Sulfonated Starch-Graft-Polyaniline@Graphene Electrically Conductive Nanocomposite: Application for Tyrosinase Immobilization
Abstract
:1. Introduction
2. Material and Approaches
2.1. Materials
2.2. Apparatus
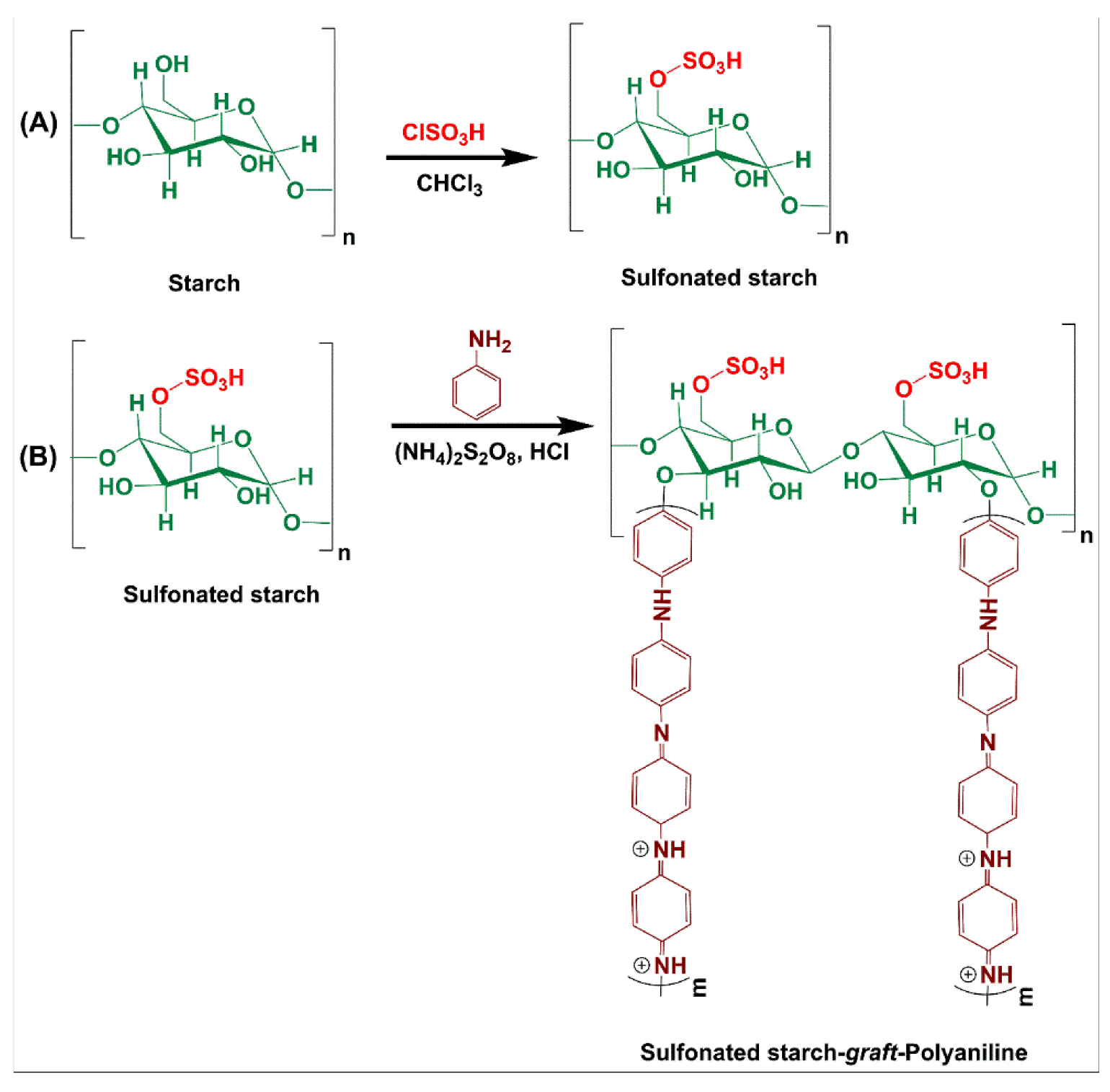
2.3. Sulfonated Starch Preparation
2.4. Preparation of Starch-Graft-Polyaniline (SSt-g-PANI)
- (1)
- G% = (W1 − W0)/W0 × 100
- (2)
- E% = (W1 − W0)/W2 × 100
2.5. Fabrication of SSt-Graft-Polyaniline@Graphene (SSt-g-PANI@G) Nanocomposite
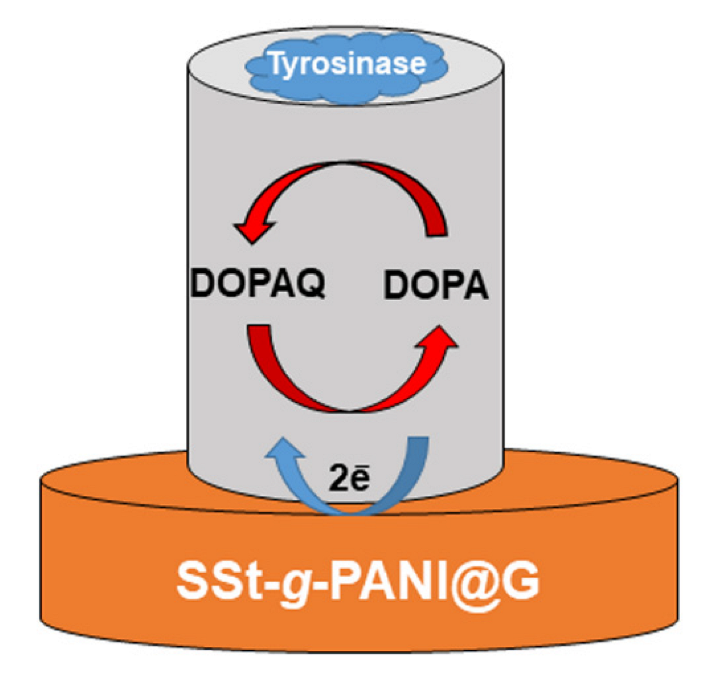
2.6. Preparation of Modified Electrode and Enzyme Immobilization
3. Results
3.1. Characterization of Nanocomposite
3.2. Electrochemical Behaviors of SSt-g-PANI@G/Tyase/GCE
3.2.1. pH Effect
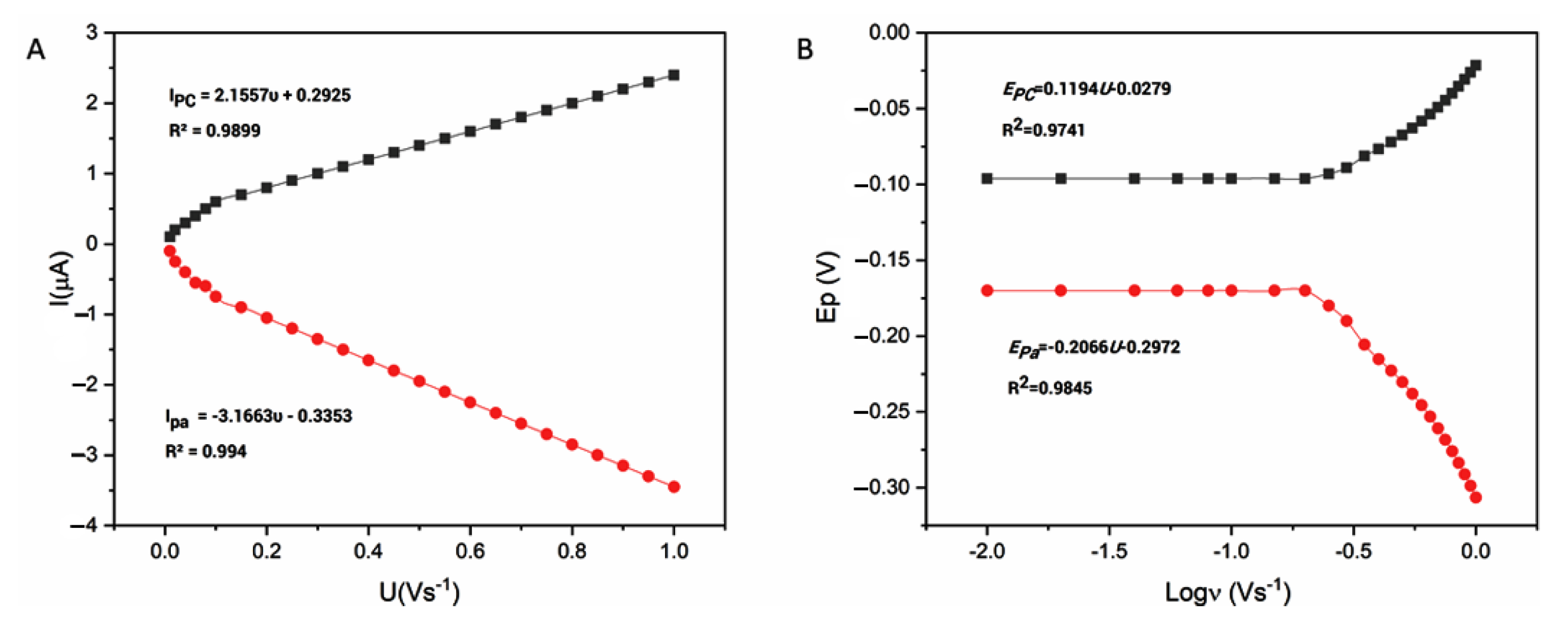
3.2.2. Scan Rate Effect
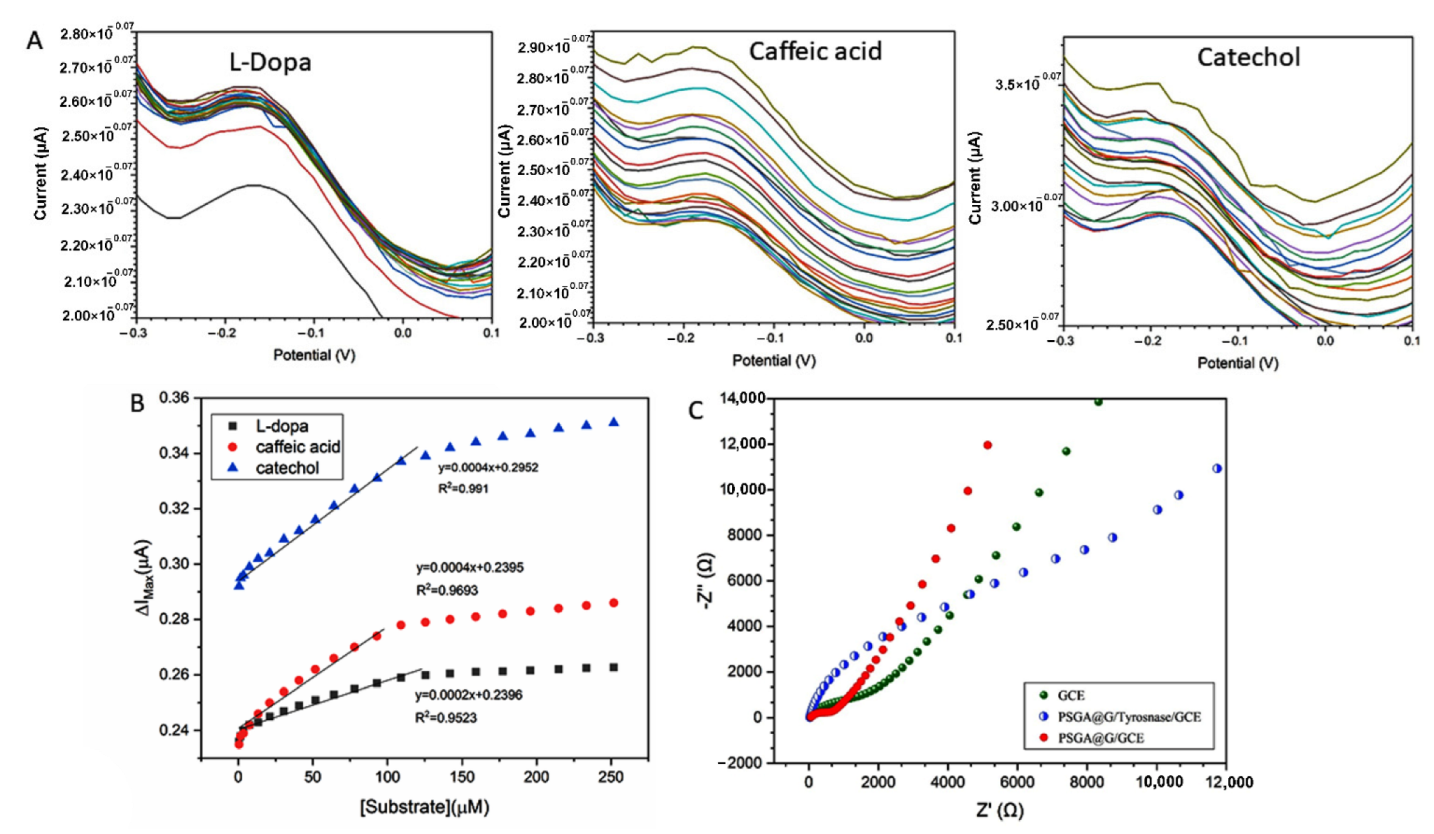
3.2.3. Catalytic Activity
3.2.4. Electrochemical Impedance Spectroscopy
3.2.5. The Temporal Stability of Sensor Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Khorsandi, D.; Zarepour, A.; Ghomi, M.; Fahimipour, A.; Tavakkoliamol, Z.; Zarrabi, A. Ionic Liquid-based Sensors. Mater. Chem. Horizons 2022, 1, 123–135. [Google Scholar]
- Ye, Q.; Zhang, Z.; Liu, J.; Wang, X. Screen printed electrodes-based biosensors modified with functional nucleic acid probes and their applications in this pandemic age: A review. Anal. Methods 2022, 14, 2961–2975. [Google Scholar] [CrossRef]
