [CrossRef]
- Zeng, F.; Qin, H.; Liu, L.; Chang, H.; Chen, Q.; Wu, L.; Zhang, L.; Wu, Z.; Xing, D. Photoacoustic-immune therapy with a multi-purpose black phosphorus-based nanoparticle. Nano Res. 2020, 13, 3403–3415. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Mehta, T.A.; Shah, N.; Parekh, K.; Dhas, N.; Patel, J.K. Surface-Modified PLGA Nanoparticles for Targeted Drug Delivery to Neurons. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 33–71. [Google Scholar]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef]
- Gadeval, A.; Maheshwari, R.; Raval, N.; Kalyane, D.; Kalia, K.; Tekade, R.K. Green graphene nanoplates for combined photo-chemo-thermal therapy of triple-negative breast cancer. Nanomedicine 2020, 15, 581–601. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications. Cancers 2010, 2, 1911–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, C.-Y.; Mathias, C.J.; Green, M.A. Folate-Receptor-Targeted Radionuclide Imaging Agents. Adv. Drug Deliv. Rev. 2004, 56, 1143–1160. [Google Scholar] [CrossRef] [PubMed]
- Sega, E.I.; Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008, 27, 655–664. [Google Scholar] [CrossRef]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.; Serie, D.J.; Sun, Z.; Moreno-Aspitia, A.; et al. Folate Receptor-α (FOLR1) Expression and Function in Triple Negative Tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Y.; Sun, C.; Yun, B.; Sun, Q.; Zhao, C.; Li, Z. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Sci. Bull. 2018, 63, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Kang, M.S.; Jeong, W.Y.; Han, D.-W.; Kim, K.S. Hyaluronic Acid-Based Theranostic Nanomedicines for Targeted Cancer Therapy. Cancers 2020, 12, 940. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Savani, R.; Turley, E. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, F.; Shan, L.; Li, R.; Jiang, S.; Zhang, P. Black phosphorus nanosheets-based platform for targeted chemo-photothermal synergistic cancer therapy. Colloids Surf. B Biointerfaces 2020, 198, 111467. [Google Scholar] [CrossRef]
- Poudel, B.K.; Hwang, J.; Ku, S.K.; Kim, J.O.; Byeon, J.H. A batch-by-batch free route for the continuous production of black phosphorus nanosheets for targeted combination cancer therapy. NPG Asia Mater. 2018, 10, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Dhas, N.; García, M.C.; Kudarha, R.; Pandey, A.; Nikam, A.N.; Gopalan, D.; Fernandes, G.; Soman, S.; Kulkarni, S.; Seetharam, R.N.; et al. Advancements in cell membrane camouflaged nanoparticles: A bioinspired platform for cancer therapy. J. Control. Release 2022, 346, 71–97. [Google Scholar] [CrossRef]
- Jha, A.; Nikam, A.N.; Kulkarni, S.; Mutalik, S.P.; Pandey, A.; Hegde, M.; Rao, B.S.S.; Mutalik, S. Biomimetic nanoarchitecturing: A disguised attack on cancer cells. J. Control. Release 2021, 329, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, H.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Li, H.; Hou, Y.; Gao, M.; Lan, X. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater. Sci. 2020, 8, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.; Alqahtani, S.; Kazi, M.; Shakeel, F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics 2021, 13, 368. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.-M. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnol. 2019, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Duan, Y.; Zhang, Q.; Komarla, A.; Gong, H.; Gao, W.; Zhang, L. Drug Targeting via Platelet Membrane–Coated Nanoparticles. Small Struct. 2020, 1, 2000018. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, Q.; Wu, B.; Zhao, Q.; Li, J.; Huang, X.; Chen, W.; Gui, R. Platelet-Membrane-Camouflaged Black Phosphorus Quantum Dots Enhance Anticancer Effect Mediated by Apoptosis and Autophagy. ACS Appl. Mater. Interfaces 2019, 11, 28254–28266. