A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications
Abstract
:1. Introduction
2. Design of the UCNPs Analysis Nanoplatform
2.1. Synthesis of the UCNPs
2.1.1. High-Temperature Decomposition Method
2.1.2. Hydrothermal Method
2.1.3. Solvothermal Method
| Synthetic Methods | UCNPs | Precursors | Surfactant | Morphology | Size | Ref. |
|---|---|---|---|---|---|---|
| High-Temperature Decomposition Method | NaYF4: Yb/Er | RE(CF3COO)3 | OA, ODE | Spherical | 10 nm–30 nm | [40] |
| NaLuF4: Yb, Er | RE(CF3COO)3 | Ship-in-a-bottle | 80 nm–240 nm | [41] | ||
| NaGdF4: Yc, Er | ||||||
| LiYF4: Yd, Er | ||||||
| NaYF4: Yb, Er | RE(CF3COO)3 | Oleylamine | Hexagonal | 10.5 nm | [20] | |
| NaYF4: Yb, Tm | ||||||
| NaYF4: Yb, Er/Tm | OA, ODE | Large spheres | 37.9 nm | [42] | ||
| Small spheres | 14.0 nm | |||||
| Rods | Length = 60.1 nm, Width = 21.5 nm | |||||
| β-NaYF4: Yb, Er/Tm | NH4F | OA, ODE | Nanoplates and | 30 nm × 30 nm × 45 nm | [43] | |
| Nanospheres | 30 nm | |||||
| Nanoellipses | 17 nm–22 nm | |||||
| Hydrothermal Method | NaYF4: Yb3+, Er3+ | NaBF4 | OA | pH = 7 Microtubes | 14.46 μm | [44] |
| pH = 9, Microrods and nanorods | 10.65/0.87 μm | |||||
| pH = 11, Microtubes | 7.90 μm | |||||
| NH4F | pH = 7, Submicrorods | 5.53 μm | ||||
| pH = 9, Submicrorods | 4.07 μm | |||||
| pH = 11, Submicrorods | 0.48 μm | |||||
| NaF | pH = 7, Microtubes | 5.76 μm | ||||
| pH = 9, Submicrorods | 0.86 μm | |||||
| pH = 11, Nanorods | 0.65 μm | |||||
| NaYF4: Yb3+, Er3+ | OA, OH− | Nanobranches | 1.5 μm | [45] | ||
| β-NaLuF4 | NaF | Aitric acid (AC) | AC = 2 mmol, regular hexagonal phase microdisks | Height: 0.79 μm Diameter: 7.58 μm | [46] | |
| AC = 3 mmol, short hexagonal phase microprisms | Height: 2.12 μm Diameter: 8.51 μm | |||||
| AC = 8 mmol, hexagonal phase microtubes with hollow | Height: 9.47 μm Diameter: 1.88 μm | |||||
| NaGdF4: Yb3+, Er3+ | NaF | CTAB | Flower-like assemblies | 200 nm–250 nm | [47] | |
| Solvothermal Method | NaYF4: Yb, Er@NaYF4 | NH4F | OA, ODE | Hexagonal nanoparticles | 9 nm | [32] |
| NaMF4 | NaF, M(NO3)3 | OA, OH− | Uniformly hexagonal nanotubes | Length: 500 nm Diameter: 250 nm | [38] |
2.2. Surface Modification Strategies for the UCNPs
2.3. Construction of the UCNPs Nano-Platforms
| The UCNPs | Organic Ligands | Modified Material | Modified Purpose | Fluorescence Sensing Platform | Applications | Ref. |
|---|---|---|---|---|---|---|
| NaYF4: Yb, Tm | NH2- | 3-aminopropyltriethoxysilane (APTES) | Coupling with aptamer | NaYF4: Yb, Tm-NH2/aptamer and SYBR Green-I | Oxytetracycline detecting | [65] |
| NaYF4: Yb, Tm | APTES | Coupling with aptamer | NaYF4: Yb, Tm-NH2@ Molecularly Imprinted Polymer-aptamer | Enrofloxacin detecting | [66] | |
| NaYF4: Yb, Er@NaGdF4 | APTES | Activate drug delivery | NaYF4: Yb, Er@NaGdF4 | Intracellular imaging | [67] | |
| NaYF4: Yb, Er@NaYF4 | PEI | Hydrophilia | NaYF4: Yb, Er@NaYF4-NH2/Calcium Red/Alizarin Red S | Sensing pH | [68] | |
| NaYF4: Yb, Tm | Polyethyleneimine | Hydrophilia | NaYF4: Yb, Tm-NH2 | Intracellular imaging | [69] | |
| NaYF4: Yb, Er | APTES | Absorb negative charges | NaYF4: Yb, Er-NH2@SiO2-NH2 and AuNPs-citrate | Cyano-containing pesticides detecting | [70] | |
| NaYF4: Yb, Er@NaYF4 | COOH- | PAA in DEG | Hydrophilia | NaYF4: Yb, Er@NaYF4-COOH, OH | -- | [71] |
| NaYF4: Yb, Tm | PAA in H2O | Covalent coupling of dopamine by amidization reaction | NaYF4: Yb, Tm-COOH-dopamine | Organophosphorus pesticide detecting | [72] | |
| NaYF4: Yb, Er | PAA in ethyl alcohol | Coupling with hydrophilic materials | NaYF4: Yb, Er-COOH-RGB inks | Drug Anti-Counterfeiting | [73] | |
| NaYF4: Yb, Er (Tm or Ho) | Lemieux-von Rudloff reagent (OA is oxidized) | Coupling with proteins | UCNPs-Strepta-vidin | DNA detecting | [22] | |
| NaYF4: Yb, Tm | Methacrylic acid (MAA) | Loading CDDP | NaYF4: Yb, Tm-COOH | Drug delivery | [74] | |
| NaYF4: Yb,Er | Adipic acid | Coupling the growth and hydrophilia | NaYF4: Yb, Er-COOH | Intracellular imaging in vitro | [75] | |
| NaYF4: Yb, Tm | COOHCOOH | PAA in diethylene glycol | Coupling with antibody | NaYF4: Yb,Tm-COOH/Magnetic polystyrene microspheres | Bisphenol A detecting | [76] |
| NaYF4: Yb,Er | -- | Poly-MAEP | Coupling with proteins | NaYF4: Yb, Er-MAEP | Cell imaging | [77] |
| NaLuF4: Yb, Er | -- | Amphiphilic phospholipid functionalized poly ethylene glycol and DSPE-PEG | Amphipathy | NaLuF4: Yb, Er-ph-PEG, DSPE-PEG | Deep-tissue bioimaging | [78] |
| NaYF4: Yb, Er | Aryl group- | Phosphoryl-functionalized pillar arene | Hydrophilia | NaYF4: Yb, Er-PP5 | pH-responsive DDS | [79] |
| NaYF4: Yb, Er | -- | α-Cyclodextrin | Hydrophilia and specific recognize Cys | α-CD-NaYF4: Yb, Er-rhodamine-oxaldehyde (RHO) | Cys detecting | [80] |
| LaF3: Yb, Ho or LaF3: Yb, Er | -- | Polyethylene glycol monomethyl ether | Amphipathy | LaF3: Yb, HO/LaF3: Yb, Er- mPEG-OH | The UCNPs epoxidation | [81] |
| NaYF4: Yb, Er | -- | Polyethylene glycol-poly (lactic-co-glycolic acid) polymer, | Positive charge and amphiphilicity | NaYF4: Yb, Er- PEG-PLGA | Drug delivery | [82] |
| NaYF4: Yb, Er, Tm | Thiazole Derivative- | α-Cyclodextrin | Hydrophilia and specific recognize Hg2+ | α-CD- NaYF4: Yb, Er, Tm | Hg2+ detecting | [83] |
2.4. Mechanisms and Techniques for Optical Analysis of UCNPs Nano-Platforms in Practical Applications
3. The UCNPs in Analytical Application of Environmental Science
3.1. Organic Contaminant Residue Analysis
| Analyte | Matrix | Analysis Platform | Technique | Linear Detection Range (ng mL−1) | LOD (nmol L−1) | Recovery (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Metribuzin | Surface and ground waters | NaYF4: Yb, Er-Near Infrared dye | Ratiometric and colorimetric | 4.93 × 10–3.21 × 102 | 68 | -- | 1 | [98] |
| Tap water, river water | NaYF4: Yb, Er-tetramethylrh odamine | Fluorescence turn on-off | 0–80 | 2.19 × 10−7 | 91.0–115.0 | 2.3–3.7 | [99] | |
| Bisphenol A | Water sample | NaYF4: Yb, Er@Mn-aptamer | Electrogenerated chemiluminescence | 0.05–100 | 1.62 × 10−7 | 98–102.50 | -- | [100] |
| River water | NaYF4: Yb, Tm-MPMs 1 | Immunofluorescence | 1 × 105–5 × 108 | 8.76 × 10−3 | 85.35–108.35 | -- | [76] | |
| Perfluorooctane sulfonate | Surface water | NaYF4: Yb, Er-BSTFA 2 | Fluorescence quenching | 1.5 × 103–5 × 104 | 2.43 | 85.8–118.6 | 9.8 | [101] |
| Polychlorinated biphenyls | Water and soil samples | NaYF4: Yb, Er-BHQ 3-1, Carboxylated MMPs 4 | Fluorescence turn on-off | 0.004–800 | 1.36 × 10−8 | 93.4–109.7 83.2–118.5 | 1.6–2.9, 2.1–3.2 | [102] |
| Ag+ | Environmental water | NaYF4: Yb, Er/GQD 5 | Fluorescence turn on-off | 0.022 1–107.8 | 0.060 | 95–102 | 2.30–3.39 | [103] |
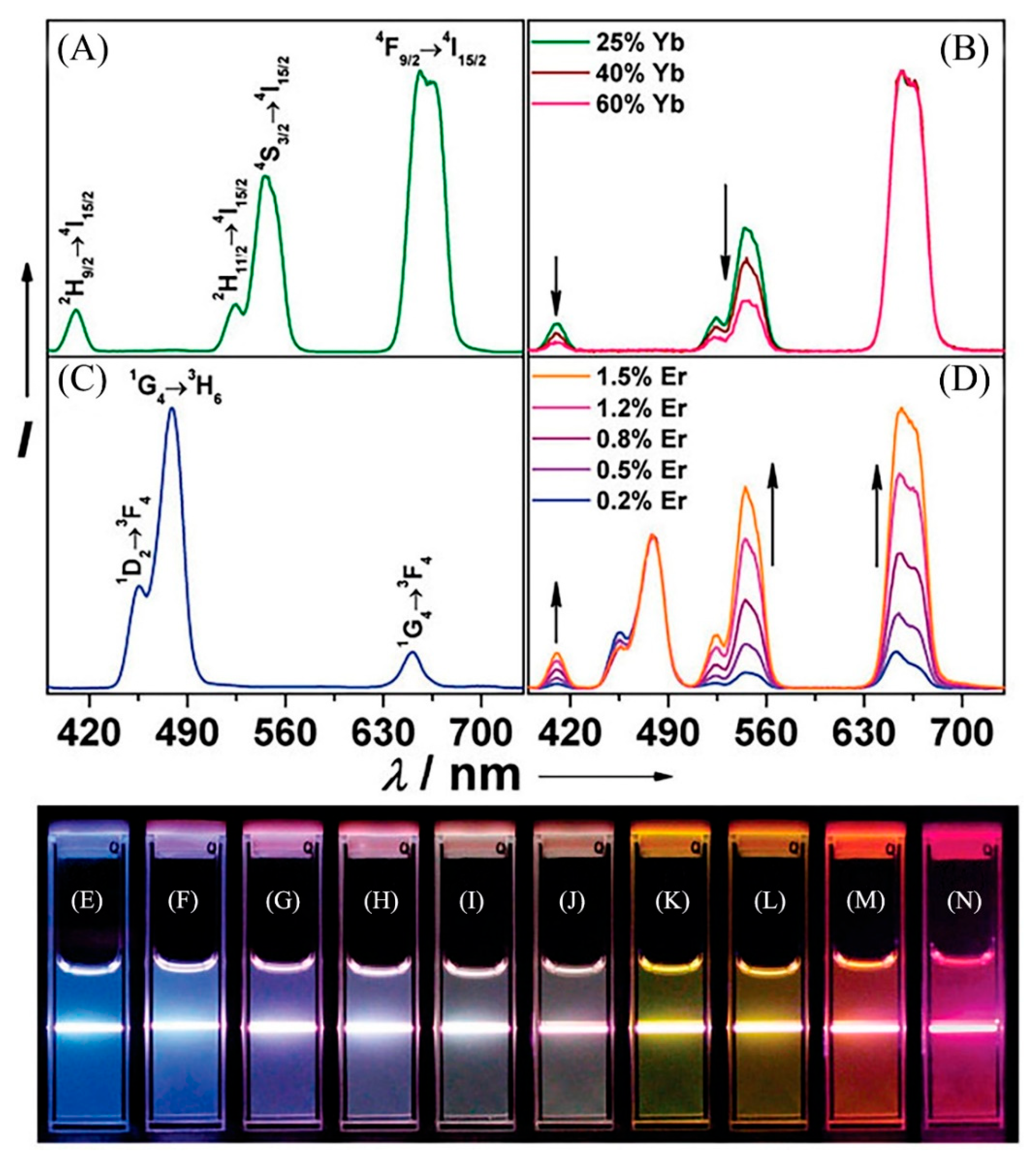
| Cr3+ | Industrial waste water | LiYF4: Yb, Ho@LiYF4@Ce3+ | Ratiometric fluorescence | 260–2600 | 4.1 × 102 | 95.7–97.2 | -- | [104] |
| Fe3+ | Waste water | NaYF4: Gd Yb, Ho/EPA 6 | Ratiometric fluorescence | 14–2800 | 2.5 × 102 | 100.9–107.3 | 0.8–1.4 | [105] |
| -- | LiYF4: Yb, Er, Ho, Tm@LiYF4: Yb | Fluorescence quenching | 0–8.6 × 106 | -- | -- | -- | [106] | |
| Pb2+ | -- | NaYF4: Yb, Er@NaYF4/AuNPs | Fluorescence turn on-off | 0–4.1 | 4.1 | -- | -- | [107] |
| Waste water | NaYF4: Gd, Yb, Ho/MNPs/AuNPs | Fluorescence turn on-off | 2.05–114.8 | 5.7 | 99.6–105.2 | 0.9–2.2 | [57] | |
| Cu2+ | Tap water | NaYF4: Yb, Er/ AuNPs-4-mercaptobenzoic acid | Fluorescence turn on-off | 1.28–64 | 18.2 | 98–106 | 1.2–1.8 | [108] |
| PO43− | Aqueous samples | ZrO2: Yb, Er@ZrO2/Fast Green alimentary dye | Fluorescence turn on-off | 1.9–95 | 20 | -- | -- | [109] |
| SO2 | SO2 gas in atmosphere | NaYF4@NaYF4: Yb, Tm/cyanine dye | Fluorescence turn on-off | 1 × 10−3–1 × 103 | 1.6 × 10−2 | -- | -- | [110] |
3.2. Inorganic Contaminant Analysis
3.2.1. Heavy Metal Ion Analysis
3.2.2. Inorganic Acid Ion
3.2.3. Air Pollutants
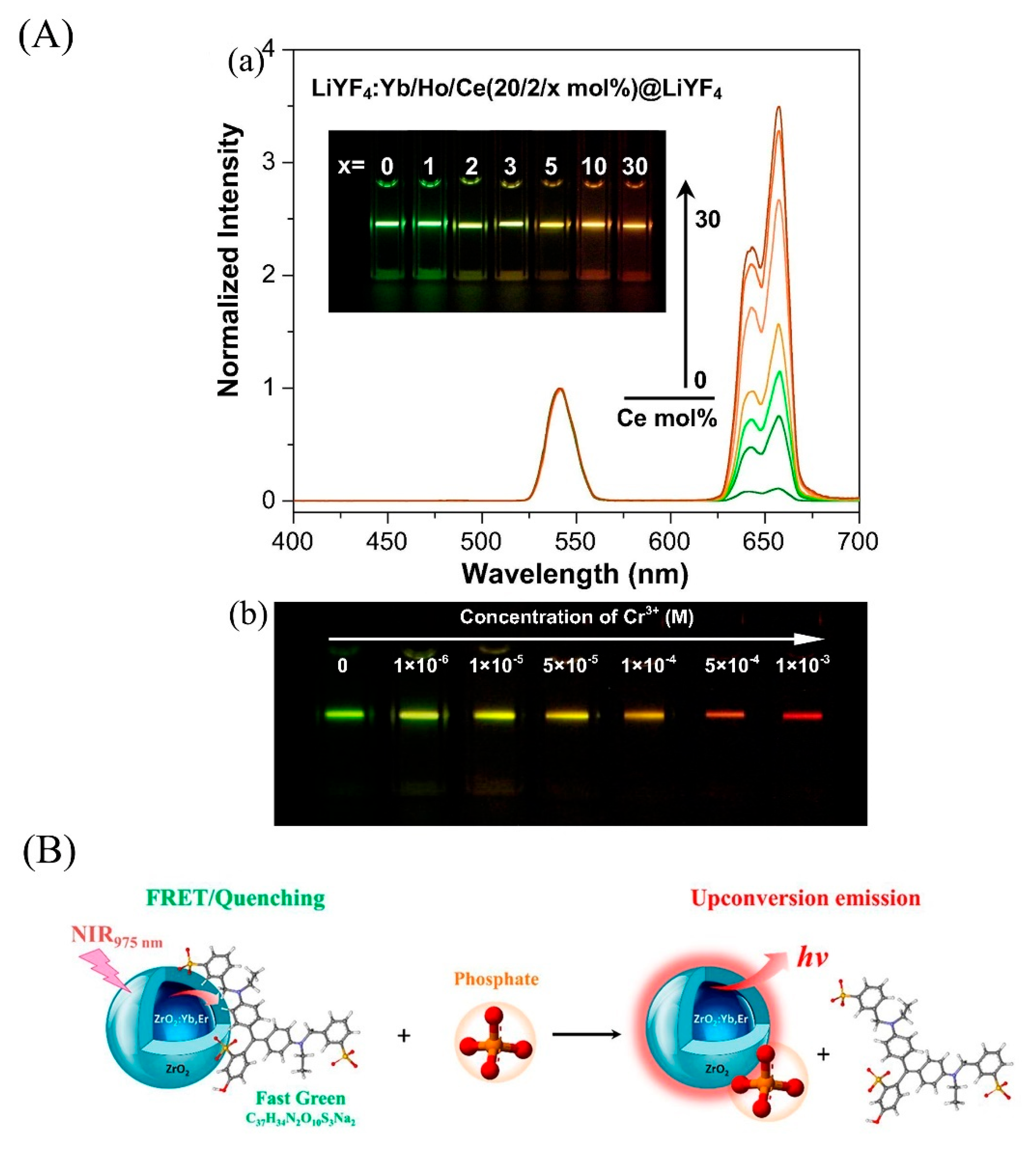
4. UCNPs in Analytical Application of Bioscience
4.1. Analysis of Biomacromolecule
4.1.1. The UCNPs Analysis of Proteins
4.1.2. The UCNPs Analysis of Nucleic Acids
4.2. Analysis of Small Biomolecules
4.2.1. The UCNPs Analysis of Amino Acid
4.2.2. The UCNPs Analysis of Peptides
4.2.3. The UCNPs Analysis of Neurotransmitter
4.3. Analysis of Microorganism