- Sohrabi, H.; Arbabzadeh, O.; Khaaki, P.; Khataee, A.; Majidi, M.R.; Orooji, Y. Patulin and Trichothecene: Characteristics, occurrence, toxic effects and detection capabilities via clinical, analytical and nanostructured electrochemical sensing/biosensing assays in foodstuffs. Crit. Rev. Food Sci. Nutr. 2022, 62, 5540–5568. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Yorganci, E.; Asav, E. Do copper ions activate tyrosinase enzyme? A biosensor model for the solution. Bioelectrochemistry 2010, 78, 155–160. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Immobilization of tyrosinase on modified diatom biosilica: Enzymatic removal of phenolic compounds from aqueous solution. J. Hazard. Mater. 2013, 244, 528–536. [Google Scholar] [CrossRef]
- Rai, R.K.; Karri, R.; Dubey, K.D.; Roy, G. Regulation of Tyrosinase Enzyme Activity by Glutathione Peroxidase Mimics. J. Agric. Food Chem. 2022, 70, 9730–9747. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Baghayeri, M. Electro-magnetic polyfuran/Fe3O4 nanocomposite: Synthesis, characterization, antioxidant activity and its application as a biosensor. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 175–183. [Google Scholar] [CrossRef]
- Kyomihumbo, H.; Feleni, U. Electroconductive green metal-polyaniline nanocomposites: Synthesis and application in sensors. Electroanalysis 2022, in press. [Google Scholar] [CrossRef]
- Nodehi, M.; Kiasadr, A.; Babaee Bachevanlo, G. Modified Glassy Carbon Electrode with Mesoporous Silica-Metformin/Multi-Walled Carbon Nanotubes as a Biosensor for Ethinylestradiol Detection. Mater. Chem. Horizons 2022, in press. [Google Scholar] [CrossRef]
