Thin-Film-Based Multifunctional System for Optical Detection and Thermal Treatment of Biological Samples
Abstract
:1. Introduction
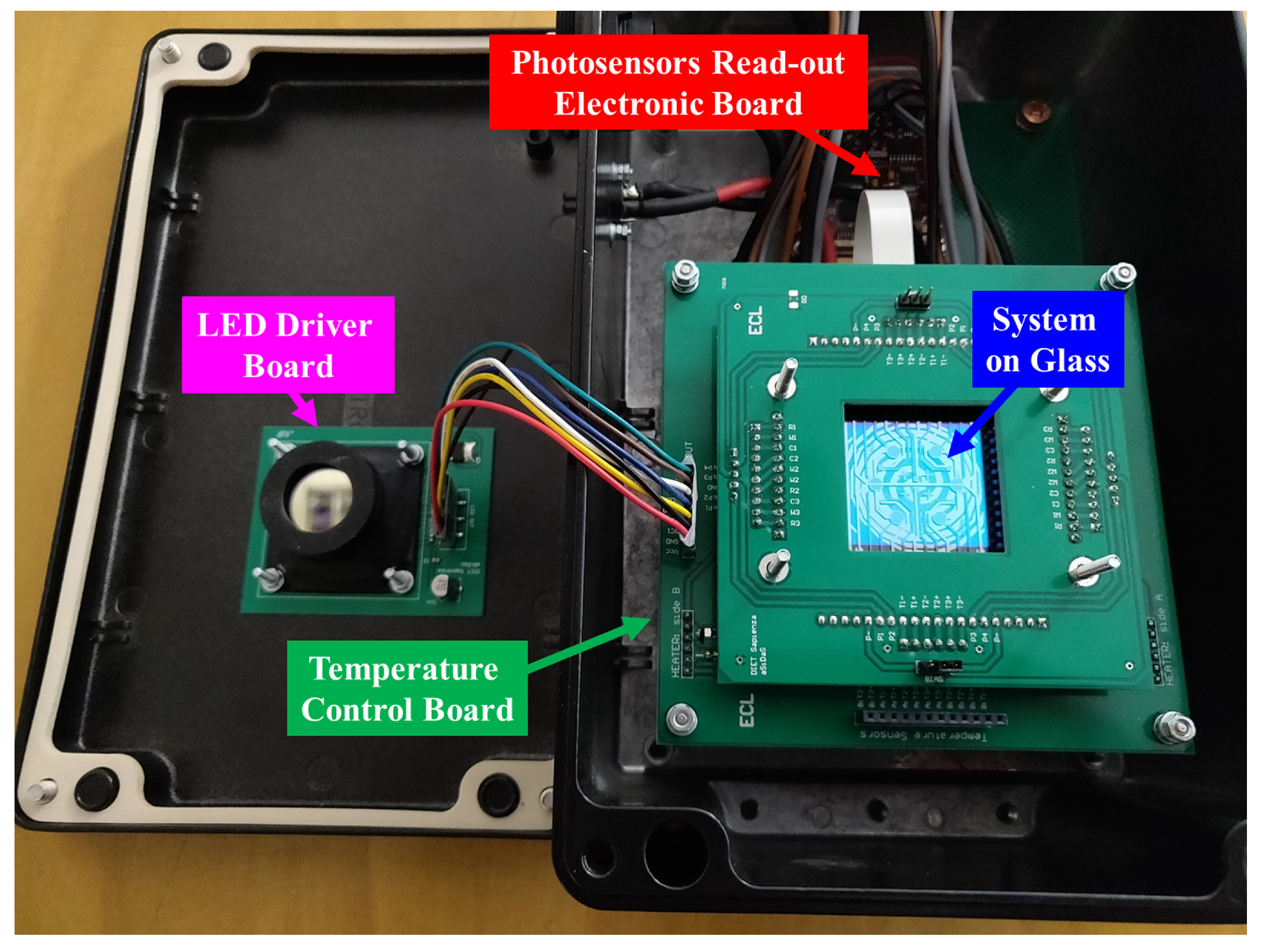
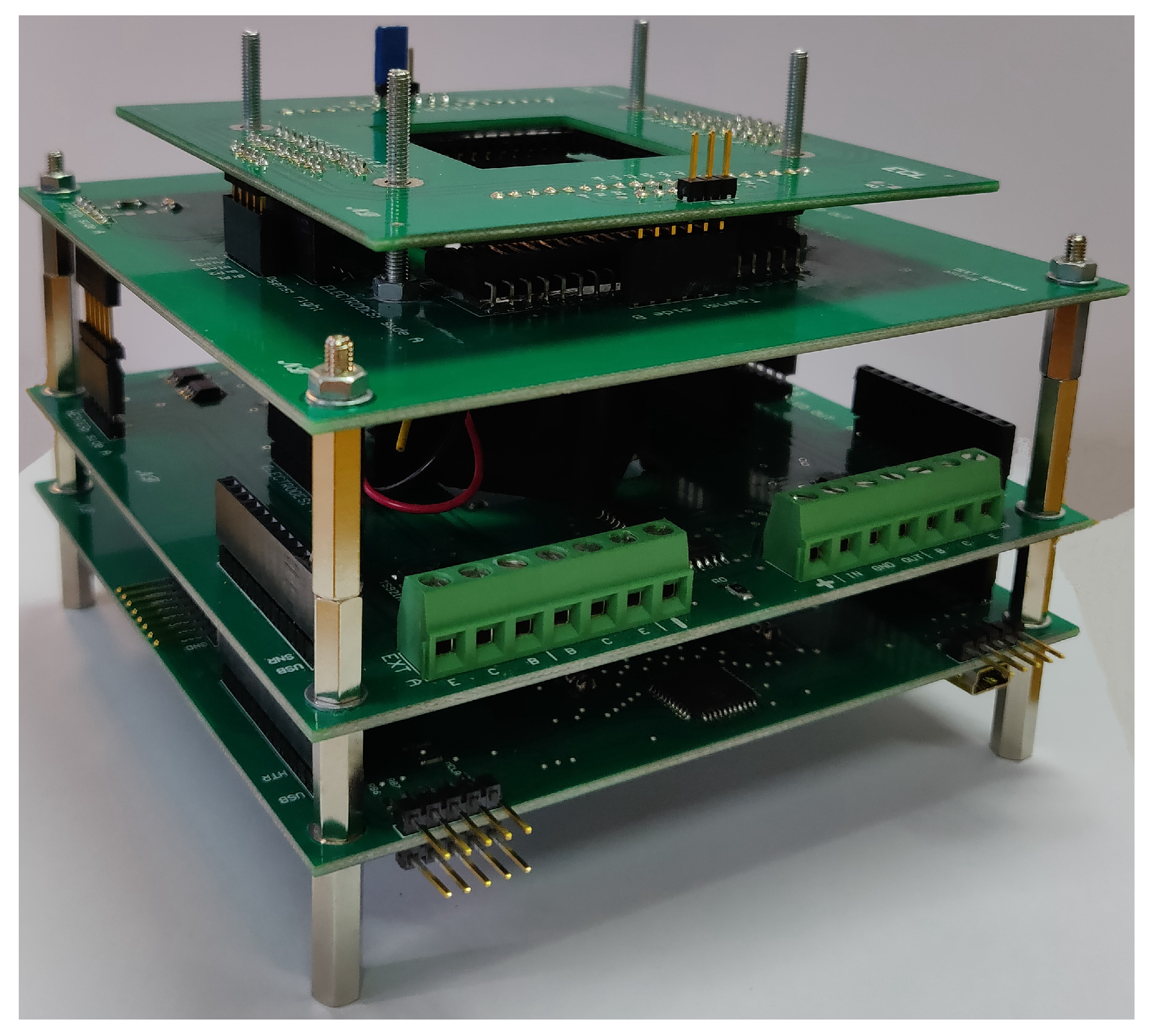
2. System Structure and Operation
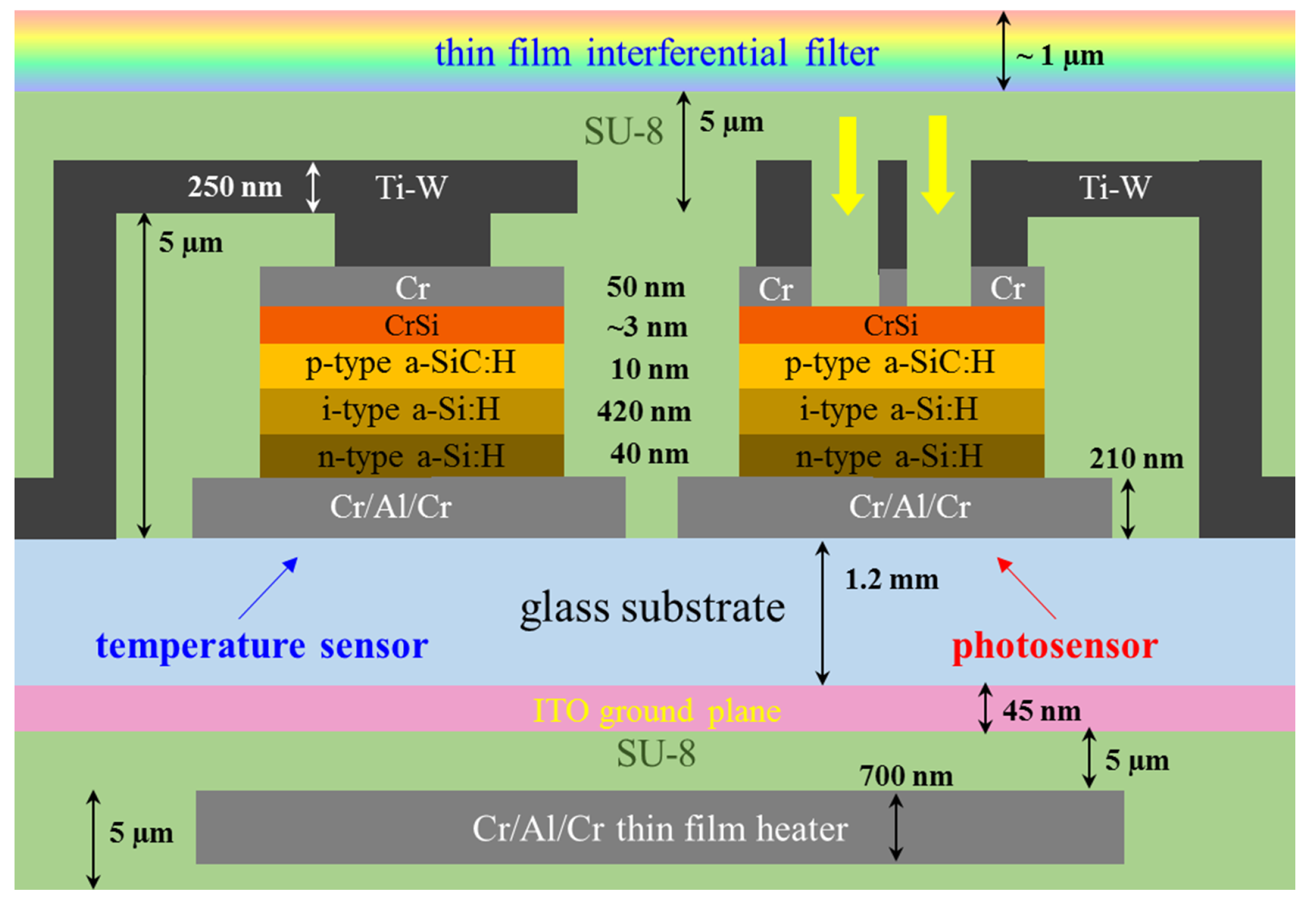
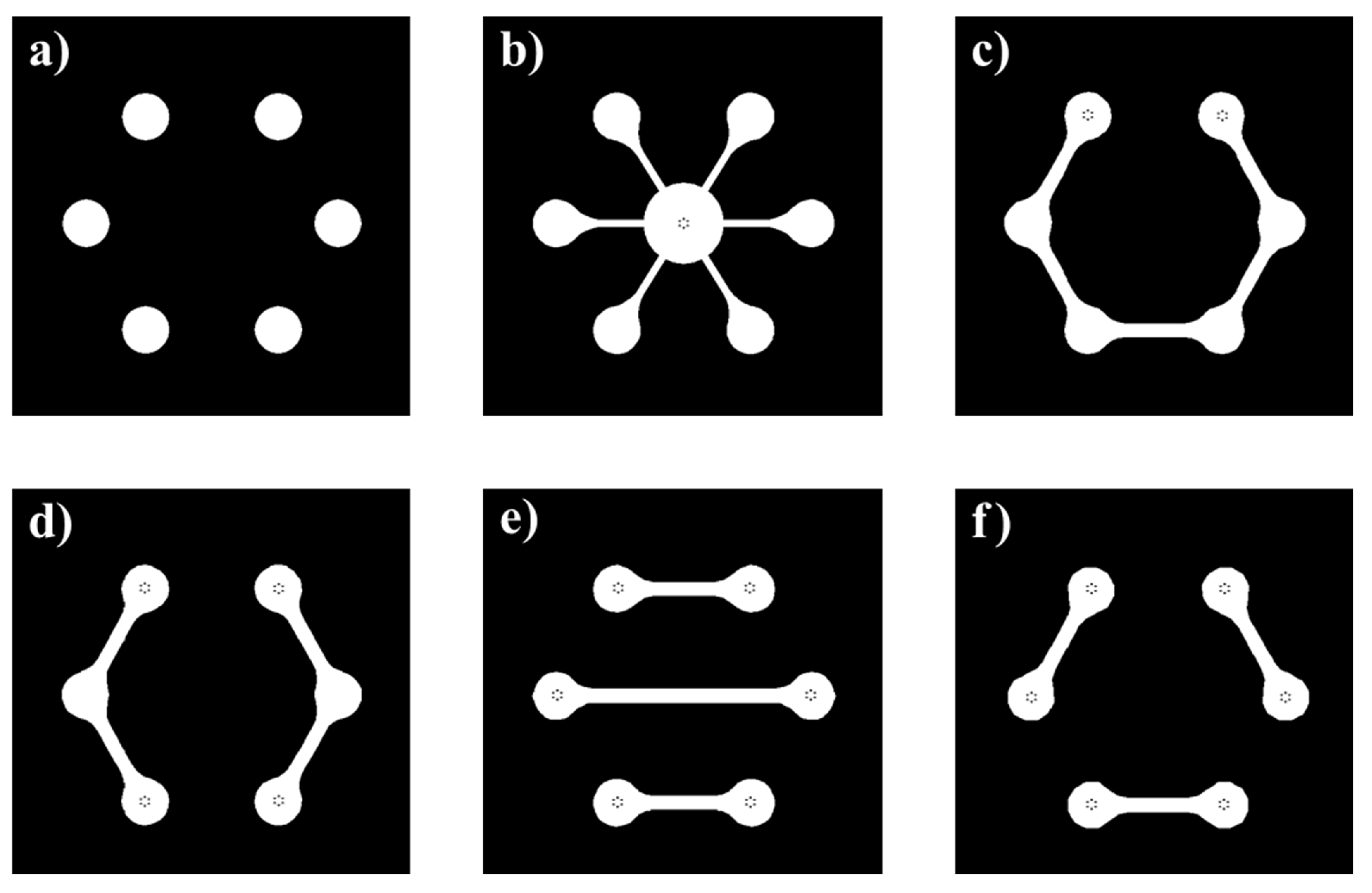
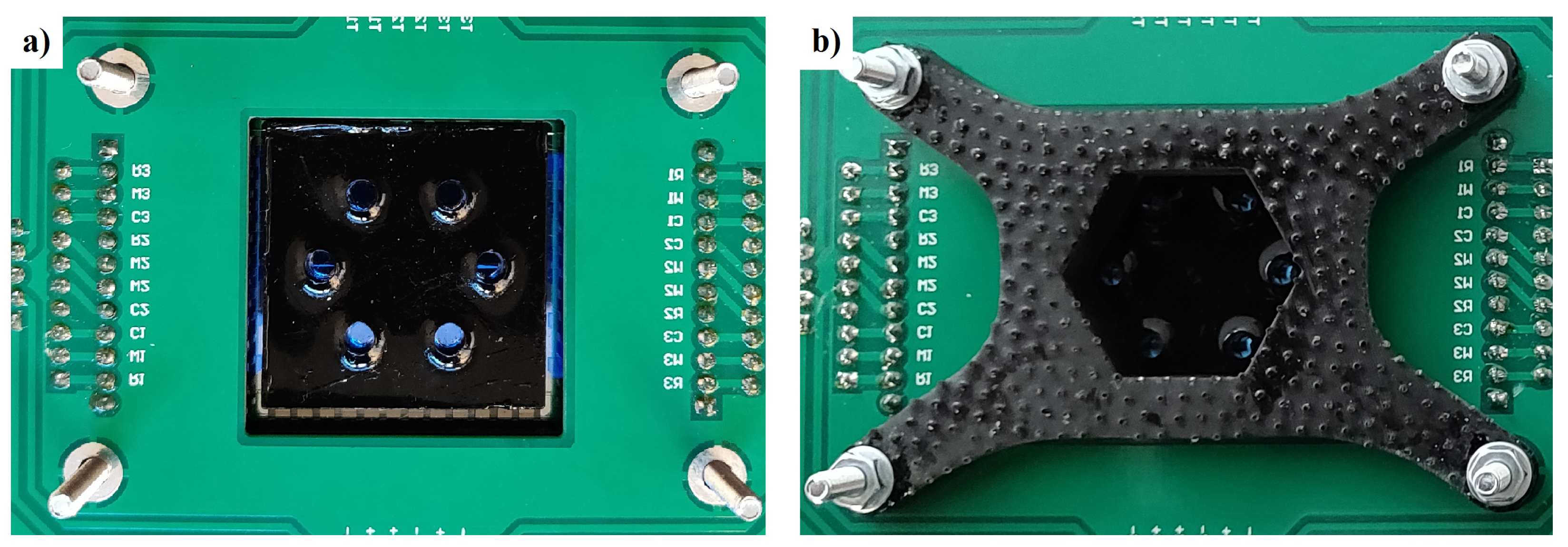
2.1. System-on-Glass
- 1.
- ultrasonic cleaning of the glass substrate;
- 2.
- sensors side:
- (a)
- deposition of a sacrificial protection layer of a 400 nm-thick titanium-tungsten (Ti-W) alloy in a three-targets Material Research Corporation RF magnetron sputtering system;
- 3.
- heater side:
- (a)
- deposition of a 45 nm-thick indium-tin oxide (ITO) layer acting as a ground plane through the same RF magnetron sputtering system;