| Detector | Fluorescence Probe | Detection Method | Highlight | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| mRNA | NaGdF4: Yb3+, Er3+/AuNPs@Pt satellite assemblies | Fluorescence analysis | In situ imaging and quantification of TK1 mRNA in live cells. | 0.67 fmol (10 μg RNA) −1 | 1.17–65.21 fmol (10 μg RNA) −1 | [129] |
| miRNA-21 | NaYF4: Yb3+, Er3+-DNA H1 | Inductively coupled plasma-mass spectrometry | Sensitivity. | 0.041 fmol L−1 | 0.1–500 fmol L−1 | [130] |
| miRNAs | NaYF4: Yb3+, Er3+-NH2/NaYF4: Yb3+, Er3+-COOH/ dye UC | Fluorescence analysis | -- | 5 × 105 fmol L−1 | 2 × 105–1.4 × 106 fmol L−1 | [131] |
| miRNA-155 | NaGdF4: Yb3+, Er3+@NaYF4-DNA/AuNPs | Fluorescence analysis | -- | 4.5 × 103 fmol L−1 | 0.1 × 105–1.5 × 106 fmol L−1 | [33] |
| miRNA-21, miRNA-10b | NaYF4: Yb3+, Tm3+, Er3+-Ti3C2 | Fluorescence analysis | Assisted single-stranded replacement double-amplified RNA detection of mRNA in cell lysates without complex equipment, allowing detection of different sequences of RNA according to test requirements. | -- | 5–1 × 105 fmol L−1 | [132] |
| miRNAs | CaF2: Yb3+, Ho3+@MSNs@SiO2-ssDNA/Polyurethane fibers@GO | Fluorescence analysis | Enrichment of RNA and greatly improves accuracy. | 2 × 104 fmol L−1 | -- | [133] |
| miRNA-21 | FexCuySe@NaYF4: Yb3+, Tm3+ | Fluorescence analysis and magnetic resonance imaging | Dual signals for in situ quantitative imaging analysis. | 0.0058 amol (ng RNA)−1 | 0.035–31.824 amol (ng RNA)−1 | [134] |
| Microorganisms/Analytes | Fluorescence Probe | Detection Mechanism | Detection Method | Linear Range (ng ml−1) | LOD (ng ml−1) | Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Dipi-colinic acid | NaYF4: Yb, Er-TPP 1/EBT 2 | Luminescence | Colorimetric assay protocol | 334–3.34 × 104 | 1.5 × 102 | Human serum | [135] |
| Deoxynivalenol | Antibody-NaYF4: Yb, Tm, Gd/Antigen-MNPs | Luminescence | Immunofluorescence analysis | 0.001–0.1 | 0.001 | Adulterated oil | [136] |
| E. coli. | cDNA-NaYF4: Yb, Er/ Aptamers-AuNPs | FRET | Fluorescence analysis | 5–106 (cfu mL−1) | 3 (cfu mL−1) | Tap/pond water and milk | [137] |
| cDNA-NaY/GdF4: Yb, Ho/Aptamer-MNPs | Luminescence | Immunofluorescence analysis | 58–58 × 106 (cfu mL−1) | 10 (cfu mL−1) | Adulterated pork | [138] | |
| Aptamer-NaYF4: Yb, Er@NaYF4/WS2 3 | FRET | Fluorescence analysis | 85–85 × 107 (cfu mL−1) | 17(cfu mL−1) | Tap water, green tea powder | [139] | |
| Fumonisin B1 | cDNA-NaGdF4: Yb, Er/AuNPs | FRET | Fluorescence analysis | 1 × 10−5–0.1 | 3 × 10−6 | Corn | [140] |
| Microcystin-LR | NaYF4: Yb, Tm@NaYF4: Yb-COOH/MoS2 | Luminescence | Fluorescence analysis | 0.01–50 | 0.002 | Tap water | [141] |
| Mycotoxins zearalenone | cDNA-NaGdF4: Yb, Er/AuNPs | FRET | Fluorescence analysis | 0.05–100 | 0.01 | Corb | [140] |
| Ochratoxin A | NaYF4: Yb, Er/GO 4 | FRET | Fluorescence analysis | 0.001–250 | 0.001 | Beer | [59] |
| NaYF4: Yb, Er@Mn/Fe3O4NPs | Luminescence | Indirect competitive immunofluorescence analysis | -- | 9.553 × 10−3 | -- | [142] | |
| Aptmer-NaYF4: Yb, Er | Luminescence | Immunofluorescence analysis | 5–100 | 1.86 | Spiked wheat and beer | [143] | |
| NaYF4: Yb, Er/AuNPs | FRET | Fluorescence analysis | 0.1–1000 | 0.022 | -- | [144] | |
| NaYF4: Yb, Tm/Polystyrene beads | Luminescence | Indirect competitive immunofluorescence analysis | -- | 0.34721 | -- | [145] | |
| Polymyxin B and polymyxin B-resistant bacteria | NaGdF4: Yb, Er/AuNPs | Luminescence | Fluorescence analysis | -- | -- | Clinic urine | [146] |
| Single Escherichia coli | KLu2F7: Yb, Er/Tapered optical fiber | Luminescence | Fluorescence analysis | -- | -- | -- | [147] |
| Staphylococcal Enterotoxin B | NaGdF4: Yb, Er/AuNR@Pt | FRET | Fluorescence analysis | 2 × 10−3–0.4 | 0.9 × 10−3 | Spiked milk | [148] |
| NaGdF4: Yb, Er/AuNPs | Luminescence | Simultaneous detection of surface-enhanced Raman, fluorescence and circular dichroism modes | 1 × 10−3–0.75 | -- | Spiked milk | [149] | |
| T-2 Toxin | NH2-NaYF4: Yb, Er@SiO2/Fe3O4 MNPs 5 | Luminescence | Fluorescence analysis | 0.1–100 | 0.035 | Beer | [140] |
| Yersinia pestis EV76 | NaYF4: Yb, Er | ECL 6 signal | Electrochemical immunoassay | -- | 1.2 × 104 (cfu∙mL−1) | Soil | [150] |
| Listeria monocytogenes | NaGdF4: Yb, Er/MNPs | Luminescence | Fluorescence analysis | 68–68 × 106 (cfu mL−1) | 8 (cfu mL−1) | Pasteurized milk | [151] |
| Staphylococcus aureus | NaYF4: Yb, Er/AuNPs | FRET | Fluorescence analysis | 47–4.7 × 107 (cfu mL−1) | 10.7 (cfu mL−1) | Pork, beef | [152] |
4.4. Analysis of Inorganic Substances in Biological Samples
4.4.1. The UCNPs Analysis of Inorganic Ions in Biological Samples
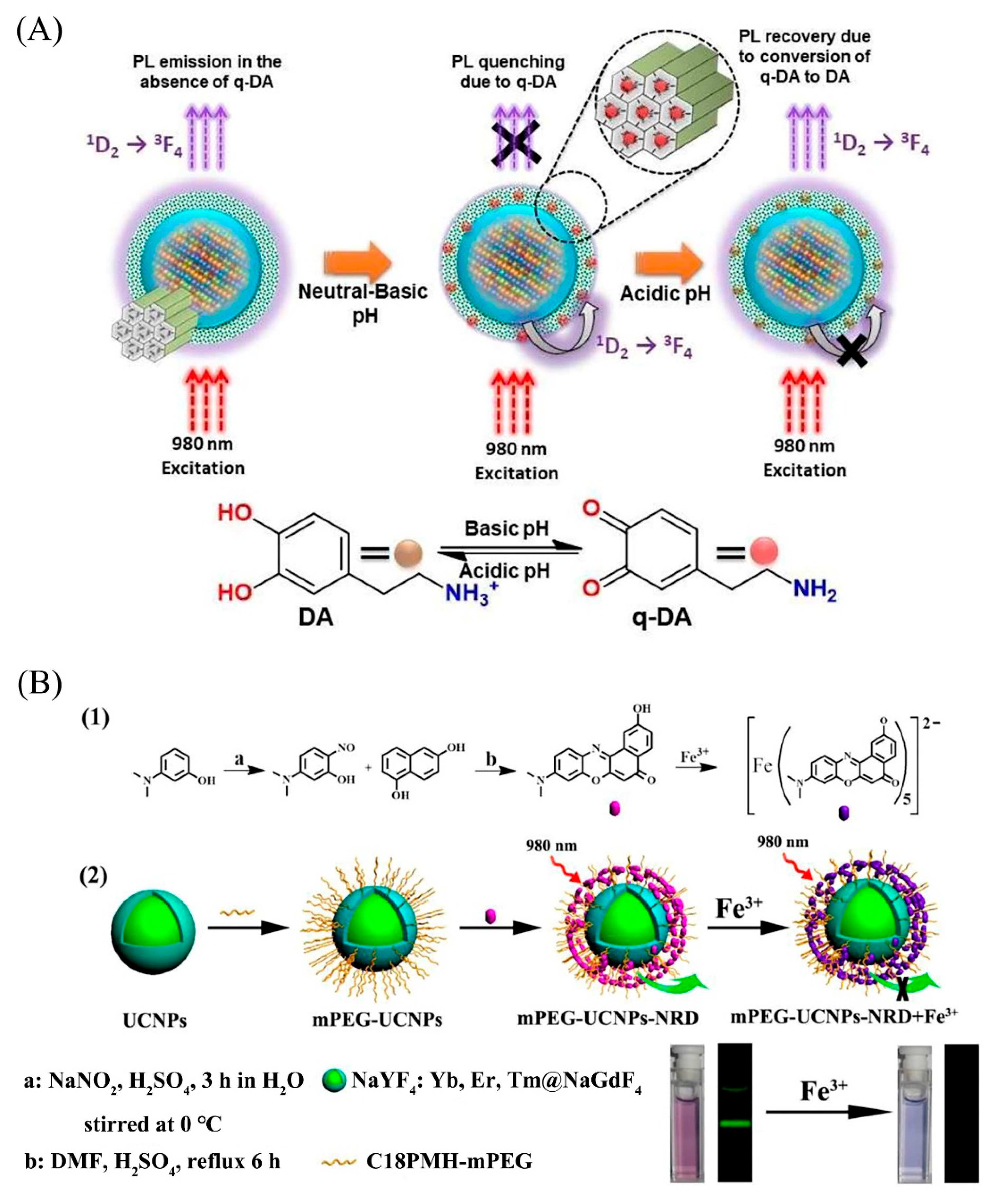
4.4.2. Upconversion Analysis of Reactive Oxygen Species
4.4.3. The UCNPs Analysis of Hydrogen Sulfide in Biological Samples
4.5. Others
5. UCNPs in Analytical Application of Food and Medical Science
5.1. Analysis of Food Samples
| Target | Samples | Platform | Characteristic | Linear Detection Range (ng mL−1) | LOD (ng mL−1) | Recovery (%) | RSD (%) (n=3) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Organophosphorus pesticides | Chlorpyrifos | Apples, cucumbers | NaYF4: Yb, Er-ChOx-Fe2+ | Accurate identification of chlorpyrifos through double-enzymes | 20–2000 | 6.7 | 89.5–97.1% | -- | [176] |
| Malathion | Tap water, matcha | NaYF4: Yb, Er-AuNPs | PDDA aptamer can specifically recognize malathion | 3.3036–330.36 | 0.47 | 99–105.25 90–111.75 | -- | [177] | |
| Parathionmet-hyl, monocrotophos, dimethoate | Apples, cucumber, capsicum | NaYF4: Yb, Er–AuNPs–AChE-acetylthiocholine (ATC) | Ensure AuNPs does not aggregate in the presence of pesticides and resulting in high efficiency of FRET | 0.002–0.2 | 6.7 × 10−4 0.023 0.067 | 96.67–110.00 | 4.78–8.43 | [178] | |
| Glyphosate | Instant tea | NaGdF4: Yb, Er-Cu2+-H2O2-TMB | Fluorescence of NaGdF4: Yb, Er at 660 nm increases linearly to form colorimetric assay | 250–1.25 × 105 | 9.8 | 96.4–100.74 | 0.56–2.91 | [90] | |
| Fenitrothion | -- | Immunochromatographic strip consists of NaYF4: Yb, Er-2, 4-D-IgG-fenitrothion-IgG | Portable sensors are prepared with unquestionable specificity | -- | 5 | -- | -- | [175] | |
| Chlorpyrifos | Balloonflower angelica | NaYF4: Yb, Tm-DA | Dopamine quinone quench FL of UCNPs through PEI and chlorpyrifos prevent oxidation of DA which recover UCNPs FL | 1.0–103 | 0.38 | 95.4–120.0 | 5.3–8.5 | [72] | |
| Diazinon | Apples | NaGdF4: Yb, Tm-Cu2+/AChE | Based on an (AChE) modulated fluorescence “off−on−off” strategy | 0.1–50 | 0.05 ng mL−1 | 93.2–102.1 | 5.7–8.3 | [179] | |
| Diazinon | Tea, apples | NaYF4: Yb, Er/Graphene Oxide | π-π interaction between UCNPs and GO | 0.05–500 | 0.023 | 86.06–104.92 86.03–95.87 | 3.43–4.85 | [180] | |
| Neonicotinoid insecticides | Imidacloprid | Water, Chinese cabbage, honey | NaYF4: Yb, Er/AuNPs | Dual signal of immunofluorescence improves the sensitivity and selectivity of imidacloprid. | 1.39–335.81 | 0.79 | 78.1–97.9 | 3.4–11.2 | [181] |
| Acetamiprid | Paddy water, pears | NaYF4: Yb, Er/cDNA-MNPs/aptamer | They label base complementary DNA, amine-functionalized UCNPs combine with negatively-charged DNA through electrostatic interaction | 0.89–114.18 | 650 | 78.2–103.5 | 2.6–10.9 | [182] | |
| Apples, strawberry | NaYF4: Yb, Er@molecularly imprinted polymer (MIP) | Quenching UCNPs emission peak at 542nm and specific recognition of MIP | 20–800 | 8.3 | 89.6–97.9 | 1.6–2.9 | [183] | ||
| Pyrethroids pesticides | Deltamethrin | Grape, cabbage | NaYF4: Yb, Er@MNPs@MIPs | Difunctional materials ensure a high degree selectivity of deltamethrin and separation | 103 –106 | 0.749 | 95.6–02% 91.8–05% | 2.97–4.07 2.42–5.20 | [184] |
| Fenpropathrin, Cypermethrin, fenvalerate | Cucumber, cabbage, apples and pears | NaYF4@NaYF4: Yb, Er@NaYF4/aminoated polystyrene magnetic microspheres-antigen | Devices for detecting multiple targets and miniaturized readout devices | -- | 0.01 0.015 0.011 | 83.4–97.8 | -- | [185] | |
| Carbamate pesticide | Carbaryl | Tea | NaErF4: Tm3+@NaYF4/polydopamine embedded sodium alginate hydrogel | UCNPS immobilized-sodium alginate hydrogel system realizes the true sense of field detectable | 0.5–200 | 0.5 | 90.51–105.33 | 2.28–4.46 | [186] |
| Benz- fungicide imidazole | CBZ | Apples, cucumber, matcha powder | NaGdF4: Yb, Er/MnO2 | The aptamer can self-assemble on the MnO2 nanosheet surface to quenching UCNPs FL | 0.1–5000 | 0.05 | 93.84–96.62 90.14–93.96 93.80–109.4 | 2.02–4.39 2.90–4.30 1.87–3.51 | [187] |