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zang, J.; Zhao, Z.; Zhang, Q.; Chen, S. The Advances of Neutrophil-Derived Effective Drug Delivery Systems: A Key Review of Managing Tumors and Inflammation. Int. J. Nanomed. 2021, ume 16, 7663–7681. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Su, Y.; Li, M.; Zhou, J.; Zhang, W.; Wang, W. A neutrophil membrane-functionalized black phosphorus riding inflammatory signal for positive feedback and multimode cancer therapy. Mater. Horiz. 2020, 7, 574–585. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Ji, W.; Zhou, S.; Li, L.; Qiu, L.; Qian, Z.; Wang, X.; Zhang, H. Biomimetic Black Phosphorus Quantum Dots-Based Photothermal Therapy Combined with Anti-PD-L1 Treatment Inhibits Recurrence and Metastasis in Triple-Negative Breast Cancer. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Soudy, R.; Byeon, N.; Raghuwanshi, Y.; Ahmed, S.; Lavasanifar, A.; Kaur, K. Engineered Peptides for Applications in Cancer-Targeted Drug Delivery and Tumor Detection. Mini-Rev. Med. Chem. 2017, 17, 1696–1712. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Lin, Y.; Chen, Y.; Chen, G.; Zhang, J.; Li, L.; Huang, A.; Zhang, L.; Ma, Y.; Xie, Z.-Y.; et al. Black Phosphorus Nanosheets Induced Oxidative Stress In Vitro and Targeted Photo-thermal Antitumor Therapy. ACS Appl. Bio Mater. 2021, 4, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Xie, L.; Zhang, Y.; Hanyu, M.; Yang, Z.; Nagatsu, K.; Suzuki, H.; Ouyang, J.; Ji, X.; Wei, J.; et al. Marriage of black phosphorus and Cu2+ as effective photothermal agents for PET-guided combination cancer therapy. Nat. Commun. 2020, 11, 2778. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Sachan, S.; Munjal, A.K.; Tiwari, R.; Dhama, K. Tumor Homing Peptides: Promising Futuristic Hope for Cancer Therapy. Top. Anti-Cancer Res. Bentham Sci. Publ. Sharjah United Arab. Emir. 2016, 5, 43–86. [Google Scholar] [CrossRef]
- Biedulska, M.; Jakóbczyk, P.; Sosnowska, M.; Dec, B.; Muchlińska, A.; Zaczek, A.J.; Nidzworski, D.; Bogdanowicz, R. Cytocompatibility of stabilized black phosphorus nanosheets tailored by directly conjugated polymeric micelles for human breast cancer therapy. Sci. Rep. 2021, 11, 9304. [Google Scholar] [CrossRef]
- Yue, H.; Huang, R.; Shan, Y.; Xing, D. Delivery of Cas13a/crRNA by self-degradable black phosphorus nanosheets to specifically inhibit Mcl-1 for breast cancer therapy. J. Mater. Chem. B 2020, 8, 11096–11106. [Google Scholar] [CrossRef]
- Zhou, W.; Cui, H.; Ying, L.; Yu, X.-F. Enhanced Cytosolic Delivery and Release of CRISPR/Cas9 by Black Phosphorus Nanosheets for Genome Editing. Angew. Chem. 2018, 130, 10425–10429. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, Y.; Shi, J.; Wang, L. NIR-Driven Water Splitting H2 Production Nanoplatform for H2-Mediated Cascade-Amplifying Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 23677–23688. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, J.; Li, Z.; Ren, Q.; Yu, X.-F.; Liu, S. Black Phosphorus-Based Multimodal Nanoagent: Showing Targeted Combinatory Therapeutics against Cancer Metastasis. Nano Lett. 2019, 19, 5587–5594. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. PH-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arambula, J.F.; Koo, S.; Kumar, R.; Singh, H.; Sessler, J.L.; Kim, J.S. Hypoxia-targeted drug delivery. Chem. Soc. Rev. 2019, 48, 771–813. [Google Scholar] [CrossRef] [PubMed]