- (b)
- deposition of the insulation layer of a 5 µm-thick SU-8 3005 film by spin coating and its patterning to expose the contacts to connect the ground plane;
- (c)
- deposition, by vacuum evaporation, of a chromium (Cr)/aluminum (Al)/ chromium (30/600/70 nm) stacked layer by means of a Balzers 510 evaporation system and its patterning to define the thin film heater by means of standard optical lithography and wet etching processes;
- (d)
- definition of a passivation layer through the spin coating of a 5 µm-thick SU-8 3005;
- 4.
- sensors side:
- (a)
- removal of the Ti-W sacrificial protection layer;
- (b)
- deposition of a Cr/Al/Cr (30/150/30 nm) stacked layer and its patterning to define the sensors bottom contacts;
- (c)
- deposition of the a-Si:H structure by means of a Glasstech Solar Incorporation three-chamber ultra-high vacuum PECVD system;
- (d)
- evaporation of a 50 nm-thick Cr layer, acting as the sensors top contact;
- (e)
- Cr wet etching and a-Si:H dry etching in Ionvac Reactive Ion Etching (RIE) system for the mesa patterning of the diodes;
- (f)
- deposition of the insulation layer by spin coating of a 5 µm-thick SU-8 3005 film and its patterning for the opening of the via holes;
- (g)
- sensors side: deposition of a 250 nm-thick Ti-W alloy and its patterning for the definition of the top contact and the connection to the pads, located on the glass edge;
- (h)
- definition of a passivation layer through the spin coating of a 5 µm-thick SU-8 3005 film;