| Rhodamine B | Opaque fishes | NaYF4: Yb, Er | Opaque fishes not absorb RB and it will be quenching UCNPs FL through FRET | -- | -- | -- | -- | [188] | |
| Norfloxacin | Milk | NaYF4: Yb, Er | Comparing analysis results of NOR strips, QD FICS and UCNPs FICS and LOD of UCNPs is lowest. | -- | 0.5 | -- | -- | [189] | |
| Kanamycin | Milk, tap water | NaGd/YF4: Yb, Er- aptamer/BHQ3-cDNA | Kanamycin disrupts the FRET between BHQ3 and UCNPs by pairing with aptamer | 24.2–2.42 × 104 | 9.15 | 87–109.6 95.6–108.8 | -- | [190] | |
| Gallic acid | Green tea, orange juice | NaErF4: Tm@SiO2@ZIF-8/TMB | The emission spectrum of NaErF4: Tm@SiO2@ZIF-8 has overlap with the absorption spectra of oxTMB | 0–5103.6 | 59.542 | 98.4–105 | 0.6–2.3 | [136] | |
| Metal ion | Cu2+ | -- | NaYF4@NaYF4: Er, Yb@NaYF4/rhodamine B hydrazide (RBH) | The distance between Er3+and RHB can enhance FL of UCNPs to improve signal sensitivity | -- | -- | --- | - | [191] |
| Pb2+ | Tea | NaYF4: Gd, Yb, Ho/MNPs-AuNPs | Addition base complementary recognition to electrostatic interaction to construct FRET sensing platform | 2.05–114.8 | 0.4674 | 101.6–107.0 | 0.8–2.1 | [57] | |
| Hg2+ | Green tea | NaYF4: Yb, Er@NaYF4/AuNPs- cysteine | Fluorescence turns off-on-off to detect Hg2+ | 0.48–480 | 1.08 | 93–102 | 1.57–2.06 | [192] | |
| Ribbon fish | T-NaYF4: Yb, Tm | UCNP-T-Hg2+-T-UCNP reticular architecture on the surface of the electrode can accumulation of UCNP | 2.01 × 10−3–0.201 | 8.04 × 10−5 | -- | -- | [193] | ||
| IAcid ion | F | Milk | NaYF4: Yb, Er, Tm/curcumin | The specific recognition of curcumin with fluoride ions caused the shift in the UCNPs characteristic peak | 475–3.8 × 103 95–2.18 × 104 | 475 | 79.58–134.02% | 0.94–22.11 | [194] |
| HSO3− | Sugar | NaYF4: Yb, Er@NaYF4/cyanine dye | NOBF4 functionalized UCNPs to electrostatic adsorption with cyanine dyes | 81–9720 | 5.67 | 99.9–103.8 | -- | [195] | |
5.2. Analysis of Medical Sample

6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Xu, J.; Gulzar, A.; Yang, D.; Gai, S.; He, F.; Yang, P. Tumor self-responsive upconversion nanomedicines for theranostic applications. Nanoscale 2019, 11, 17535–17556. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.-R.; Wang, M.; Hsu, C.-Y.; Chen, N.; Zhang, Y. Small Upconverting Fluorescent Nanoparticles for Biosensing and Bioimaging. Adv. Opt. Mater. 2016, 4, 984–997. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Yang, P.; He, F.; Xu, L. Upconversion processes: Versatile biological applications and biosafety. Nanoscale 2017, 9, 12248–12282. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Upconversion Multicolor Fine-Tuning: Visible to Near-Infrared Emission from Lanthanide-Doped NaYF4 Nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Jahromi, E.Z.; Bidari, A.; Assadi, Y.; Hosseini, M.R.M.; Jamali, M.R. Dispersive liquid–liquid microextraction combined with graphite furnace atomic absorption spectrometry: Ultra trace determination of cadmium in water samples. Anal. Chim. Acta 2007, 585, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Batista, É.A.; Silva, G.; Sgobbi, L.; Machado, F.; Macedo, I.; Moreno, E.; Neto, J.; Scalize, P.; Gil, E. Enzymatic Electroanalytical Biosensor Based on Maramiellus colocasiae Fungus for Detection of Phytomarkers in Infusions and Green Tea Kombucha. Biosensors 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hou, X.; Zhen, C.; Wang, A.X. Sub-Part-Per-Billion Level Sensing of Fentanyl Residues from Wastewater Using Portable Surface-Enhanced Raman Scattering Sensing. Biosensors 2021, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 56. [Google Scholar] [CrossRef]
- Lv, G.; Liu, S.; Liu, M.; Liao, L.; Wu, L.; Mei, L.; Li, Z.; Pan, C. Detection and quantification of phenol in liquid and gas phases using a clay/dye composite. J. Ind. Eng. Chem. 2018, 62, 284–290. [Google Scholar] [CrossRef]
- Han, C.; Li, H. Host-molecule-coated quantum dots as fluorescent sensors. Anal. Bioanal. Chem. 2009, 397, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, B.; Yan, Y.; Tang, K.; Ding, C.-F. Binary magnetic metal-organic frameworks composites: A promising affinity probe for highly selective and rapid enrichment of mono- and multi-phosphopeptides. Mikrochim. Acta 2019, 186, 832. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Boyer, J.-C.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+ via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Song, Y.; Gong, G.; Du, J.; Xie, S.; Ouyang, M.; Feng, Y.; Xu, J.; Xu, L. Synthesis and Inkjet Printing of NaYF4:Ln3+@NaYF4 Core–Shell Nanoparticles with Enhanced Upconversion Fluorescence for Anti-Counterfeiting Applications. J. Nanosci. Nanotechnol. 2020, 20, 1511–1519. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Synthesis of Hexagonal-Phase NaYF4:Yb,Er and NaYF4:Yb,Tm Nanocrystals with Efficient Up-Conversion Fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Li, C.; Quan, Z.; Yang, J.; Yang, A.P.; Lin, J. Highly Uniform and Monodisperse β-NaYF4:Ln3+ (Ln = Eu, Tb, Yb/Er, and Yb/Tm) Hexagonal Microprism Crystals: Hydrothermal Synthesis and Luminescent Properties. Inorg. Chem. 2007, 46, 6329–6337. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Hu, H.; Yu, M.; Li, F.; Zhang, Q.; Zhou, Z.; Yi, T.; Huang, C. Versatile Synthesis Strategy for Carboxylic Acid−functionalized Upconverting Nanophosphors as Biological Labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Q.; Wang, Y. Enhanced optical temperature sensing and upconversion emissions based on the Mn2+ codoped NaGdF4:Yb3+,Ho3+ nanophosphor. New J. Chem. 2018, 43, 5011–5019. [Google Scholar] [CrossRef]
- Liangab, S.; Sunac, C.; Yangc, P.; Ping’Anmaab, P.; Huanga, S.; Chengab, Z.; Yubd, X.; Linab, J. Core-shell structured upconversion nanocrystal-dendrimer composite as a carrier for mitochondria targeting and catalase enhanced anti-cancer photodynamic therapy. Biomaterials 2020, 240, 119850. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Xu, D.; Ding, Y.; Hong, X.; Liu, Y. An “off-on” colorimetric and fluorometric assay for Cu(II) based on the use of NaYF4:Yb(III),Er(III) upconversion nanoparticles functionalized with branched polyethylenimine. Mikrochim. Acta 2018, 185, 211. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yang, F.; Jing, Q.; Zhao, C.; Wang, M.; Du, M.; Guo, Y.; Zhou, Q.; Li, Z. Optical multi-functionalities of Er3+- and Yb3+-sensitized strontium bismuth titanate nanoparticles. J. Alloy. Compd. 2019, 801, 100. [Google Scholar] [CrossRef]
- Su, S.; Mo, Z.; Tan, G.; Wen, H.; Chen, X.; Hakeem, D.A. PAA Modified Upconversion Nanoparticles for Highly Selective and Sensitive Detection of Cu2+ Ions. Front. Chem. 2021, 8, 619764. [Google Scholar] [CrossRef]
- Liu, J.; Bu, W.; Zhang, S.; Chen, F.; Xing, H.; Pan, L.; Zhou, L.; Peng, W.; Shi, J. Controlled Synthesis of Uniform and Monodisperse Upconversion Core/Mesoporous Silica Shell Nanocomposites for Bimodal Imaging. Chem. A Eur. J. 2012, 18, 2335–2341. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Xu, J.; Yang, D.; Liu, B.; Gai, S.; He, F.; Yang, P. Multimodal imaging and photothermal therapy were simultaneously achieved in the core–shell UCNR structure by using single near-infrared light. Dalton Trans. 2017, 46, 12147–12157. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Kong, X.; Tian, L.; Wang, Y.; Tu, L.; Zhao, J.; Zhang, H. Controlled Synthesis, Formation Mechanism, and Great Enhancement of Red Upconversion Luminescence of NaYF4:Yb3+, Er3+ Nanocrystals/Submicroplates at Low Doping Level. J. Phys. Chem. B 2008, 112, 15666–15672. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Dual-mode, tunable color, enhanced upconversion luminescence and magnetism of multifunctional BaGdF5:Ln3+ (Ln = Yb/Er/Eu) nanophosphors. Phys. Chem. Chem. Phys. 2016, 18, 21518–21526. [Google Scholar] [CrossRef] [PubMed]