- Bugárová, N.; Špitálsky, Z.; Mičušík, M.; Bodík, M.; Šiffalovič, P.; Koneracká, M.; Závišová, V.; Kubovčíková, M.; Kajanová, I.; Zaťovičová, M. A Multifunctional Graphene Oxide Platform for Targeting Cancer. Cancers 2019, 11, 753. [Google Scholar] [CrossRef]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano-Micro Lett. 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Liu, Z.; Huang, C.; Zeng, N.; Jiang, W.; Li, Q. Novel Engineered Bacterium/Black Phosphorus Quantum Dot Hybrid System for Hypoxic Tumor Targeting and Efficient Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 10564–10573. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Zhong, Z.; Huang, H.; Shao, J.; Xie, X.; Zhang, Y.; Wang, W.; Dong, X. Platinum nanoenzyme functionalized black phosphorus nanosheets for photothermal and enhanced-photodynamic therapy. Chem. Eng. J. 2021, 409, 127381. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Dhas, N.; Kudarha, R.; Pandey, A.; Nikam, A.N.; Sharma, S.; Singh, A.; Garkal, A.; Hariharan, K.; Singh, A.; Bangar, P.; et al. Stimuli responsive and receptor targeted iron oxide based nanoplatforms for multimodal therapy and imaging of cancer: Conjugation chemistry and alternative therapeutic strategies. J. Control. Release 2021, 333, 188–245. [Google Scholar] [CrossRef]
- Radiopharmaceuticals for Sentinel Lymph Node Detection: Status and Trends|IAEA. Available online: https://www.iaea.org/publications/10713/radiopharmaceuticals-for-sentinel-lymph-node-detection-status-and-trends (accessed on 4 April 2022).
- Zhang, S.; Yang, J.; Xu, R.; Wang, F.; Li, W.; Ghufran, M.; Zhang, Y.-W.; Yu, Z.; Zhang, G.; Qin, Q.; et al. Extraordinary Photoluminescence and Strong Temperature/Angle-Dependent Raman Responses in Few-Layer Phosphorene. ACS Nano 2014, 8, 9590–9596. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tong, L.; Li, Z.; Yang, N.; Fu, H.; Wu, L.; Cui, H.; Zhou, W.; Wang, J.; Wang, H.; et al. Stable and Multifunctional Dye-Modified Black Phosphorus Nanosheets for Near-Infrared Imaging-Guided Photothermal Therapy. Chem. Mater. 2017, 29, 7131–7139. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, F.; Liu, H.; Zhang, H.; Han, Y.; Liu, Z.; Wang, W.; Sun, Q.; Zhao, C.; Li, Z. Cholesterol-Modified Black Phosphorus Nanospheres for the First NIR-II Fluorescence Bioimaging. ACS Appl. Mater. Interfaces 2019, 11, 21399–21407. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Shen, W.; Li, P.; Fang, F.; Qin, H.; Li, X.K.; Pan, D.; Shen, L. Carbon Dot-Passivated Black Phosphorus Nanosheet Hybrids for Synergistic Cancer Therapy in the NIR-II Window. ACS Appl. Mater. Interfaces 2019, 11, 44949–44960. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Jia, Y.; Zhang, W.; Zhao, H.; Fan, W.; Zhang, W.; Zhong, H.; Ni, Y.; Guo, Z. A two-dimensional fingerprint nanoprobe based on black phosphorus for bio-SERS analysis and chemo-photothermal therapy. Nanoscale 2018, 10, 18795–18804. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Luo, M.; Liu, G.; Wang, X.; Tao, W.; Lin, Y.; Ji, X.; Nie, L.; Mei, L. Polydopamine-Modified Black Phosphorous Nanocapsule with Enhanced Stability and Photothermal Performance for Tumor Multimodal Treatments. Adv. Sci. 2018, 5, 1800510. [Google Scholar] [CrossRef] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Lee, C.; Jung, H.S.; Jeon, M.; Kim, K.S.; Yun, S.H.; Kim, C.; Hahn, S.K. Biodegradable Photonic Melanoidin for Theranostic Applications. ACS Nano 2015, 10, 822–831. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Li, Z.; Cui, H.; Zhou, Y.; Li, W.; Tao, W.; Zhang, H.; Wang, H.; Chu, P.K.; et al. TiL4-Coordinated Black Phosphorus Quantum Dots as an Efficient Contrast Agent for In Vivo Photoacoustic Imaging of Cancer. Small 2017, 13, 1602896. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Y.; Deng, L.; Zhang, H.; Sun, Q.; Zhao, C.; Li, Z. Blood Circulation, Biodistribution, and Pharmacokinetics of Dextran-Modified Black Phosphorus Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 673–682. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent Functionalization of Graphene Oxide with Biocompatible Poly(ethylene glycol) for Delivery of Paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Guo, W.; Jin, Y.; Miao, W.; Wang, C.; Zhang, H.; Hu, Y.; Huang, H. Decomposable black phosphorus nano-assembly for controlled delivery of cisplatin and inhibition of breast cancer metastasis. J. Control. Release 2021, 335, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.R.; de Melo Alves Paiva, R. Gene Therapy: Advances, Challenges and Perspectives. Einstein Sao Paulo 2017, 15, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther.-Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Niidome, T.; Huang, L. Gene Therapy Progress and Prospects: Nonviral vectors. Gene Ther. 2002, 9, 1647–1652. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-Z.; Li, J.-Q.; Wang, Z.-Z.; Dong, D.-W.; Qi, X.-R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef]
- Bates, K.; Kostarelos, K. Carbon nanotubes as vectors for gene therapy: Past achievements, present challenges and future goals. Adv. Drug Deliv. Rev. 2013, 65, 2023–2033. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.-T.; Lu, F.; Liang, Y.; Li, Z. SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhong, L.; Liu, Y.; Xu, X.; Xing, C.; Wang, M.; Bai, S.-M.; Lu, C.-H.; Yang, H.-H. A black phosphorus nanosheet-based siRNA delivery system for synergistic photothermal and gene therapy. Chem. Commun. 2018, 54, 3142–3145. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Huang, P.; Rong, P.; Lin, J.; Li, W.; Yan, X.; Zhang, M.G.; Nie, L.; Niu, G.; Lu, J.; Wang, W.; et al. Triphase Interface Synthesis of Plasmonic Gold Bellflowers as Near-Infrared Light Mediated Acoustic and Thermal Theranostics. J. Am. Chem. Soc. 2014, 136, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Soman, S.; Mutalik, S.; Barnwal, R.P.; Singh, G.; et al. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2020, 32, 1703284. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Lin, C.-H.; Chen, Y.-C.; Chen, H.-H.; Lin, Y.-J.; Lin, K.-Y.A. Dopamine-Modified Zero-Valent Iron Nanoparticles for Dual-Modality Photothermal and Photodynamic Breast Cancer Therapy. ChemMedChem 2020, 15, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal Response of Human and Murine Cancer Cells to Multiwalled Carbon Nanotubes after Laser Irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Zhong, H.; Chen, H.; Guo, Y.; Guo, Z.; Huang, D.; Zhang, W.; Wu, Q.; Yang, B.; Liu, Z. Black phosphorus–polypyrrole nanocomposites for high-performance photothermal cancer therapy. New J. Chem. 2019, 43, 8620–8626. [Google Scholar] [CrossRef]
- Piskorz, J.; Lijewski, S.; Gierszewski, M.; Gorniak, K.; Sobotta, L.; Wicher, B.; Tykarska, E.; Düzgüneş, N.; Konopka, K.; Sikorski, M.; et al. Sulfanyl porphyrazines: Molecular barrel-like self-assembly in crystals, optical properties and in vitro photodynamic activity towards cancer cells. Dye. Pigment. 2017, 136, 898–908. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Zhu, X.; Cai, G.; Wu, J.; Chen, M.; Du, P.; Chen, Y.; Liu, W.; Yang, S. Enhancing the photodynamic therapy efficacy of black phosphorus nanosheets by covalently grafting fullerene C60. Chem. Sci. 2020, 11, 11435–11442. [Google Scholar] [CrossRef]