- (i)
- optional deposition of a multilayer stacked structure of titanium oxide (TiO2)/ silicon oxide (SiO2) dielectric layers acting as thin film interferential filter.
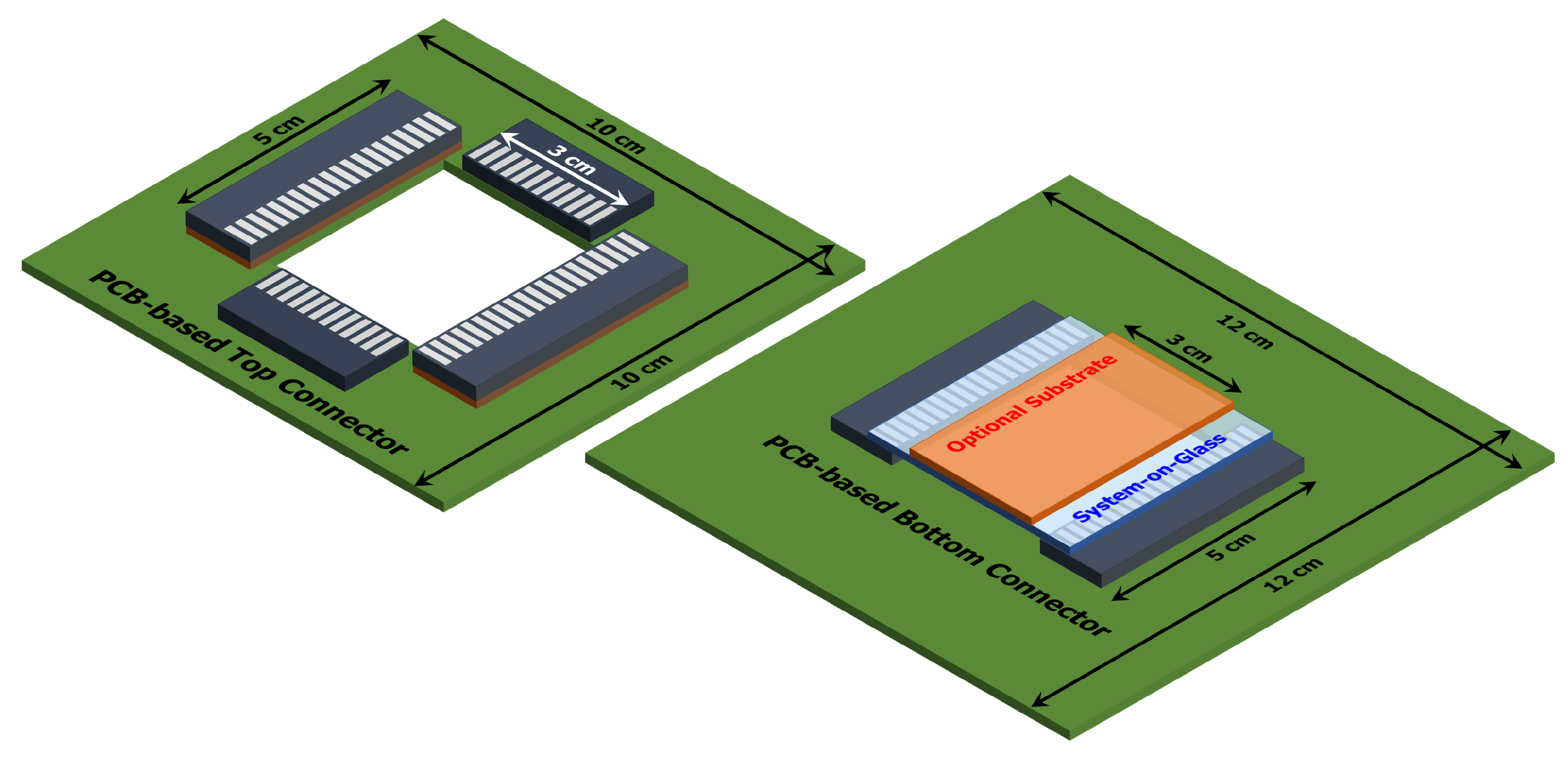
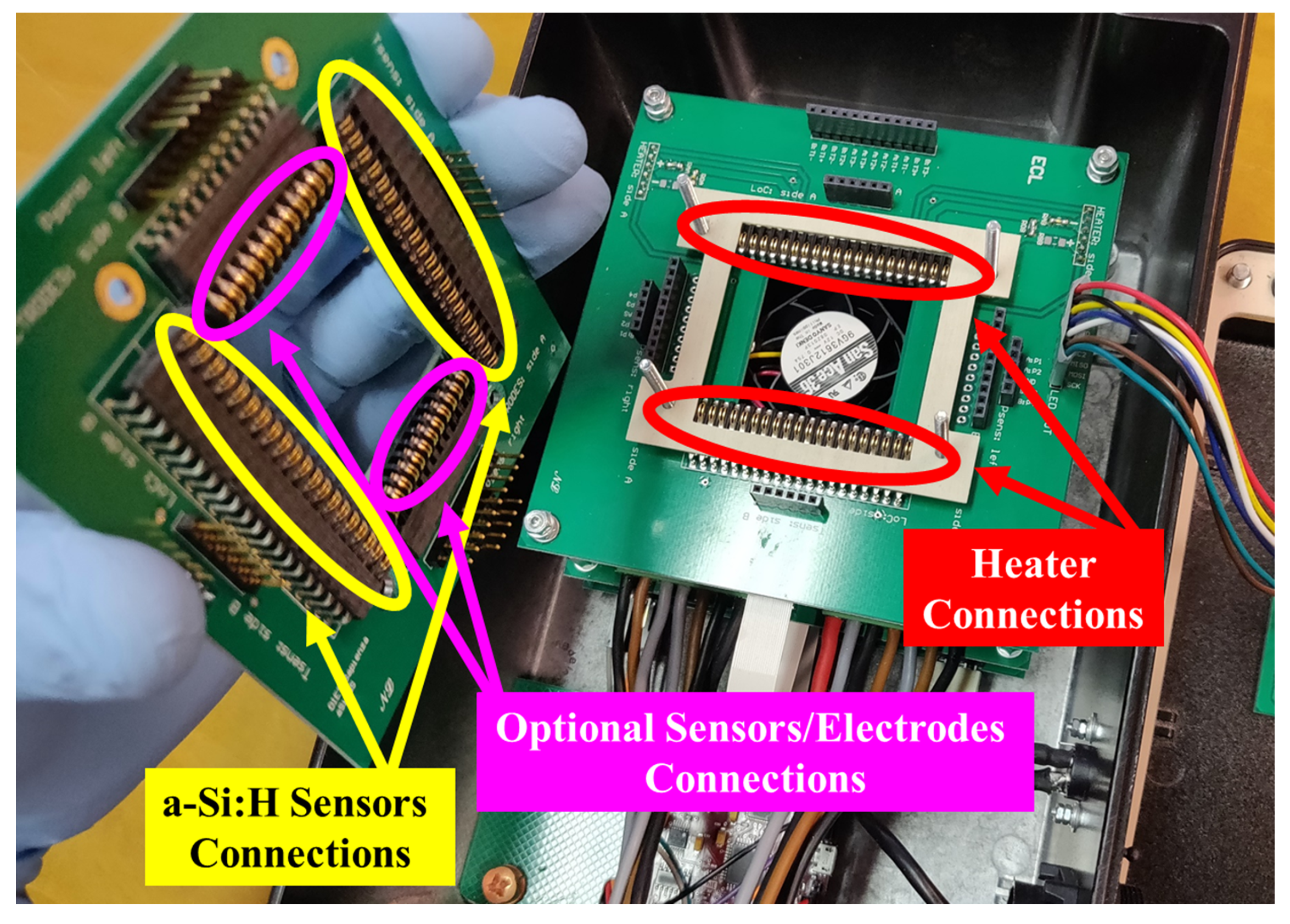
2.2. SoG Connection System
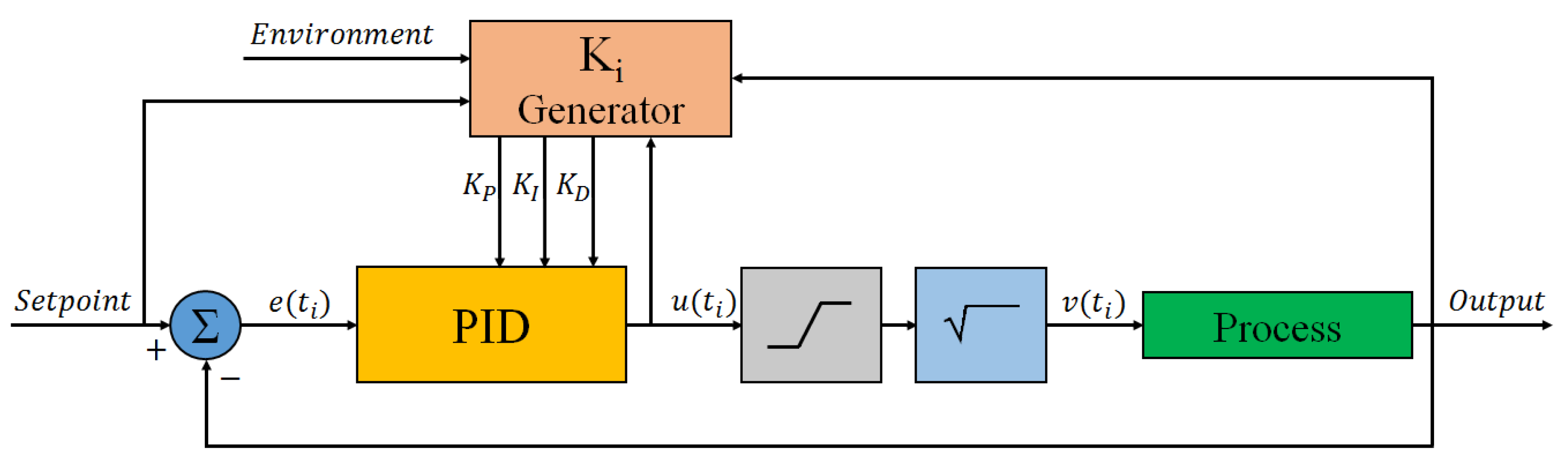
2.3. Thermal Treatment Module
- a power circuit that drives the heater with the current required to achieve the desired temperature;
- a power circuit that drives a fan used to enhance the cooling of the LoC if it is required by the specific application;
- an electronic circuit that biases the temperature sensor at a constant current and measures the voltage across the diode junction.