- Kargirwar, S.R.; Thakare, S.R.; Choudhary, M.D.; Kondawar, S.B.; Dhakate, S.R. Morphology and electrical conductivity of self-doping polyanilines synthesized via self-assembly process. Adv. Mater. Lett. 2011, 2, 397–401. [Google Scholar] [CrossRef]
- Ponnamma, D.; Yin, Y.; Salim, N.; Parameswaranpillai, J.; Thomas, S.; Hameed, N. Recent progress and multifunctional applications of 3D printed graphene nanocomposites. Compos. Part B Eng. 2021, 204, 108493. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2020, 33, 2001105. [Google Scholar] [CrossRef]
- Bilisik, K.; Akter, M. Graphene nanocomposites: A review on processes, properties, and applications. J. Ind. Text. 2022, 51, 3718S–3766S. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Cellulose sulfuric acid (CSA) and starch sulfuric acid (SSA) as solid and heterogeneous catalysts in green organic synthesis: Recent advances. Ark. Online J. Org. Chem. 2015, 2015, 136–159. [Google Scholar] [CrossRef]
- Han, R.; Li, Y.; Chen, M.; Li, W.; Ding, C.; Luo, X. Antifouling Electrochemical Biosensor Based on the Designed Functional Peptide and the Electrodeposited Conducting Polymer for CTC Analysis in Human Blood. Anal. Chem. 2022, 94, 2204–2211. [Google Scholar] [CrossRef]
- Jo, H.J.; Ryu, J.S.; Robby, A.I.; Kim, Y.S.; Chung, H.J.; Park, S.Y. Rapid and Selective Electrochemical Sensing of Bacterial Pneumonia in Human Sputum Based On Conductive Polymer Dot Electrodes. Sensors Actuators B Chem. 2022, 368, 132084. [Google Scholar] [CrossRef]