- Märkl, S.; Schroter, A.; Hirsch, T. Small and Bright Water-Protected Upconversion Nanoparticles with Long-Time Stability in Complex, Aqueous Media by Phospholipid Membrane Coating. Nano Lett. 2020, 20, 8620–8625. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Chen, H. Turn-on detection of MicroRNA155 based on simple UCNPs-DNA-AuNPs luminescence energy transfer probe and duplex-specific nuclease signal amplification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117345. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Sangeetha, N.M.; Boyer, J.-C.; van Veggel, F.C.J.M. Facile ligand-exchange with polyvinylpyrrolidone and subsequent silica coating of hydrophobic upconverting β-NaYF4:Yb3+/Er3+ nanoparticles. Nanoscale 2010, 2, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.; Ullah, S.; Correales, Y.E.S.; Caetano, L.G.; Ribeiro, S.J.L. Enhanced NIR-I emission from water-dispersible NIR-II dye-sensitized core/active shell upconverting nanoparticles. J. Mater. Chem. C 2018, 6, 4777–4785. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhang, M.; Wu, F.; Zheng, T.; Sheng, B.; Liu, Y.; Shen, J.; Zhou, N.; Sun, Y. A theranostic nanocomposite with integrated black phosphorus nanosheet, Fe3O4@MnO2-doped upconversion nanoparticles and chlorin for simultaneous multimodal imaging, highly efficient photodynamic and photothermal therapy. Chem. Eng. J. 2020, 391, 123525. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Hu, R.; Fang, Z.-Q.; Shi, G.; Zhang, S.; Zhang, M. A multifunctional upconversion nanoparticles probe for Cu2+ sensing and pattern recognition of biothiols. Chin. Chem. Lett. 2021, 33, 3782–3786. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.; Shi, Y.; Xie, S.; Li, Y.; Xu, L.; Tu, B.; Zhao, D. Uniform Nanostructured Arrays of Sodium Rare-Earth Fluorides for Highly Efficient Multicolor Upconversion Luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef]
- Bu, W.; Chen, Z.; Chen, F.; Shi, J. Oleic Acid/Oleylamine Cooperative-Controlled Crystallization Mechanism for Monodisperse Tetragonal Bipyramid NaLa(MoO4)2 Nanocrystals. J. Phys. Chem. C 2009, 113, 12176–12185. [Google Scholar] [CrossRef]
- Liu, S.; De, G.; Xu, Y.; Wang, X.; Liu, Y.; Cheng, C.; Wang, J. Size, phase-controlled synthesis, the nucleation and growth mechanisms of NaYF4:Yb/Er nanocrystals. J. Rare Earths 2018, 36, 1060–1066. [Google Scholar] [CrossRef]
- Lu, S.; Tu, D.; Li, X.; Li, R.; Chen, X. A facile “ship-in-a-bottle” approach to construct nanorattles based on upconverting lanthanide-doped fluorides. Nano Res. 2015, 9, 187–197. [Google Scholar] [CrossRef]
- Na, H.; Woo, K.; Lim, K.; Jang, H.S. Rational morphology control of β-NaYF4:Yb,Er/Tm upconversion nanophosphors using a ligand, an additive, and lanthanide doping. Nanoscale 2013, 5, 4242–4251. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4:Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef]
- Ding, M.; Yin, S.; Chen, D.; Zhong, J.; Ni, Y.; Lu, C.; Xu, Z.; Ji, Z. Hexagonal NaYF4:Yb3+/Er3+ nano/micro-structures: Controlled hydrothermal synthesis and morphology-dependent upconversion luminescence. Appl. Surf. Sci. 2015, 333, 23–33. [Google Scholar] [CrossRef]
- Li, S.; Ye, S.; Chen, X.; Liu, T.; Guo, Z.; Wang, D. OH− ions-controlled synthesis and upconversion luminescence properties of NaYF4:Yb3+,Er3+ nanocrystals via oleic acid-assisted hydrothermal process. J. Rare Earths 2017, 35, 753–760. [Google Scholar] [CrossRef]
- Lin, H.; Xu, D.; Li, A.; Teng, D.; Yang, S.; Zhang, Y. Morphology evolution and pure red upconversion mechanism of β-NaLuF4 crystals. Sci. Rep. 2016, 6, 28051. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Sun, R.; Gao, G.; Zhang, C.; Chen, B.; Yan, N.; Yin, T.; Liu, Y.; Zhang, J.; Yang, Y.; et al. An Anion-Induced Hydrothermal Oriented-Explosive Strategy for the Synthesis of Porous Upconversion Nanocrystals. Theranostics 2015, 5, 456–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 710–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, Y. Monodisperse Silica-Coated Polyvinylpyrrolidone/NaYF4 Nanocrystals with Multicolor Upconversion Fluorescence Emission. Angew. Chem. Int. Ed. 2006, 45, 7732–7735. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, G.; Bi, H.; Xu, J.; Dong, S.; Jia, T.; Wang, Z.; Zhao, R.; Sun, Q.; Gai, S.; et al. An intelligent nanoplatform for imaging-guided photodynamic/photothermal/chemo-therapy based on upconversion nanoparticles and CuS integrated black phosphorus. Chem. Eng. J. 2020, 382, 122822. [Google Scholar] [CrossRef]
- He, B.; Zhou, L. Efficient tailoring of the surface of upconversion nanoparticles via surface-initiated cationic ring-opening polymerization. RSC Adv. 2015, 5, 97764–97772. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Sun, Y.; Li, Z.; Song, H.; Zhang, B.; Zhou, G.; Zhou, H.; Hu, J. Fluorescence resonance energy transfer between NH2–NaYF4:Yb,Er/NaYF4@SiO2 upconversion nanoparticles and gold nanoparticles for the detection of glutathione and cadmium ions. Talanta 2019, 207, 120294. [Google Scholar] [CrossRef]
- Shi, H.; Nie, Q.; Yang, M.; Wang, C.; Liu, E.; Ji, Z.; Fan, J. A ratiometric fluorescence probe for melamine detection based on luminescence resonance energy transfer between the NaYF4:Yb, Er upconversion nanoparticles and gold nanoparticles. J. Photochem. Photobiol. A Chem. 2020, 389, 112259. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, X.; Han, Y.; Zhang, H. Detection of procalcitonin (PCT) using the double antibody sandwich method based on fluorescence resonance energy transfer between upconversion nanoparticles and quantum dots. Anal. Methods 2018, 10, 1015–1022. [Google Scholar] [CrossRef]
- Tan, H.; Gong, G.; Xie, S.; Song, Y.; Zhang, C.; Li, N.; Zhang, D.; Xu, L.; Xu, J.; Zheng, J. Upconversion Nanoparticles@Carbon Dots@Meso-SiO2 Sandwiched Core–Shell Nanohybrids with Tunable Dual-Mode Luminescence for 3D Anti-Counterfeiting Barcodes. Langmuir 2019, 35, 11503–11511. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hassan, M.; Li, H.; Chen, Q. Fluorometric determination of lead(II) by using aptamer-functionalized upconversion nanoparticles and magnetite-modified gold nanoparticles. Mikrochim. Acta 2020, 187, 85. [Google Scholar] [CrossRef]
- Hu, J.; Wang, R.; Fan, R.; Huang, Z.; Liu, Y.; Guo, G.; Fu, H. Enhanced luminescence in Yb3+ doped core-shell upconversion nanoparticles for sensitive doxorubicin detection. J. Lumin. 2020, 217, 116812. [Google Scholar] [CrossRef]
- Yan, Z.; Fang, G.-Z. Molecularly imprinted polymer based on upconversion nanoparticles for highly selective and sensitive determination of Ochratoxin A. J. Central South Univ. 2019, 26, 515–523. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Gao, J.; Liu, X.; Gao, X.; Lu, X.; Fang, G.; Wang, J.; Li, J. Upconversion particle@Fe3O4@molecularly imprinted polymer with controllable shell thickness as high-performance fluorescent probe for sensing quinolones. Talanta 2018, 181, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Fang, G.; Pan, M.; Yang, Y.; Bai, X.; Wang, S. A molecularly imprinted electrochemiluminescence sensor based on upconversion nanoparticles enhanced by electrodeposited rGO for selective and ultrasensitive detection of clenbuterol. Biosens. Bioelectron. 2018, 102, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Demirçalı, A. Novel heterocyclic disazo dyes containing pyrazole and phenylpyrazole. part 1: Synthesis, characterization, solvent polarity and acid-base sensitive characteristics. J. Mol. Struct. 2021, 1231, 129960. [Google Scholar] [CrossRef]
- Levina, E.N. Fluorescein-labeled antibodies in detection of Bacillus anthracis. I. J. Microbiol. Epidemiol. Immunobiol. 1958, 29, 9–15. [Google Scholar]
- Guan, Y.; Qu, S.; Li, B.; Zhang, L.; Ma, H.; Zhang, L. Ratiometric fluorescent nanosensors for selective detecting cysteine with upconversion luminescence. Biosens. Bioelectron. 2016, 77, 124–130. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, C.; Wu, S.; Duan, N.; Wang, Z. Upconversion luminescence resonance energy transfer-based aptasensor for the sensitive detection of oxytetracycline. Anal. Biochem. 2015, 489, 44–49. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Su, L.; Gao, X.; Tang, Y.; Ma, T.; Zhu, L.; Li, J. Novel hybrid probe based on double recognition of aptamer-molecularly imprinted polymer grafted on upconversion nanoparticles for enrofloxacin sensing. Biosens. Bioelectron. 2017, 87, 203–208. [Google Scholar] [CrossRef]