2.4. Optical Detection Module
2.5. Microfluidics
3. Evaluation of System Performances
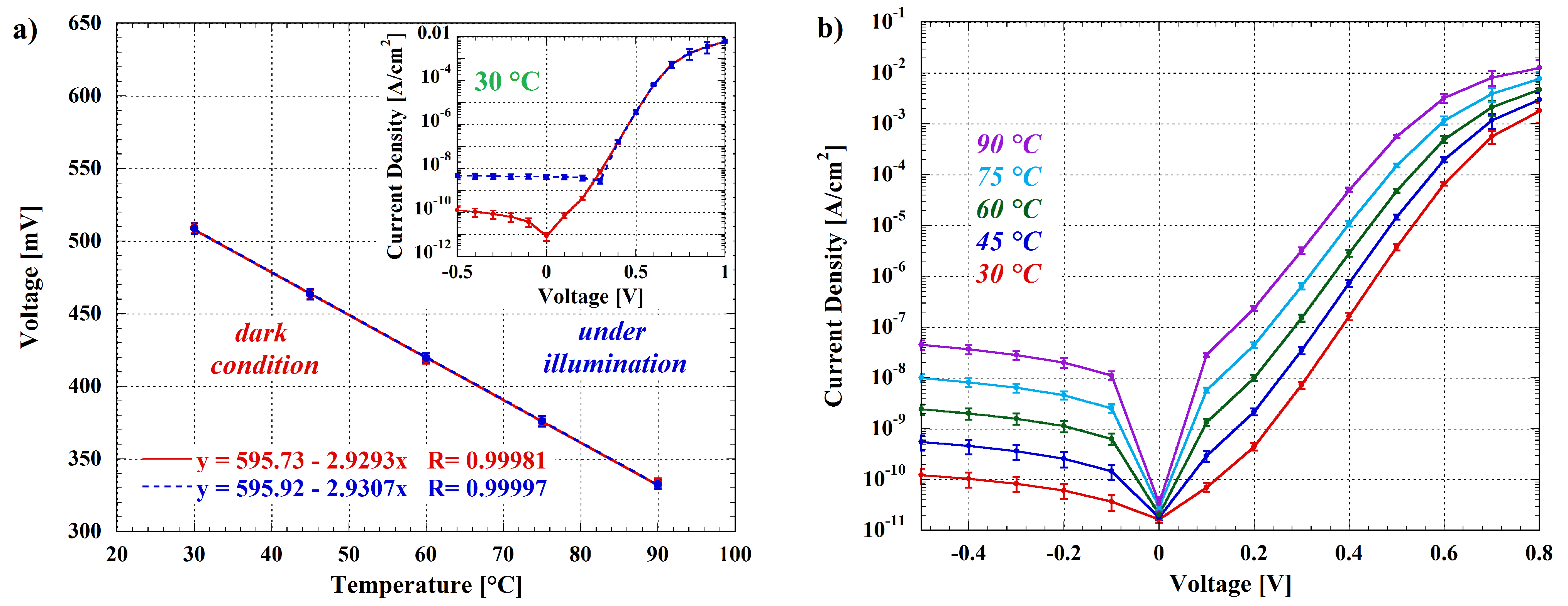
3.1. Room-Temperature Luminescence Detection
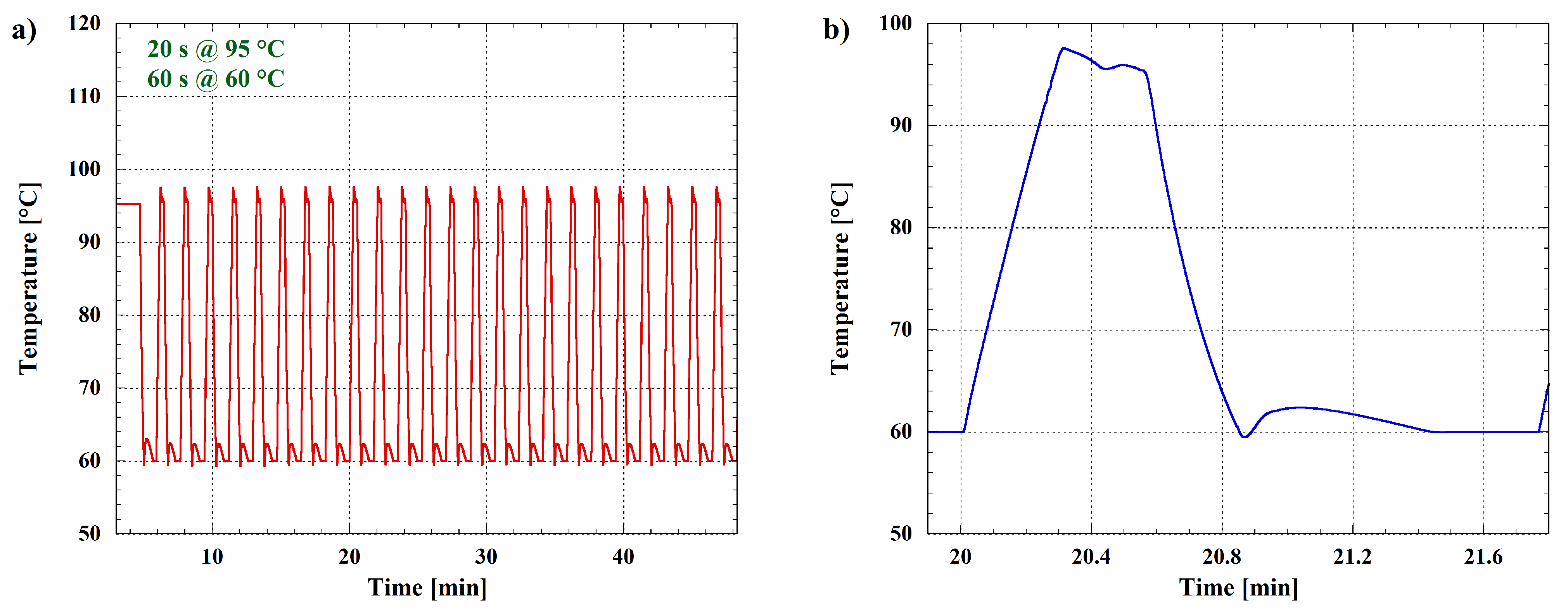
3.2. Luminescence Detection under Thermal Treatment of the Sample
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a-Si:H | hydrogenated amorphous silicon |
| Al | aluminum |
| BART | Bioluminescent Assay in Real Time |
| CL | chemiluminescence |
| COC | Cyclic Olefin Copolymer |
| Cr | chromium |
| ECL | electrochemiluminescence |
| GUI | Graphical User Interface |
| HDA | helicase-dependent amplification |
| ITO | indium-tin oxide |
| JV | current density–voltage |
| LAMP | loop-mediated isothermal amplification |
| LED | light-emitting diode |
| LoC | Lab-on-Chip |
| MDA | multiple displacement amplification |
| PCB | printed circuit board |
| PCR | polymerase chain reaction |
| PDMS | Polydimethylsiloxane |
| PECVD | Plasma-Enhanced Chemical Vapor Deposition |
| PID | proportional–integral–derivative |
| RCA | rolling-circle amplification |
| RIE | Reactive Ion Etching |
| SDA | strand-displacement amplification |
| SiO2 | silicon oxide |
| SoG | System-on-Glass |
| TCL | thermochemiluminescence |
| Ti-W | titanium-tungsten |
| TiO2 | titanium oxide |
| UV | ultraviolet |
| VT | voltage–temperature |
| WGA | whole-genome amplification |
References
- Abgrall, P.; Gue, A. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef] [Green Version]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrucci, G.; Caputo, D.; Lovecchio, N.; Costantini, F.; Legnini, I.; Bozzoni, I.; Nascetti, A.; De Cesare, G. Multifunctional system-on-glass for lab-on-chip applications. Biosens. Bioelectron. 2017, 93, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-chip technology for chronic disease diagnosis. Npj Digit. Med. 2018, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinton-Bailey, G.S.; Grand, M.M.; Beaton, A.D.; Nightingale, A.M.; Owsianka, D.R.; Slavik, G.J.; Connelly, D.P.; Cardwell, C.L.; Mowlem, M.C. A lab-on-chip analyzer for in situ measurement of soluble reactive phosphate: Improved phosphate blue assay and application to fluvial monitoring. Environ. Sci. Technol. 2017, 51, 9989–9995. [Google Scholar] [CrossRef]
- Malara, P.; Crescitelli, A.; Di Meo, V.; Giorgini, A.; Avino, S.; Esposito, E.; Ricciardi, A.; Cusano, A.; Rendina, I.; De Natale, P.; et al. Resonant enhancement of plasmonic nanostructured fiber optic sensors. Sens. Actuators B Chem. 2018, 273, 1587–1592. [Google Scholar] [CrossRef]
- Dhar, B.C.; Lee, N.Y. Lab-on-a-chip technology for environmental monitoring of microorganisms. BioChip J. 2018, 12, 173–183. [Google Scholar] [CrossRef]
- Patel, S.; Nanda, R.; Sahoo, S.; Mohapatra, E. Biosensors in health care: The milestones achieved in their development towards lab-on-chip-analysis. Biochem. Res. Int. 2016, 2016, 3130469. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Poltronieri, P. Fluorescence-free biosensor methods in detection of food pathogens with a special focus on Listeria monocytogenes. Biosensors 2017, 7, 63. [Google Scholar] [CrossRef]
- Iemmolo, R.; La Cognata, V.; Morello, G.; Guarnaccia, M.; Arbitrio, M.; Alessi, E.; Cavallaro, S. Development of a Pharmacogenetic Lab-on-Chip Assay Based on the In-Check Technology to Screen for Genetic Variations Associated to Adverse Drug Reactions to Common Chemotherapeutic Agents. Biosensors 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, V.; Moccia, M.; Sanità, G.; Crescitelli, A.; Lamberti, A.; Galdi, V.; Rendina, I.; Esposito, E. Advanced DNA Detection via Multispectral Plasmonic Metasurfaces. Front. Bioeng. Biotechnol. 2021, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Manessis, G.; Gelasakis, A.I.; Bossis, I. Point-of-Care Diagnostics for Farm Animal Diseases: From Biosensors to Integrated Lab-On-Chip Devices. Biosensors 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluidics 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Erickson, D.; O’Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef] [Green Version]
- Ter Schiphorst, J.; Saez, J.; Diamond, D.; Benito-Lopez, F.; Schenning, A.P. Light-responsive polymers for microfluidic applications. Lab Chip 2018, 18, 699–709. [Google Scholar] [CrossRef]
- Podbiel, D.; Laermer, F.; Zengerle, R.; Hoffmann, J. Fusing MEMS technology with lab-on-chip: Nanoliter-scale silicon microcavity arrays for digital DNA quantification and multiplex testing. Microsystems Nanoeng. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Wartmann, D.; Rothbauer, M.; Kuten, O.; Barresi, C.; Visus, C.; Felzmann, T.; Ertl, P. Automated, miniaturized, and integrated quality control-on-chip (QC-on-a-chip) for cell-based cancer therapy applications. Front. Mater. 2015, 2, 60. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.P.; Madaboosi, N.; Soares, R.R.; Fernandes, J.T.S.; Novo, P.; Moulas, G.; Chu, V. Lab-on-chip systems for integrated bioanalyses. Essays Biochem. 2016, 60, 121–131. [Google Scholar]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating biosensors in organs-on-chip devices: A perspective on current strategies to monitor microphysiological systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef]
- Sano, T.; Zhang, H.; Losakul, R.; Schmidt, H. All-In-One Optofluidic Chip for Molecular Biosensing Assays. Biosensors 2022, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Chittick, R.; Alexander, J.; Sterling, H. The preparation and properties of amorphous silicon. J. Electrochem. Soc. 1969, 116, 77. [Google Scholar] [CrossRef]
- Street, R. Technology and Applications of Amorphous Silicon; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume 37. [Google Scholar]
- Treacy, M.; Borisenko, K. The local structure of amorphous silicon. Science 2012, 335, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Lovecchio, N.; Caputo, D.; Costantini, F.; Di Meo, V.; Nascetti, A.; de Cesare, G. On the stability of amorphous silicon temperature sensors. IEEE Trans. Electron Devices 2020, 67, 3348–3354. [Google Scholar] [CrossRef]