- Gholami, M.D.; O’mullane, A.P.; Sonar, P.; Ayoko, G.A.; Izake, E.L. Antibody coated conductive polymer for the electrochemical immunosensing of Human Cardiac Troponin I in blood plasma. Anal. Chim. Acta 2021, 1185, 339082. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S. Ultrasonic-assisted fabrication of starch/MWCNT-glucose nanocomposites for drug delivery. Ultrason. Sonochem. 2018, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.K.; Pradhan, A.K.; Sahu, H.S. Synthesis of gas barrier starch by dispersion of functionalized multiwalled carbon nanotubes. Carbohydr. Polym. 2013, 94, 663–668. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.; Hashim, M.A. Functionalization of graphene using deep eutectic solvents. Nanoscale Res. Lett. 2015, 10, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Tanrıverdi, E.E.; Uzumcu, A.T.; Kavas, H.; Demir, A.; Baykal, A. Conductivity study of polyaniline-cobalt ferrite (PANI-CoFe2O4) nanocomposite. Nano Micro Lett. 2011, 3, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tang, G.-S.; Yu, Z.-Z.; Qi, J.-S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radicals and electrical exfoliation in different electrolyte systems. RSC Adv. 2019, 9, 3693–3703. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.-Q.; Wang, X.-X.; Yuan, J.; Wang, W.-Z.; Cao, M.-S. Temperature dependent microwave absorption of ultrathin graphene composites. J. Mater. Chem. C 2015, 3, 10017–10022. [Google Scholar] [CrossRef]
- Boomi, P.; Poorani, G.P.; Palanisamy, S.; Selvam, S.; Ramanathan, G.; Ravikumar, S.; Barabadi, H.; Prabu, H.G.; Jeyakanthan, J.; Saravanan, M. Evaluation of antibacterial and anticancer potential of polyaniline-bimetal nanocomposites synthesized from chemical reduction method. J. Clust. Sci. 2019, 30, 715–726. [Google Scholar] [CrossRef]