- Li, R.; Shan, L.; Yao, Y.; Peng, F.; Jiang, S.; Yang, D.; Ling, G.; Zhang, P. Black phosphorus nanosheets and docetaxel micelles co-incorporated thermoreversible hydrogel for combination chemo-photodynamic therapy. Drug Deliv. Transl. Res. 2021, 11, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, T.; Fan, F.; Gao, F.; Ji, H.; Yang, L. Piezoelectric Materials as Sonodynamic Sensitizers to Safely Ablate Tumors: A Case Study Using Black Phosphorus. J. Phys. Chem. Lett. 2020, 11, 1228–1238. [Google Scholar] [CrossRef]
- Ouyang, J.; Deng, L.; Chen, W.; Sheng, J.; Liu, Z.; Wang, L.; Liu, Y.-N. Two dimensional semiconductors for ultrasound-mediated cancer therapy: The case of black phosphorus nanosheets. Chem. Commun. 2018, 54, 2874–2877. [Google Scholar] [CrossRef]
- Qin, L.; Ling, G.; Peng, F.; Zhang, F.; Jiang, S.; He, H.; Yang, D.; Zhang, P. Black phosphorus nanosheets and gemcitabine encapsulated thermo-sensitive hydrogel for synergistic photothermal-chemotherapy. J. Colloid Interface Sci. 2019, 556, 232–238. [Google Scholar] [CrossRef]
- Shou, X.; Liu, Y.; Wu, D.; Zhang, H.; Zhao, Y.; Sun, W.; Shen, X. Black phosphorus quantum dots doped multifunctional hydrogel particles for cancer immunotherapy. Chem. Eng. J. 2021, 408, 127349. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile synthesis of black phosphorus–Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.A. Cellular mechanisms of multidrug resistance of tumor cells. Biochem. Biokhimiia 2000, 65, 95–106. [Google Scholar]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.R.; Singhal, A.K.; Liu, H.; Nordquist, R.E. Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res. 2001, 61, 459–461. [Google Scholar]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Fu, Q.; Liu, Y.; Wang, J.; Song, J.; Yang, H. NIR/ROS-Responsive Black Phosphorus QD Vesicles as Immunoadjuvant Carrier for Specific Cancer Photodynamic Immunotherapy. Adv. Funct. Mater. 2020, 30, 1905758. [Google Scholar] [CrossRef]
- Qunfang, Z.; Liu, W.; Zhao, Y.; Gao, J.; Xia, T.; Shi, J.; Hu, L.; Zhou, W.; Gao, J.; Wang, H.; et al. Improved Biocompatibility of Black Phosphorus Nanosheets by Chemical Modification. Angew. Chem. Int. Ed. 2017, 56, 14488–14493. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Zhang, X.; Zhang, S.; Lei, L.; Ma, W.; Li, D.; Wang, W.; Zhao, Q.; Xing, B. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol. Environ. Saf. 2018, 161, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Z.; Su, C.; Yang, B.; Fei, X.; Li, Y.; Hou, Y.; Zhao, H.; Guo, Y.; Zhuang, Z.; et al. Biodegradable Black Phosphorus-based Nanomaterials in Biomedicine: Theranostic Applications. Curr. Med. Chem. 2019, 26, 1788–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef]
- Plutnar, J.; Sofer, Z.; Pumera, M. Products of Degradation of Black Phosphorus in Protic Solvents. ACS Nano 2018, 12, 8390–8396. [Google Scholar] [CrossRef]
- Abellán, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U.; Maultzsch, J.; Varela, M.; Hauke, F.; Hirsch, A. Fundamental Insights into the Degradation and Stabilization of Thin Layer Black Phosphorus. J. Am. Chem. Soc. 2017, 139, 10432–10440. [Google Scholar] [CrossRef] [Green Version]
- Sutrisno, L.; Chen, H.; Chen, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Composite scaffolds of black phosphorus nanosheets and gelatin with controlled pore structures for photothermal cancer therapy and adipose tissue engineering. Biomaterials 2021, 275, 120923. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Yi, X.; Xu, Y.; Niu, C.; Zhang, W.; Wang, L.; Sheng, J.; Deng, L.; Liu, Y.-N.; et al. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Adv. Mater. 2018, 30, 1703458. [Google Scholar] [CrossRef]



| Properties | Exhibited by BP | Related Biomedical Applications |
|---|---|---|