- i Cabarrocas, P.R. Plasma enhanced chemical vapor deposition of amorphous, polymorphous and microcrystalline silicon films. J. Non-Cryst. Solids 2000, 266, 31–37. [Google Scholar] [CrossRef]
- Bakos, T.; Maroudas, D. First-principles analysis of precursor-surface reaction pathways relevant to plasma deposition of silicon thin films. IEEE Trans. Plasma Sci. 2005, 33, 230–231. [Google Scholar] [CrossRef]
- Lovecchio, N.; Costantini, F.; Parisi, E.; Nardecchia, M.; Tucci, M.; Nascetti, A.; de Cesare, G.; Caputo, D. Integrated optoelectronic device for detection of fluorescent molecules. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1337–1344. [Google Scholar] [CrossRef]
- Arora, A.; Simone, G.; Salieb-Beugelaar, G.B.; Kim, J.T.; Manz, A. Latest developments in micro total analysis systems. Anal. Chem. 2010, 82, 4830–4847. [Google Scholar] [CrossRef]
- Schumacher, S.; Nestler, J.; Otto, T.; Wegener, M.; Ehrentreich-Förster, E.; Michel, D.; Wunderlich, K.; Palzer, S.; Sohn, K.; Weber, A.; et al. Highly-integrated lab-on-chip system for point-of-care multiparameter analysis. Lab Chip 2012, 12, 464–473. [Google Scholar] [CrossRef]
- Knipp, D.; Street, R.A.; Stiebig, H.; Krause, M.; Lu, J.P.; Ready, S.; Ho, J. Vertically integrated amorphous silicon color sensor arrays. IEEE Trans. Electron Devices 2006, 53, 1551–1558. [Google Scholar] [CrossRef]
- Lin, C.L.; Deng, M.Y.; Wu, C.E.; Hsu, C.C.; Lee, C.L. Hydrogenated amorphous silicon gate driver with low leakage for thin-film transistor liquid crystal display applications. IEEE Trans. Electron Devices 2017, 64, 3193–3198. [Google Scholar] [CrossRef]
- Mutitu, J.G.; Shi, S.; Barnett, A.; Prather, D.W. Hybrid dielectric-metallic back reflector for amorphous silicon solar cells. Energies 2010, 3, 1914–1933. [Google Scholar] [CrossRef] [Green Version]
- Contreras, J.; Costa, D.; Pereira, S.; Fortunato, E.; Martins, R.; Wierzbicki, R.; Heerlein, H.; Ferreira, I. Micro cantilever movement detection with an amorphous silicon array of position sensitive detectors. Sensors 2010, 10, 8173–8184. [Google Scholar] [CrossRef] [Green Version]
- Costantini, F.; Tiggelaar, R.M.; Salvio, R.; Nardecchia, M.; Schlautmann, S.; Manetti, C.; Gardeniers, H.J.; De Cesare, G.; Caputo, D.; Nascetti, A. An all-glass microfluidic network with integrated amorphous silicon photosensors for on-chip monitoring of enzymatic biochemical assay. Biosensors 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, D.R.; Soares, R.R.; Chu, V.; Conde, J.P. Performance of hydrogenated amorphous silicon thin film photosensors at ultra-low light levels: Towards attomole sensitivities in lab-on-chip biosensing applications. IEEE Sens. J. 2017, 17, 6895–6903. [Google Scholar] [CrossRef]
- Costantini, F.; Petrucci, G.; Lovecchio, N.; Nardecchia, M.; Nascetti, A.; de Cesare, G.; Tedeschi, L.; Domenici, C.; Ruggi, A.; Placidi, P.; et al. Integrated sensor system for DNA amplification and separation based on thin film technology. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1141–1148. [Google Scholar] [CrossRef]
- Bruijns, B.; Costantini, F.; Lovecchio, N.; Tiggelaar, R.; Di Timoteo, G.; Nascetti, A.; de Cesare, G.; Gardeniers, J.; Caputo, D. On-chip real-time monitoring of multiple displacement amplification of DNA. Sensors Actuators B Chem. 2019, 293, 16–22. [Google Scholar] [CrossRef]
- Nikolaidou, K.; Condelipes, P.G.; Caneira, C.R.; Krack, M.; Fontes, P.M.; Oliveira, H.M.; Kovačič, M.; Krč, J.; Topič, M.; Cardoso, S.; et al. Monolithically integrated optical interference and absorption filters on thin film amorphous silicon photosensors for biological detection. Sensors Actuators B Chem. 2022, 356, 131330. [Google Scholar] [CrossRef]
- Lovecchio, N.; de Cesare, G.; Nascetti, A.; Costantini, F.; Caputo, D. Large-Area Thin Film Heater for Thermal Treatments in Lab-on-Chip. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Online, 20–25 June 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 639–642. [Google Scholar]
- Caputo, D.; De Cesare, G.; Ceccarelli, M.; Nascetti, A.; Tucci, M.; Meda, L.; Losurdo, M.; Bruno, G. Characterization of chromium silicide thin layer formed on amorphous silicon films. J. Non-Cryst. Solids 2008, 354, 2171–2175. [Google Scholar] [CrossRef]
- Kong, T.F.; Shen, X.; Marcos; Yang, C. Lab-on-chip microfluidic impedance measurement for laminar flow ratio sensing and differential conductivity difference detection. Appl. Phys. Lett. 2017, 110, 233501. [Google Scholar] [CrossRef]
- Smolka, M.; Puchberger-Enengl, D.; Bipoun, M.; Klasa, A.; Kiczkajlo, M.; Śmiechowski, W.; Sowiński, P.; Krutzler, C.; Keplinger, F.; Vellekoop, M. A mobile lab-on-a-chip device for on-site soil nutrient analysis. Precis. Agric. 2017, 18, 152–168. [Google Scholar] [CrossRef]
- Reddy, B.; Dorvel, B.R.; Go, J.; Nair, P.R.; Elibol, O.H.; Credo, G.M.; Daniels, J.S.; Chow, E.K.; Su, X.; Varma, M.; et al. High-k dielectric Al2O3 nanowire and nanoplate field effect sensors for improved pH sensing. Biomed. Microdevices 2011, 13, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputo, D.; de Cesare, G.; Lovecchio, N.; Scipinotti, R.; Nascetti, A. Electrowetting-on-dielectric system based on polydimethylsiloxane. In Proceedings of the 5th IEEE International Workshop on Advances in Sensors and Interfaces IWASI, Bari, Italy, 13–14 June 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 99–103. [Google Scholar]
- Jin, K.; Hu, C.; Hu, S.; Hu, C.; Li, J.; Ma, H. “One-to-three” droplet generation in digital microfluidics for parallel chemiluminescence immunoassays. Lab Chip 2021, 21, 2892–2900. [Google Scholar] [CrossRef]
- Stefano, J.S.; Orzari, L.O.; Silva-Neto, H.A.; de Ataíde, V.N.; Mendes, L.F.; Coltro, W.K.T.; Paixão, T.R.L.C.; Janegitz, B.C. Different approaches for fabrication of low-cost electrochemical sensors. Curr. Opin. Electrochem. 2022, 32, 100893. [Google Scholar] [CrossRef]