- Naderizadeh, S.; Shakeri, A.; Mahdavi, H.; Nikfarjam, N.; Taheri Qazvini, N. Hybrid Nanocomposite Films of Starch, Poly (vinyl alcohol)(PVA), Starch Nanocrystals (SNCs), and Montmorillonite (Na-MMT): Structure–Properties Relationship. Starch-Stärke 2019, 71, 1800027. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Aravind, S.S.J.; Wu, H.; Forys, J.; Venkataraman, V.; Ramanujachary, K.; Hu, X. Tunable green graphene-silk biomaterials: Mechanism of protein-based nanocomposites. Mater. Sci. Eng. C 2017, 79, 728–739. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Swaminathan, M.; Houas, A. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. 2017, 60, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; McMahill, P.; Mason, H.S.; Moss, T.H. The oxidation state of copper in resting tyrosinase. J. Biol. Chem. 1974, 249, 6062–6066. [Google Scholar] [CrossRef]
- Dwivedi, P.; Singh, R.; Chauhan, Y.S. Crossing the Nernst limit (59 mV/pH) of sensitivity through tunneling transistor-based biosensor. IEEE Sens. J. 2020, 21, 3233–3240. [Google Scholar] [CrossRef]
- Kano, K.; Konse, T.; Uno, B.; Kubota, T. Electrochemical Study of the Mechanism and Kinetics of Oxygen Reduction Mediated by Anthracycline Antibiotics Adsorbed on Electrode Surface. In Redox Chemistry and Interfacial Behavior of Biological Molecules; Springer: Berlin/Heidelberg, Germany, 1988; pp. 267–280. [Google Scholar]
- Bond, A.M. Modern Polarographic Methods in Analytical Chemistry; CRC Press: Boca Raton, FL, USA, 2020; ISBN 1000110451. [Google Scholar]