- Tenga, B.; Dingbc, B.; Shaob, S.; Wangd, Z.; Tonga, W.; Wanga, S.; Chengbc, Z.; Linbc, J.; Ping’Anmaabc, P. Intracellular RNA and nuclear DNA-dual-targeted tumor therapy via upconversion nanoplatforms with UCL/MR dual-mode bioimaging. Chem. Eng. J. 2020, 405, 126606. [Google Scholar] [CrossRef]
- Tsai, E.S.; Himmelstoß, S.F.; Wiesholler, L.M.; Hirsch, T.; Hall, E.A.H. Upconversion nanoparticles for sensing pH. Anal. 2019, 144, 5547–5557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-C.; Yang, Z.-L.; Dong, W.; Tang, R.-J.; Sun, L.-D.; Yan, C.-H. Bioimaging and toxicity assessments of near-infrared upconversion luminescent NaYF4:Yb,Tm nanocrystals. Biomaterials 2011, 32, 9059–9067. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Jiang, S.; Fu, Z. A facile luminescence resonance energy transfer method for detecting cyano-containing pesticides in herbal medicines. Microchem. J. 2019, 152, 104451. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Li, X.; Chen, D. Monodisperse, size-tunable and highly efficient β-NaYF4:Yb,Er(Tm) up-conversion luminescent nanospheres: Controllable synthesis and their surface modifications. J. Mater. Chem. 2009, 19, 3546–3553. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.; Wang, Y.; Ouyang, H.; Fu, Z. Dopamine-functionalized upconversion nanoparticles as fluorescent sensors for organophosphorus pesticide analysis. Talanta 2018, 195, 706–712. [Google Scholar] [CrossRef]
- You, M.; Lin, M.; Wang, S.; Wang, X.; Zhang, G.; Hong, Y.; Dong, Y.; Jin, G.; Xu, F. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale 2016, 8, 10096–10104. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Geng, Z.; Wang, K.; Wang, Z. One-step synthesis of carboxyl-functionalized rare-earth fluoride nanoparticles for cell imaging and drug delivery. RSC Adv. 2015, 5, 33999–34007. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, T.; Hu, Y.; Qin, Y.; Wei, W. In Situ Synthesis of Dicarboxylic Acid Functionalized Upconversion Nanoparticles for Bioimaging Applications. ChemPhotoChem 2018, 3, 145–150. [Google Scholar] [CrossRef]
- Sheng, W.; Duan, W.; Shi, Y.; Chang, Q.; Zhang, Y.; Lu, Y.; Wang, S. Sensitive detection of bisphenol A in drinking water and river water using an upconversion nanoparticles-based fluorescence immunoassay in combination with magnetic separation. Anal. Methods 2018, 10, 5313–5320. [Google Scholar] [CrossRef]
- Chen, Y.; D’Amario, C.; Gee, A.; Duong, H.T.; Shimoni, O.; Valenzuela, S.M. Dispersion stability and biocompatibility of four ligand-exchanged NaYF4: Yb, Er upconversion nanoparticles. Acta Biomater. 2019, 102, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Huang, S.; Xu, C. Intensely red-emitting luminescent upconversion nanoparticles for deep-tissue multimodal bioimaging. Talanta 2018, 184, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Sun, X.; Yu, H.; Yan, F.; Meguellati, K.; Cheng, Z.; Zhang, H.; Yang, Y.-W. Facile surface functionalization of upconversion nanoparticles with phosphoryl pillar[5]arenes for controlled cargo release and cell imaging. Chem. Commun. 2018, 54, 12990–12993. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Shan, C.; Li, B.; Zhang, L.; Ma, H.; Luo, Y.; Song, H. Assembling of a functional cyclodextrin-decorated upconversion luminescence nanoplatform for cysteine-sensing. Chem. Commun. 2015, 51, 14054–14056. [Google Scholar] [CrossRef]
- Hu, H.; Yu, M.; Li, F.; Chen, Z.; Gao, X.; Xiong, L.; Huang, C. Facile Epoxidation Strategy for Producing Amphiphilic Up-Converting Rare-Earth Nanophosphors as Biological Labels. Chem. Mater. 2008, 20, 7003–7009. [Google Scholar] [CrossRef]
- Bai, X.; Xu, S.; Liu, J.; Wang, L. Upconversion luminescence tracking of gene delivery via multifunctional nanocapsules. Talanta 2016, 150, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhou, Y.; Zhang, X.; Liu, X.; Zhang, Y.; Marks, R.; Zhang, H.; Zhang, Q. Thiazole derivative-modified upconversion nanoparticles for Hg2+ detection in living cells. Nanoscale 2015, 8, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Chen, G.; Yang, C. Sensing Using Rare-Earth-Doped Upconversion Nanoparticles. Theranostics 2013, 3, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dumlupinar, G.; Andersson-Engels, S.; Melgar, S. Emerging applications of upconverting nanoparticles in intestinal infection and colorectal cancer. Int. J. Nanomed. 2019, 14, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta 2019, 1069, 32. [Google Scholar] [CrossRef]
- Liu, X.; Kong, X.; Zhang, Y.; Tu, L.; Wang, Y.; Zeng, Q.; Li, C.; Shi, Z.; Zhang, H. Breakthrough in concentration quenching threshold of upconversion luminescence via spatial separation of the emitter doping area for bio-applications. Chem. Commun. 2011, 47, 11957–11959. [Google Scholar] [CrossRef]
- Achatz, D.E.; Ali, R.; Wolfbeis, O.S. Luminescent Chemical Sensing, Biosensing, and Screening Using Upconverting Nanoparticles. In Luminescence Applied in Sensor Science; Prodi, L., Montalti, M., Zaccheroni, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 300, pp. 29–50. [Google Scholar]
- Petrakova, V.; Benson, V.; Buncek, M.; Fiserova, A.; Ledvina, M.; Stursa, J.; Cigler, P.; Nesladek, M. Imaging of transfection and intracellular release of intact, non-labeled DNA using fluorescent nanodiamonds. Nanoscale 2016, 8, 12002–12012. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yang, L.; Sharma, A.S.; Chen, M.; Chen, Q. A system composed of polyethylenimine-capped upconversion nanoparticles, copper(II), hydrogen peroxide and 3,3′,5,5′-tetramethylbenzidine for colorimetric and fluorometric determination of glyphosate. Mikrochim. Acta 2019, 186, 835. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Fang, A.; Wen, Y.; Li, H.; Zhang, Y.; Yao, S. Rapid and highly-sensitive uric acid sensing based on enzymatic catalysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Mikrochim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Fang, A.; Wu, Q.; Lu, Q.; Chen, H.; Li, H.; Liu, M.; Zhang, Y.; Yao, S. Upconversion ratiometric fluorescence and colorimetric dual-readout assay for uric acid. Biosens. Bioelectron. 2016, 86, 664–670. [Google Scholar] [CrossRef]
- Tan, C.; Atas, E.; Müller, J.G.; Pinto, M.R.; Kleiman, V.D.; Schanze, K.S. Amplified Quenching of a Conjugated Polyelectrolyte by Cyanine Dyes. J. Am. Chem. Soc. 2004, 126, 13685–13694. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Jeon, E.; Kim, S.; Lee, J. Lanthanide-Doped Upconversion Nanomaterials: Recent Advances and Applications. BioChip J. 2020, 14, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhao, W. Nanomaterials for luminescent detection of water and humidity. Analyst 2018, 144, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shikha, S.; Liu, J.; Zhang, J.; Mei, Q.; Zhang, Y. Upconversion Nanoprobes: Recent Advances in Sensing Applications. Anal. Chem. 2018, 91, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Alminderej, F.M.; Ali, R.; Abdallah, O.I. Optical sensor film for metribuzin pesticide detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 229, 117971. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bai, J.; Ren, S.; Wang, J.; Gao, Y.; Li, S.; Peng, Y.; Ning, B.; Gao, Z. An ultrasensitive sensor based on quantitatively modified upconversion particles for trace bisphenol A detection. Anal. Bioanal. Chem. 2018, 411, 171–179. [Google Scholar] [CrossRef]
- Guo, X.; Wu, S.; Duan, N.; Wang, Z. Mn2+-doped NaYF4:Yb/Er upconversion nanoparticle-based electrochemiluminescent aptasensor for bisphenol A. Anal. Bioanal. Chem. 2016, 408, 3823–3831. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Guo, H.Q.; Lin, L.K.; Hu, S.Y.; Ruan, J.J.; Liu, W.S.; Yan, L.S.; Li, K.X. An Upconversion Fluorescent Method for Rapid Detection of Perfluorooctane Sulfonate in Water Samples Based on Fluorine-Fluorine Interaction. Chin. J. Anal. Chem. 2019, 47, 380–387. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, J.; Huo, B.; Yuan, S.; Zhang, M.; Sun, X.; Peng, Y.; Li, S.; Wang, J.; Ning, B.; et al. Upconversion Fluorescent Aptasensor for Polychlorinated Biphenyls Detection Based on Nicking Endonuclease and Hybridization Chain Reaction Dual-Amplification Strategy. Anal. Chem. 2018, 90, 9936–9942. [Google Scholar] [CrossRef]