- Kwok, K.E.; Ping, M.C.; Li, P. A model-based augmented PID algorithm. J. Process Control 2000, 10, 9–18. [Google Scholar] [CrossRef]
- Lovecchio, N.; Caputo, D.; Petrucci, G.; Nascetti, A.; Nardecchia, M.; Costantini, F.; Cesare, G.D. Amorphous Silicon Temperature Sensors Integrated with Thin Film Heaters for Thermal Treatments of Biomolecules. In Proceedings of the Convegno Nazionale Sensori; Springer: Berlin/Heidelberg, Germany, 2016; pp. 183–193. [Google Scholar]
- Chao, C.T.; Sutarna, N.; Chiou, J.S.; Wang, C.J. An optimal fuzzy PID controller design based on conventional PID control and nonlinear factors. Appl. Sci. 2019, 9, 1224. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, J.R.; Cruz-Miguel, E.E.; Carrillo-Serrano, R.V.; Mendoza-Mondragón, F.; Toledano-Ayala, M.; Rodríguez-Reséndiz, J. A PID-type fuzzy logic controller-based approach for motion control applications. Sensors 2020, 20, 5323. [Google Scholar] [CrossRef]
- Lovecchio, N. Design and Development of a Lab-on-Chip for Biomedical Analysis Based on ElectroWetting on Dielectric Technique. Ph.D. Thesis, Sapienza University of Rome, Rome, Italy, 2018. [Google Scholar]
- Nascetti, A.; Colonia, G.; Caputo, D.; De Cesare, G. SOPHIE: A general purpose sub-picoamps current readout electronics. In Sensors; Springer: Berlin/Heidelberg, Germany, 2015; pp. 285–289. [Google Scholar]
- De Cesare, G.; Nascetti, A.; Caputo, D. Amorphous silicon pin structure acting as light and temperature sensor. Sensors 2015, 15, 12260–12272. [Google Scholar] [CrossRef] [Green Version]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low cost lab on chip for the colorimetric detection of nitrate in mineral water products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef] [Green Version]
- Chiriacò, M.S.; Bianco, M.; Nigro, A.; Primiceri, E.; Ferrara, F.; Romano, A.; Quattrini, A.; Furlan, R.; Arima, V.; Maruccio, G. Lab-on-chip for exosomes and microvesicles detection and characterization. Sensors 2018, 18, 3175. [Google Scholar] [CrossRef] [Green Version]
- Walter, J.G.; Alwis, L.S.; Roth, B.; Bremer, K. All-optical planar polymer waveguide-based biosensor chip designed for smartphone-assisted detection of vitamin D. Sensors 2020, 20, 6771. [Google Scholar] [CrossRef] [PubMed]
- Tsopela, A.; Laborde, A.; Salvagnac, L.; Ventalon, V.; Bedel-Pereira, E.; Séguy, I.; Temple-Boyer, P.; Juneau, P.; Izquierdo, R.; Launay, J. Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens. Bioelectron. 2016, 79, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, A.; Rouillard, C.; Mathault, J.; Boisvert, M.; Tessier, F.; Landari, H.; Melki, I.; Laprise-Pelletier, M.; Boisselier, E.; Fortin, M.A.; et al. Towards a multifunctional electrochemical sensing and niosome generation lab-on-chip platform based on a plug-and-play concept. Sensors 2016, 16, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jović, M.; Zhu, Y.; Lesch, A.; Bondarenko, A.; Cortés-Salazar, F.; Gumy, F.; Girault, H.H. Inkjet-printed microtiter plates for portable electrochemical immunoassays. J. Electroanal. Chem. 2017, 786, 69–76. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Tijero, M.; Martin, A.; Acosta, V.; Berganzo, J.; Castillejo, A.; Bouali, M.M.; Soto, J.L. Optimizing polymer lab-on-chip platforms for ultrasonic manipulation: Influence of the substrate. Micromachines 2015, 6, 574–591. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Lee, Y.C.; Lai, Y.H.; Lim, J.C.; Huang, N.T.; Lin, C.T.; Huang, J.J. Review of integrated optical biosensors for point-of-care applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef]
- Di Meo, V.; Crescitelli, A.; Moccia, M.; Sandomenico, A.; Cusano, A.M.; Portaccio, M.; Lepore, M.; Galdi, V.; Esposito, E. Pixeled metasurface for multiwavelength detection of vitamin D. Nanophotonics 2020, 9, 3921–3930. [Google Scholar] [CrossRef]
- Dawson, H.; Elias, J.; Etienne, P.; Calas-Etienne, S. The Rise of the OM-LoC: Opto-Microfluidic Enabled Lab-on-Chip. Micromachines 2021, 12, 1467. [Google Scholar] [CrossRef]
- Al Mughairy, B.; Al-Lawati, H.A. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. TrAC Trends Anal. Chem. 2020, 124, 115802. [Google Scholar] [CrossRef]
- Novo, P.; Moulas, G.; Prazeres, D.M.F.; Chu, V.; Conde, J.P. Detection of ochratoxin A in wine and beer by chemiluminescence-based ELISA in microfluidics with integrated photodiodes. Sensors Actuators B Chem. 2013, 176, 232–240. [Google Scholar] [CrossRef]
- Costantini, F.; Lovecchio, N.; Ruggi, A.; Manetti, C.; Nascetti, A.; Reverberi, M.; de Cesare, G.; Caputo, D. Fluorescent label-free aptasensor integrated in a lab-on-chip system for the detection of ochratoxin a in beer and wheat. ACS Appl. Bio Mater. 2019, 2, 5880–5887. [Google Scholar] [CrossRef]
- Nascetti, A.; Mirasoli, M.; Marchegiani, E.; Zangheri, M.; Costantini, F.; Porchetta, A.; Iannascoli, L.; Lovecchio, N.; Caputo, D.; de Cesare, G.; et al. Integrated chemiluminescence-based lab-on-chip for detection of life markers in extraterrestrial environments. Biosens. Bioelectron. 2019, 123, 195–203. [Google Scholar] [CrossRef]
- Nandimandalam, M.; Costantini, F.; Lovecchio, N.; Iannascoli, L.; Nascetti, A.; de Cesare, G.; Caputo, D.; Manetti, C. Split Aptamers Immobilized on Polymer Brushes Integrated in a Lab-on-Chip System Based on an Array of Amorphous Silicon Photosensors: A Novel Sensor Assay. Materials 2021, 14, 7210. [Google Scholar] [CrossRef]
- Shin, Y.H.; Gutierrez-Wing, M.T.; Choi, J.W. Recent progress in portable fluorescence sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Măriuţa, D.; Colin, S.; Barrot-Lattes, C.; Le Calvé, S.; Korvink, J.G.; Baldas, L.; Brandner, J.J. Miniaturization of fluorescence sensing in optofluidic devices. Microfluid. Nanofluidics 2020, 24, 1–28. [Google Scholar] [CrossRef]