- Laviron, E.J.J. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Faridnouri, H.; Ghourchian, H.; Hashemnia, S. Direct electron transfer enhancement of covalently bound tyrosinase to glassy carbon via Woodward’s reagent K. Bioelectrochemistry 2011, 82, 1–9. [Google Scholar] [CrossRef]
- VanArsdale, E.; Hörnström, D.; Sjöberg, G.; Järbur, I.; Pitzer, J.; Payne, G.F.; van Maris, A.J.A.; Bentley, W.E. A coculture based tyrosine-tyrosinase electrochemical gene circuit for connecting cellular communication with electronic networks. ACS Synth. Biol. 2020, 9, 1117–1128. [Google Scholar] [CrossRef]
- Sýs, M.; Vytřas, K. Tyrosinase electrochemical biosensors monitoring medicinally significant substances. Curr. Med. Chem. 2018, 25, 3988–4006. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Karve, M.; Zinjarde, S. Selective electrochemical sensing of bisphenol derivatives using novel bioelectrode of agarose-guar gum-graphene oxide immobilized with tyrosinase. J. Environ. Chem. Eng. 2022, 10, 107360. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kane, B.J.; Khanwalker, M.; Sode, K. Development of an electrochemical impedance spectroscopy based biosensor for detection of ubiquitin C-Terminal hydrolase L1. Biosens. Bioelectron. 2022, 208, 114232. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, I.K.; Yeganeh, M.; Shoushtari, M.T.; Zadeh, M.K. Electrochemical Impedance Spectroscopy (EIS) for Biosensing. In Nanosensors for Smart Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 533–554. [Google Scholar]