- He, L.; Yang, L.; Zhu, H.; Dong, W.; Ding, Y.; Zhu, J.-J. A highly sensitive biosensing platform based on upconversion nanoparticles and graphene quantum dots for the detection of Ag+. Methods Appl. Fluoresc. 2017, 5, 024010. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Zhang, C.; Yang, L.; Zhao, T.; Zhang, R.; Jiang, C. Upconversion color tuning in Ce3+-doped LiYF4:Yb3+/Ho3+@LiYF4 nanoparticles towards ratiometric fluorescence detection of chromium(III). J. Colloid Interface Sci. 2017, 493, 10–16. [Google Scholar] [CrossRef]
- Chen, M.; Kutsanedzie, F.Y.H.; Cheng, W.; Agyekum, A.A.; Li, H.; Chen, Q. A nanosystem composed of upconversion nanoparticles and N, N-diethyl-p-phenylenediamine for fluorimetric determination of ferric ion. Mikrochim. Acta 2018, 185, 378. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Wu, C.-H.; Yang, C.-H.; Wang, T.-L. Multiple doping effect of LiYF4:Yb3+/Er3+/Ho3+/Tm3+@LiYF4:Yb3+ core/shell nanoparticles and its application in Hg2+ sensing detection. J. Alloys Compd. 2019, 806, 272–282. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, M.; Chen, Z.; Deng, Z.; Liu, N.; Fan, J.; Zhang, W. A Fluorescence Resonance Energy Transfer Probe Based on DNA-Modified Upconversion and Gold Nanoparticles for Detection of Lead Ions. Front. Chem. 2020, 8, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Ma, Q.; Yu, W.; Dong, X.; Hong, X. “Off-On” typed upconversion fluorescence resonance energy transfer probe for the determination of Cu2+ in tap water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120920. [Google Scholar] [CrossRef]
- Ramírez-García, G.; Cervantes, E.D.; Mounzer, O.; De la Rosa, E.; Luke, T.L.; de la Cruz, F.N. A Turn-On Luminescence Method for Phosphate Determination Based on Fast Green-Functionalized ZrO2:Yb,Er@ZrO2Core@Shell Upconversion Nanoparticles. Anal. Chem. 2019, 91, 14657–14665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ling, X.; Mei, Q.; He, H.; Deng, S.; Zhang, Y. Surface lanthanide activator doping for constructing highly efficient energy transfer-based nanoprobes for the on-site monitoring of atmospheric sulfur dioxide. Analyst 2019, 145, 537–543. [Google Scholar] [CrossRef]
- Tian, L.; Guo, H.; Li, J.; Yan, L.; Zhu, E.; Liu, X.; Li, K. Fabrication of a near-infrared excitation surface molecular imprinting ratiometric fluorescent probe for sensitive and rapid detecting perfluorooctane sulfonate in complex matrix. J. Hazard. Mater. 2021, 413, 125353. [Google Scholar] [CrossRef]
- Ge, X.; Wei, R.; Sun, L. Lanthanide nanoparticles with efficient near-infrared-II emission for biological applications. J. Mater. Chem. B 2020, 8, 10257–10270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, X.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Recent advances on inorganic lanthanide-doped NIR-II fluorescence nanoprobes for bioapplication. J. Lumin. 2020, 228, 117627. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, J.; Yin, B.; Li, H.; Zhang, Y.; Yao, S. A novel label-free upconversion fluorescence resonance energy transfer-nanosensor for ultrasensitive detection of protamine and heparin. Anal. Biochem. 2015, 477, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, B.; Chen, B.; He, M.; Hu, B. Upconversion nanoparticle as elemental tag for the determination of alpha-fetoprotein in human serum by inductively coupled plasma mass spectrometry. Analyst 2016, 142, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Liu, D.; Jiang, Y.; Chen, X.; Shao, L.; Li, J.; Sheng, K.; Zhang, X.; Song, H. Near-infrared-light-triggered photoelectrochemical biosensor for detection of alpha-fetoprotein based on upconversion nanophosphors. Sens. Actuators B Chem. 2019, 286, 468–475. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, L.; Zeng, R.; Su, L.; Tang, D. Near-Infrared Light-Excited Core–Core–Shell UCNP@Au@CdS Upconversion Nanospheres for Ultrasensitive Photoelectrochemical Enzyme Immunoassay. Anal. Chem. 2018, 90, 9568–9575. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.-J.; Hu, J.; Li, Y.; Wang, Y.; Zhao, L.-X. Affinity binding-mediated fluorometric protein assay based on the use of target-triggered DNA assembling probes and aptamers labelled with upconversion nanoparticles: Application to the determination of platelet derived growth factor-BB. Mikrochim. Acta 2019, 187, 9. [Google Scholar] [CrossRef]
- Chen, H.; Pang, X.; Ni, Z.; Liu, M.; Zhang, Y.; Yao, S. Upconversion nanoparticles with bright red luminescence for highly sensitive quantifying alkaline phosphatase activity based on target-triggered fusing reaction. Anal. Chim. Acta 2019, 1095, 146–153. [Google Scholar] [CrossRef]
- Liang, M.-Y.; Zhao, B.; Xiong, Y.; Chen, W.-X.; Huo, J.-Z.; Zhang, F.; Wang, L.; Li, Y. A “turn-on” sensor based on MnO2coated UCNPs for detection of alkaline phosphatase and ascorbic acid. Dalton Trans. 2019, 48, 16199–16210. [Google Scholar] [CrossRef]
- Fang, A.; Chen, H.; Li, H.; Liu, M.; Zhang, Y.; Yao, S. Glutathione regulation-based dual-functional upconversion sensing-platform for acetylcholinesterase activity and cadmium ions. Biosens. Bioelectron. 2017, 87, 545–551. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Yang, H.; Chen, J.; Yang, H. A novel arginine bioprobe based on up-conversion fluorescence resonance energy transfer. Anal. Chim. Acta 2019, 1079, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Y.; Wang, L.; Chen, H. Detection of tyramine and tyrosinase activity using red region emission NaGdF4:Yb,Er@NaYF4 upconversion nanoparticles. Talanta 2019, 197, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.T.; Huy, B.T.; Tawfik, S.M.; Zayakhuu, G.; Cho, H.H.; Lee, Y.-I. Highly selective and sensitive optosensing of glutathione based on fluorescence resonance energy transfer of upconversion nanoparticles coated with a Rhodamine B derivative. Arab. J. Chem. 2020, 13, 2671–2679. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. NaYbF4@CaF2 core–satellite upconversion nanoparticles: One-pot synthesis and sensitive detection of glutathione. Nanoscale 2018, 10, 19898–19905. [Google Scholar] [CrossRef]
- Pulgarín, J.A.M.; Molina, A.A.; García, E.J.; Gómez, L.G. A sensitive resonance Rayleigh scattering sensor for dopamine in urine using upconversion nanoparticles. J. Raman Spectrosc. 2019, 51, 406–413. [Google Scholar] [CrossRef]
- Kumar, B.; Murali, A.; Giri, S. Upconversion Nanoplatform for FRET-Based Sensing of Dopamine and pH. ChemistrySelect 2019, 4, 5407–5414. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Han, L.; Shen, Y.; Fang, Y.; Xia, Y.; Yao, X.; Wu, F.; Li, C.; Chen, J.; et al. Upconversion luminescence–based aptasensor for the detection of thyroid-stimulating hormone in serum. Mikrochim. Acta 2022, 189, 179. [Google Scholar] [CrossRef]
- Gao, R.; Hao, C.; Xu, L.; Xu, C.; Kuang, H. Spiny Nanorod and Upconversion Nanoparticle Satellite Assemblies for Ultrasensitive Detection of Messenger RNA in Living Cells. Anal. Chem. 2018, 90, 5414–5421. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.-Q.; Cheng, Z.-H.; Wei, X.; Yang, T.; Yu, Y.-L.; Chen, M.-L.; Wang, J.-H. Highly Sensitive Detection of MicroRNA-21 with ICPMS via Hybridization Accumulation of Upconversion Nanoparticles. Anal. Chem. 2018, 90, 12116–12122. [Google Scholar] [CrossRef]
- Zhu, D.; Miao, Z.Y.; Hu, Y.; Zhang, X.J. Single-step, homogeneous and sensitive detection for microRNAs with dual-recognition steps based on luminescence resonance energy transfer (LRET) using upconversion nanoparticles. Biosens. Bioelectron. 2017, 100, 475–481. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Q.; Zhang, Y.; Yao, S. Strand displacement dual amplification miRNAs strategy with FRET between NaYF4:Yb,Tm/Er upconversion nanoparticles and Ti3C2 nanosheets. Sensors Actuators B Chem. 2019, 297, 126751. [Google Scholar] [CrossRef]