| Bandgap (eV) | 0.3–2.0 | Broad range of bandgap results in excellent optical absorption along UV, visible, and IR spectrum for the detection of biomolecules, such as proteins and various inorganic ions, which makes BP a right choice for biosensing, phototherapy, and photoacoustic imaging [65]. |
| Electrical conductivity | Ambipolar | As BP is ambipolar, it can detect both positively and negatively charged bioanalytes for efficient biosensing [66]. |
| Carrier mobility (cm2·V−1·s−1) | 1000 | Excellent carrier mobility exhibited by BP nanomaterials makes them suitable for gas sensing based on electrical conductivity measurement [67]. |
| Biocompatibility | Excellent | BP shows better biocompatibility and comparatively less cytotoxicity [68]. |
| In vivo biodegradability | Excellent | BPNSs can degrade to nontoxic phosphate and phosphonate in vivo, and hence, they do not produce any immune response or have toxic potential [69]. |
| Surface area (m2·g−1) | ~2630 | BPNSs exhibit a large surface area with single-atomic thickness and contain a large number of spots for anchoring the chemotherapeutic agents [70]. They can load drugs that weigh more than the carriers; hence, BPNSs are considered good candidates to be used as drug carriers for chemo-photothermal therapy [24,71]. |
| Type of BP Nanosystem | Targeting Ligand | Modification | Therapy | Cell Line | Animal Model | Results | Reference |
|---|---|---|---|---|---|---|---|
| BPNS | - | PEITC | PTT/PDT/gene therapy | MCF-7/ADR | BALB/c nude mice | Inhibition of drug-resistant cancer | [82] |
| BPQDs | - | PNIPAM | PTT/immunotherapy | MDA-MB231 | BALB/c nude mice | Stimulation of γδ T-cell for cancer immunotherapy | [168] |
| BPQDs | - | PLGA | PTT | 4T1-LG12 | BALB/c mice | Apoptosis-dependent tumor cell death | [121] |
| BPNS | - | Nile blue | PTT | MCF-7 | BALB/c nude mice | Tumor ablation under NIR | [134] |
| BPNS | - | - | PTT/PDT | 4T1 | BALB/c mice | Enhanced drug release on NIR irradiation | [62] |
| BPQDs | - | PLGA | PTT | MCF-7 | BALB/c nude mice | Better PTT and tumor-targeting efficiency | [63] |
| BPNS | - | Au | PTT | 4T1 | BALB/c mice | Enhanced photothermal conversion efficiency | [169] |
| BPNS | - | PEI | PTT/gene therapy | MCF-7 | BALB/c nude mice | Better PTT and tumor-targeting efficiency | [152] |
| BPNS | - | Polypyrrole | PTT | 4T1 | BALB/c mice | Efficient performance of NIR PTT | [159] |
| BPNS | - | PEOz | PTT | MCF-7 | Female severe combined immunodeficient (SCID) mice | Targeted long circulation and better cellular uptake | [19] |
| BPNS | HA | PAMAM | PTT | 4T1 | BALB/c mice | Better therapeutic effect | [100] |
| BPNS | HA | - | PTT | 4T1 | Balb/c mice | pH/NIR-triggered drug release | [80] |
| BPNPs | FA | DEX | PTT | 4T1 | BALB/c mice | Excellent photothermal conversion efficiency | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soman, S.; Kulkarni, S.; Pandey, A.; Dhas, N.; Subramanian, S.; Mukherjee, A.; Mutalik, S. 2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements. Biosensors 2022, 12, 1009. https://doi.org/10.3390/bios12111009
Soman S, Kulkarni S, Pandey A, Dhas N, Subramanian S, Mukherjee A, Mutalik S. 2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements. Biosensors. 2022; 12(11):1009. https://doi.org/10.3390/bios12111009
Chicago/Turabian StyleSoman, Soji, Sanjay Kulkarni, Abhijeet Pandey, Namdev Dhas, Suresh Subramanian, Archana Mukherjee, and Srinivas Mutalik. 2022. "2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements" Biosensors 12, no. 11: 1009. https://doi.org/10.3390/bios12111009
APA StyleSoman, S., Kulkarni, S., Pandey, A., Dhas, N., Subramanian, S., Mukherjee, A., & Mutalik, S. (2022). 2D Hetero-Nanoconstructs of Black Phosphorus for Breast Cancer Theragnosis: Technological Advancements. Biosensors, 12(11), 1009. https://doi.org/10.3390/bios12111009