- Raoof, J.-B.; Ojani, R.; Baghayeri, M. Simultaneous electrochemical determination of glutathione and tryptophan on a nano-TiO2/ferrocene carboxylic acid modified carbon paste electrode. Sens. Actuators B Chem. 2009, 143, 261–269. [Google Scholar] [CrossRef]


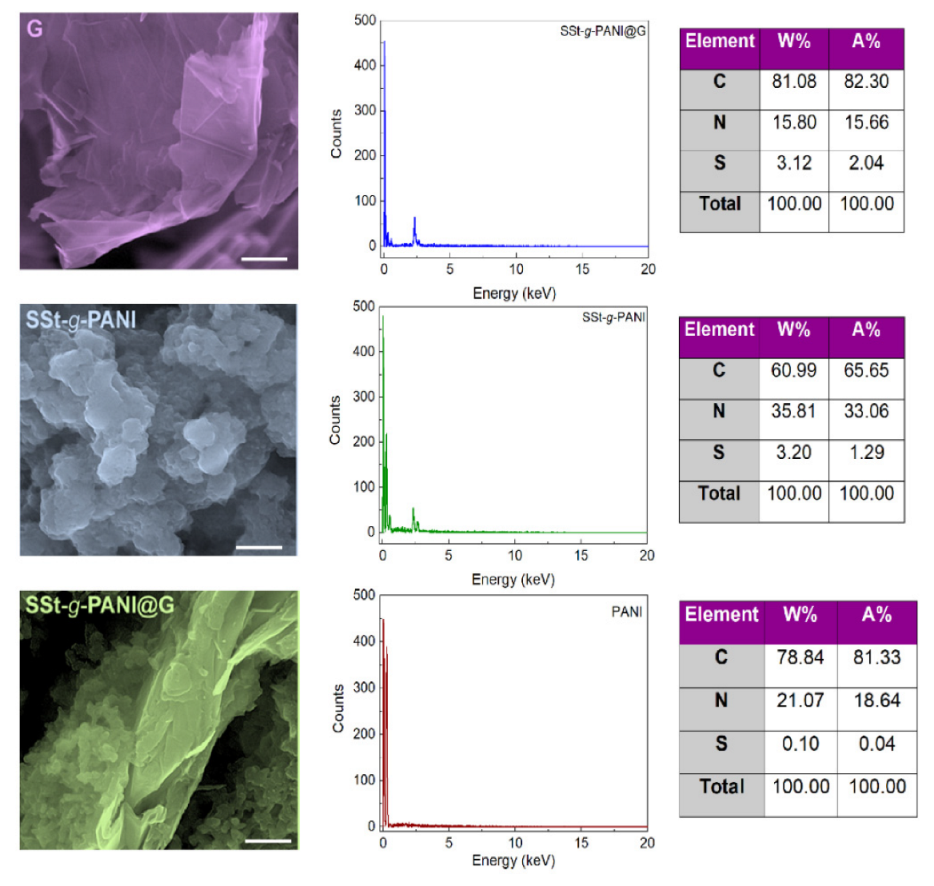

| Sample | %C | %O | %H | %N | %S |
|---|---|---|---|---|---|
| SSt | 58.71 | 31.04 | 8.14 | - | 2.11 |
| SSt-g-PANI | 46.40 | 38.60 | 6.04 | 5.78 | 3.18 |
| SSt-g-PANI@G | 60.82 | 21.94 | 6.46 | 7.68 | 3.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliya, M.; Zare, E.N.; Faridnouri, H.; Ghomi, M.; Makvandi, P. Sulfonated Starch-Graft-Polyaniline@Graphene Electrically Conductive Nanocomposite: Application for Tyrosinase Immobilization. Biosensors 2022, 12, 939. https://doi.org/10.3390/bios12110939
Aliya M, Zare EN, Faridnouri H, Ghomi M, Makvandi P. Sulfonated Starch-Graft-Polyaniline@Graphene Electrically Conductive Nanocomposite: Application for Tyrosinase Immobilization. Biosensors. 2022; 12(11):939. https://doi.org/10.3390/bios12110939
Chicago/Turabian StyleAliya, Marzieh, Ehsan Nazarzadeh Zare, Hassan Faridnouri, Matineh Ghomi, and Pooyan Makvandi. 2022. "Sulfonated Starch-Graft-Polyaniline@Graphene Electrically Conductive Nanocomposite: Application for Tyrosinase Immobilization" Biosensors 12, no. 11: 939. https://doi.org/10.3390/bios12110939
APA StyleAliya, M., Zare, E. N., Faridnouri, H., Ghomi, M., & Makvandi, P. (2022). Sulfonated Starch-Graft-Polyaniline@Graphene Electrically Conductive Nanocomposite: Application for Tyrosinase Immobilization. Biosensors, 12(11), 939. https://doi.org/10.3390/bios12110939