- Gu, T.; Ren, Z.; Li, X.; Huang, J.; Han, G. A flexible smart membrane consisting of GO composite fibres and upconversion MSNs for microRNA detection. Chem. Commun. 2019, 55, 9104–9107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hao, C.; Zhang, H.; Wu, X.; Xu, L.; Sun, M.; Xu, C.; Kuang, H. Dual-Modal FexCuySe and Upconversion Nanoparticle Assemblies for Intracellular MicroRNA-21 Detection. ACS Appl. Mater. Interfaces 2020, 13, 41405–41413. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-H.; Liu, X.; Zhang, S.-Q.; Yang, T.; Chen, M.-L.; Wang, J.-H. Placeholder Strategy with Upconversion Nanoparticles−Eriochrome Black T Conjugate for a Colorimetric Assay of an Anthrax Biomarker. Anal. Chem. 2019, 91, 12094–12099. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, W.; Sun, C.; Li, H.; Ouyang, Q. Synthesis of improved upconversion nanoparticles as ultrasensitive fluorescence probe for mycotoxins. Anal. Chim. Acta 2016, 938, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control 2019, 107, 106761. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.; Hassan, M.; Ouyang, Q.; Li, H.; Chen, Q. A highly sensitive upconversion nanoparticles-WS2 nanosheet sensing platform for Escherichia coli detection. Sens. Actuators B Chem. 2020, 320, 128434. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Jin, Z.; Xu, E. A fluorometric method for aptamer-based simultaneous determination of two kinds of the fusarium mycotoxins zearalenone and fumonisin B1 making use of gold nanorods and upconversion nanoparticles. Mikrochim. Acta 2020, 187, 179. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhao, S.; Wu, S.; Wang, Z. Upconversion nanoparticles grafted molybdenum disulfide nanosheets platform for microcystin-LR sensing. Biosens. Bioelectron. 2017, 90, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, Y.; Su, L.; Chang, J.; Wang, H. Application of upconversion luminescent-magnetic microbeads with weak background noise and facile separation in ochratoxin A detection. J. Nanoparticle Res. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Wang, W.; Yu, Q.; Wang, Z. A test strip for ochratoxin A based on the use of aptamer-modified fluorescence upconversion nanoparticles. Mikrochim. Acta 2018, 185, 497. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jo, E.-J.; Lee, K.J.; Park, J.; Jung, G.Y.; Shin, Y.-B.; Lee, L.P.; Kim, M.-G. Gold nanocap-supported upconversion nanoparticles for fabrication of a solid-phase aptasensor to detect ochratoxin A. Biosens. Bioelectron. 2019, 150, 111885. [Google Scholar] [CrossRef]
- Yang, M.; Cui, M.; Wang, W.; Yang, Y.; Chang, J.; Hao, J.; Wang, H. Background-free upconversion-encoded microspheres for mycotoxin detection based on a rapid visualization method. Anal. Bioanal. Chem. 2020, 412, 81–91. [Google Scholar] [CrossRef]
- Sun, M.; Qu, A.; Hao, C.; Wu, X.; Xu, L.; Xu, C.; Kuang, H. Chiral Upconversion Heterodimers for Quantitative Analysis and Bioimaging of Antibiotic-Resistant Bacteria In Vivo. Adv. Mater. 2018, 30, e1804241. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Xu, D.; Zhang, Y.; Chen, C.-H.; Li, B. Single Upconversion Nanoparticle-Bacterium Cotrapping for Single-Bacterium Labeling and Analysis. Small 2017, 13, 3418. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B. A fluorometric assay for staphylococcal enterotoxin B by making use of platinum coated gold nanorods and of upconversion nanoparticles. Mikrochim. Acta 2018, 185, 516. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B.; Jin, Z.; Xu, E. Triple-Mode Aptasensor for Sensitive and Reliable Determination of Staphylococcal Enterotoxin B. Food Anal. Methods 2020, 13, 1255–1261. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Wang, J.; Zhou, L.; Yang, R. A novel electro-driven immunochromatography assay based on upconversion nanoparticles for rapid pathogen detection. Biosens. Bioelectron. 2020, 152, 112037. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2020, 122, 107808. [Google Scholar] [CrossRef]
- Ouyang, Q.; Yang, Y.; Ali, S.; Wang, L.; Li, H.; Chen, Q. Upconversion nanoparticles-based FRET system for sensitive detection of Staphylococcus aureus. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119734. [Google Scholar] [CrossRef]
- Wei, R.; Wei, Z.; Sun, L.; Zhang, J.Z.; Liu, J.; Ge, X.; Shi, L. Nile Red Derivative-Modified Nanostructure for Upconversion Luminescence Sensing and Intracellular Detection of Fe3+ and MR Imaging. ACS Appl. Mater. Interfaces 2015, 8, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, F.; Wang, L.; Chen, H. A single-particle enumeration method for the detection of Fe2+ based on a near-infrared core–shell upconversion nanoparticle and IR-808 dye composite nanoprobe. Analyst 2019, 145, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xu, S.; Yu, Y.; Gao, Y.; Yin, Z.; Li, J.; Zhang, X.; Yu, H.; Chen, B. Turn-on fluorescence ferrous ions detection based on MnO2 nanosheets modified upconverion nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120275. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Q.; Yao, S.; Jiang, G. Recent progress in the application of nanomaterials in the analysis of emerging chemical contaminants. Anal. Methods 2017, 9, 2768–2783. [Google Scholar] [CrossRef]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Ferlazzo, A.; Celesti, C.; Branca, C.; Neri, G. Electrochemical and Fluorescent Properties of Crown Ether Functionalized Graphene Quantum Dots for Potassium and Sodium Ions Detection. Nanomaterials 2021, 11, 2897. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lu, Q.; Huang, L.; Liu, B.; Liu, M.; Zhang, Y.; Liu, J. DNA Triplex and Quadruplex Assembled Nanosensors for Correlating K + and pH in Lysosomes. Angew. Chem. Int. Ed. 2020, 60, 5453–5458. [Google Scholar] [CrossRef]
- Hao, C.; Wu, X.; Sun, M.; Zhang, H.; Yuan, A.; Xu, L.; Xu, C.; Kuang, H. Chiral Core–Shell Upconversion Nanoparticle@MOF Nanoassemblies for Quantification and Bioimaging of Reactive Oxygen Species in Vivo. J. Am. Chem. Soc. 2019, 141, 19373–19378. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, X.; Cao, C.; Lu, W.; Yuan, W.; Liu, Q.; Feng, W.; Li, F. Dye-sensitized upconversion nanocomposites for ratiometric semi-quantitative detection of hypochlorite in vivo. Nanoscale 2019, 11, 2959–2965. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Q.; He, K.; Liu, M.; Zhang, Y.; Yao, S. A cyclic signal amplification strategy to fluorescence and colorimetric dual-readout assay for the detection of H2O2-related analytes and application to colorimetric logic gate. Sensors Actuators B Chem. 2018, 260, 908–917. [Google Scholar] [CrossRef]
- Singh, P.; Singh, P.; Prakash, R.; Rai, S.B. Photo-physical studies of ultrasmall upconversion nanoparticles embedded organometallic complexes: Probing a dual mode optical sensor for hydrogen peroxide. Opt. Mater. 2019, 98, 109459. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, M.; Wang, P.; Gu, Y. FRET-Based Upconversion Nanoprobe Sensitized by Nd3+ for the Ratiometric Detection of Hydrogen Peroxide in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 7441–7449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Hao, C.; Wu, X.; Sun, M.; Qu, A.; Xu, L.; Kuang, H.; Xu, C. Chiral Cu xOS@ZIF-8 Nanostructures for Ultrasensitive Quantification of Hydrogen Sulfide In Vivo. Adv. Mater. 2020, 32, e1906580. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, W.; Ji, C.; Wang, L. Dye-sensitized core–shell NaGdF4:Yb,Er@NaGdF4:Yb,Nd upconversion nanoprobe for determination of H2S. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 248, 119281. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Qu, X.; Cheng, S.; Xian, Y. Cationic cyanine chromophore-assembled upconversion nanoparticles for sensing and imaging H2S in living cells and zebrafish. Biosens. Bioelectron. 2018, 126, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Q.; Zhai, X.; Mao, F.; Jiang, A.; Zhou, J. Rationally designed pure-inorganic upconversion nanoprobes for ultra-highly selective hydrogen sulfide imaging and elimination in vivo. Chem. Sci. 2018, 10, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ling, B.; Chen, H.; Wang, L. Mn2+-doped NaYF4:Yb,Er upconversion nanoparticles for detection of uric acid based on the Fenton reaction. Talanta 2018, 180, 120–126. [Google Scholar] [CrossRef]
- Mo, J.; Shen, L.; Xu, Q.; Zeng, J.; Sha, J.; Hu, T.; Bi, K.; Chen, Y. An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection. Nanomaterials 2019, 9, 1700. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Tawfik, S.M.; Thangadurai, D.T.; Lee, Y.-I. Highly sensitive and selective detection of Alprenolol using upconversion nanoparticles functionalized with amphiphilic conjugated polythiophene. Microchem. J. 2021, 164, 106010. [Google Scholar] [CrossRef]
- Giust, D.; Lucío, M.I.; El-Sagheer, A.H.; Brown, T.; Williams, L.E.; Muskens, O.L.; Kanaras, A.G. Graphene Oxide–Upconversion Nanoparticle Based Portable Sensors for Assessing Nutritional Deficiencies in Crops. ACS Nano 2018, 12, 6273–6279. [Google Scholar] [CrossRef]
- Modlitbová, P.; Hlaváček, A.; Švestková, T.; Pořízka, P.; Šimoníková, L.; Novotný, K.; Kaiser, J. The effects of photon-upconversion nanoparticles on the growth of radish and duckweed: Bioaccumulation, imaging, and spectroscopic studies. Chemosphere 2019, 225, 723–734. [Google Scholar] [CrossRef]
- Popov, A.; Gurkov, A.; Borvinskaya, E.; Sadovoy, A.; Bykov, A.; Timofeyev, M.; Meglinski, I. Eco-photonics: Micro-encapsulated probe as implantable sensor for monitoring the physiological state of water organisms. In Proceedings of the 2018 International Conference Laser Optics (ICLO), St. Petersburg, Russia, 4–8 June 2018; p. 547. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Guo, T.; Zhang, W.; Li, B.; Liu, L.; Hua, R. Green upconversion nanoparticles for 2, 4- dichlorophenoxyacetic acid and fenitrothion detection. J. Alloy. Compd. 2018, 771, 187–194. [Google Scholar] [CrossRef]
- Lin, X.; Yu, Q.; Yang, W.; He, C.; Zhou, Y.; Duan, N.; Wu, S. Double-enzymes-mediated fluorescent assay for sensitive determination of organophosphorus pesticides based on the quenching of upconversion nanoparticles by Fe3+. Food Chem. 2020, 345, 128809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sheng, R.; Wang, P.; Ouyang, Q.; Wang, A.; Ali, S.; Zareef, M.; Hassan, M. Ultra-sensitive detection of malathion residues using FRET-based upconversion fluorescence sensor in food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118654. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, H.; Hassan, M.; Guo, Z.; Zhang, Z.-Z.; Chen, Q. Fabricating an Acetylcholinesterase Modulated UCNPs-Cu2+ Fluorescence Biosensor for Ultrasensitive Detection of Organophosphorus Pesticides-Diazinon in Food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef]
- Rong, Y.; Li, H.; Ouyang, Q.; Ali, S.; Chen, Q. Rapid and sensitive detection of diazinon in food based on the FRET between rare-earth doped upconversion nanoparticles and graphene oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118500. [Google Scholar] [CrossRef]
- Si, F.; Zou, R.; Jiao, S.; Qiao, X.; Guo, Y.; Zhu, G. Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotoxicol. Environ. Saf. 2018, 148, 862–868. [Google Scholar] [CrossRef]
- Sun, N.; Ding, Y.; Tao, Z.; You, H.; Hua, X.; Wang, M. Development of an upconversion fluorescence DNA probe for the detection of acetamiprid by magnetic nanoparticles separation. Food Chem. 2018, 257, 289–294. [Google Scholar] [CrossRef]
- Yu, Q.; He, C.; Li, Q.; Zhou, Y.; Duan, N.; Wu, S. Fluorometric determination of acetamiprid using molecularly imprinted upconversion nanoparticles. Mikrochim. Acta 2020, 187, 222. [Google Scholar] [CrossRef]
- Guo, T.; Wang, C.; Zhou, H.; Zhang, Y.; Ma, L. A multifunctional near-infrared fluorescent sensing material based on core-shell upconversion nanoparticles@magnetic nanoparticles and molecularly imprinted polymers for detection of deltamethrin. Mikrochim. Acta 2021, 188, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, J.; Zhu, W.; Zuo, Y.; Song, Y. Detection of Pyrethroids in Food by Immunofluorescence Enhanced Method Based on Three-Layer Core-Shell Structure Upconversion Materials. Foods 2022, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhao, X.; Yan, X.; Han, X.; Zhu, Z.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; Liu, X.; et al. Background-free sensing platform for on-site detection of carbamate pesticide through upconversion nanoparticles-based hydrogel suit. Biosens. Bioelectron. 2021, 194, 113598. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wu, X.; Hu, S.; Chen, Z.; Yan, H.; Xi, Z.; Yu, Y.; Dai, G.; Liu, Y. Enhanced upconversion luminescence through core/shell structures and its application for detecting organic dyes in opaque fishes. Photochem. Photobiol. Sci. 2016, 15, 260–265. [Google Scholar] [CrossRef]
- Hu, G.; Gao, S.; Han, X.; Yang, L. Comparison of Immunochromatographic Strips Using Colloidal Gold, Quantum Dots, and Upconversion Nanoparticles for Visual Detection of Norfloxacin in Milk Samples. Food Anal. Methods 2020, 13, 1069–1077. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Hassan, M.; Li, H.; Ouyang, Q.; Chen, Q. Fluorescence resonance energy transfer-based aptasensor for sensitive detection of kanamycin in food. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120147. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Tong, L.; Zhong, H.; Xia, H.; Hua, R.; Chen, B. Improved LRET-based detection characters of Cu2+ using sandwich structured NaYF4@NaYF4:Er3+/Yb3+@NaYF4 nanoparticles as energy donor. Sensors Actuators B Chem. 2018, 257, 829–838. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, G.; Wei, Y.; Zhang, L.; Shen, Y. Engineering of an Upconversion Luminescence Sensing Platform Based on the Competition Effect for Mercury-Ion Monitoring in Green Tea. J. Agric. Food Chem. 2021, 69, 8565–8570. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, Z.; Ren, D.; Shang, Y.; Hu, Y.; Yi, L. Electrochemiluminescence sensor based on the target recognition-induced aggregation of sensing units for Hg2+ determination. Sensors Actuators B Chem. 2021, 337, 129821. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, Q.; Li, H.; Zhang, Z.; Chen, Q. Development of an Inner Filter Effects-Based Upconversion Nanoparticles–Curcumin Nanosystem for the Sensitive Sensing of Fluoride Ion. ACS Appl. Mater. Interfaces 2017, 9, 18314–18321. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xia, W.; Gao, Q.; Wang, L. Sensitive quantitative image analysis of bisulfite based on near-infrared upconversion luminescence total internal reflection platform. Talanta 2020, 224, 121928. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Wang, B.; Chen, Q. A turn-on upconversion fluorescence sensor for acrylamide in potato chips based on fluorescence resonance energy transfer and thiol-ene Michael addition. Food Chem. 2021, 351, 129215. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Li, H.; Yin, M.; Liu, H.; Deng, Q.; Wang, S. Upconversion fluorescent nanoparticles based-sensor array for discrimination of the same variety red grape wines. RSC Adv. 2019, 9, 7349–7355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Sheng, W.; Chen, H.; Zhang, B.; Huang, N.; Wang, S. Upconversion Nanoparticles-Based Fluorescence Immunoassay for the Sensitive Detection of 2-Amino-3-methylimidazo [4,5-f] Quinoline (IQ) in Heat Processed Meat. Sensors 2021, 22, 8. [Google Scholar] [CrossRef]
- del Barrio, M.; Cases, R.; Cebolla, V.; Hirsch, T.; de Marcos, S.; Wilhelm, S.; Galbán, J. A reagentless enzymatic fluorescent biosensor for glucose based on upconverting glasses, as excitation source, and chemically modified glucose oxidase. Talanta 2016, 160, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Han, Z.; Yang, L.; Liu, J. Highly-reproducible Raman scattering of NaYF4:Yb,Er@SiO2@Ag for methylamphetamine detection under near-infrared laser excitation. Analyst 2015, 140, 5268–5275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yao, T.; Wang, S.; Feng, R.; Chen, L.; Zhu, V.; Hu, G.; Zhang, H.; Yang, G. Upconversion luminescence nanoparticles-based immunochromatographic assay for quantitative detection of triamcinolone acetonide in cosmetics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 302–308. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Yi, J.; Li, X.; He, F.; Niu, N.; Chen, L. A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications. Biosensors 2022, 12, 1036. https://doi.org/10.3390/bios12111036
Jiang W, Yi J, Li X, He F, Niu N, Chen L. A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications. Biosensors. 2022; 12(11):1036. https://doi.org/10.3390/bios12111036
Chicago/Turabian StyleJiang, Wei, Jiaqi Yi, Xiaoshuang Li, Fei He, Na Niu, and Ligang Chen. 2022. "A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications" Biosensors 12, no. 11: 1036. https://doi.org/10.3390/bios12111036
APA StyleJiang, W., Yi, J., Li, X., He, F., Niu, N., & Chen, L. (2022). A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications. Biosensors, 12(11), 1036. https://doi.org/10.3390/bios12111036