- Bassani, T.; Dias, P.; Branco, G.; da Silva, W.J.; Neto, H.V.; Schneider, F.K. Fluorescence-based lab-on-chip spot design for improved signal detection. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: Piscataway, NJ, USA; pp. 543–546. [Google Scholar]
- Li, Q.; Liu, Y.; Zhao, B.; Lei, J.; Lu, S.; Gong, W.; Liang, K.; Wu, J.; Hong, X.; Xiao, Y. A single-molecular ruthenium (ii) complex-based NIR-II fluorophore for enhanced chemo-photothermal therapy. Chem. Commun. 2022, 58, 6546–6549. [Google Scholar] [CrossRef]
- Wu, M.; Yin, C.; Fu, L.; Liu, T.; Jiang, M.; Sun, Q.; Chen, L.; Niu, N. A biocompatible ruthenium-based composite fluorescent probe using bovine serum albumin as a scaffold for ethylene gas detection and its fluorescence imaging in plant tissues. Chem. Eng. J. 2022, 435, 135045. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; Liang, Y.; Su, Y.; Shang, Q.; Zhang, C. A simple and sensitive paper-based bipolar electrochemiluminescence biosensor for detection of oxidase-substrate biomarkers in serum. J. Electrochem. Soc. 2018, 165, B361. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. Multiplexed and simultaneous biosensing in a 3D-printed portable six-well smartphone operated electrochemiluminescence standalone point-of-care platform. Microchim. Acta 2022, 189, 1–9. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Mukundan, A.; Chen, W.C.; Wu, M.T.; Hsieh, S.C.; Wang, H.C. Design of a Lab-On-Chip for Cancer Cell Detection through Impedance and Photoelectrochemical Response Analysis. Biosensors 2022, 12, 405. [Google Scholar] [CrossRef]
- Visco, R.; Chandross, E. On the mechanism of the electrochemiluminescence of aromatic hydrocarbons in aprotic solvents. Electrochim. Acta 1968, 13, 1187–1196. [Google Scholar] [CrossRef]
- Liu, C.C. Electrochemical based biosensors. Biosensors 2012, 2, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Valenti, G.; Scarabino, S.; Goudeau, B.; Lesch, A.; Jovic, M.; Villani, E.; Sentic, M.; Rapino, S.; Arbault, S.; Paolucci, F.; et al. Single cell electrochemiluminescence imaging: From the proof-of-concept to disposable device-based analysis. J. Am. Chem. Soc. 2017, 139, 16830–16837. [Google Scholar] [CrossRef]
- Ko, M.; Kim, H.U.; Jeon, N. Boosting Electrochemical Activity of Porous Transparent Conductive Oxides Electrodes Prepared by Sequential Infiltration Synthesis. Small 2022, 18, 2105898. [Google Scholar] [CrossRef]
- Andronico, L.A.; Chen, L.; Mirasoli, M.; Guardigli, M.; Quintavalla, A.; Lombardo, M.; Trombini, C.; Chiu, D.T.; Roda, A. Thermochemiluminescent semiconducting polymer dots as sensitive nanoprobes for reagentless immunoassay. Nanoscale 2018, 10, 14012–14021. [Google Scholar] [CrossRef]
- Sze, S.M.; Li, Y.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Schweitzer, B.; Kingsmore, S. Combining nucleic acid amplification and detection. Curr. Opin. Biotechnol. 2001, 12, 21–27. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; von Stetten, F. A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2019, 144, 31–67. [Google Scholar] [CrossRef] [Green Version]
- Walker, F.M.; Hsieh, K. Advances in directly amplifying nucleic acids from complex samples. Biosensors 2019, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Louie, M.; Louie, L.; Simor, A.E. The role of DNA amplification technology in the diagnosis of infectious diseases. CMAJ 2000, 163, 301–309. [Google Scholar]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Schindler, D. Genetic engineering and synthetic genomics in yeast to understand life and boost biotechnology. Bioengineering 2020, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Newbold, L.K.; Robinson, A.; Rasnaca, I.; Lahive, E.; Soon, G.H.; Lapied, E.; Oughton, D.; Gashchak, S.; Beresford, N.A.; Spurgeon, D.J. Genetic, epigenetic and microbiome characterisation of an earthworm species (Octolasion lacteum) along a radiation exposure gradient at Chernobyl. Environ. Pollut. 2019, 255, 113238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, W.; Sun, R.; Liu, B.; Zhang, X.; Yang, H. Upconverting nanoparticles based nanodevice for DNAzymes amplified miRNAs detection and artificially controlled chemo-gene therapy. Biosens. Bioelectron. 2022, 214, 114549. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR past, present and future. BioTechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Leonardo, S.; Toldrà, A.; Campàs, M. Biosensors based on isothermal DNA amplification for bacterial detection in food safety and environmental monitoring. Sensors 2021, 21, 602. [Google Scholar] [CrossRef]
- Bodulev, O.; Sakharov, I.Y. Isothermal nucleic acid amplification techniques and their use in bioanalysis. Biochemistry 2020, 85, 147–166. [Google Scholar] [CrossRef]
- Mattes, W. The basics of the polymerase chain reaction. In PCR Protocols in Molecular Toxicology; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–40. [Google Scholar]
- Pumford, E.A.; Lu, J.; Spaczai, I.; Prasetyo, M.E.; Zheng, E.M.; Zhang, H.; Kamei, D.T. Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens. Bioelectron. 2020, 170, 112674. [Google Scholar] [CrossRef]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Costantini, F.; Roda, A.; Gallinella, G.; Caputo, D.; et al. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sensors Actuators B Chem. 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Saiki, R.K. The design and optimization of the PCR. In PCR Technology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 7–16. [Google Scholar]
- Gendron, L.; Verreault, D.; Veillette, M.; Moineau, S.; Duchaine, C. Evaluation of filters for the sampling and quantification of RNA phage aerosols. Aerosol Sci. Technol. 2010, 44, 893–901. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Nakajima, M. Recent lab-on-chip developments for novel drug discovery. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1381. [Google Scholar] [CrossRef]
- Serioli, L.; Laksafoss, T.Z.; Haagensen, J.A.; Sternberg, C.; Soerensen, M.P.; Molin, S.; Zor, K.; Boisen, A. Bacterial cell cultures in a lab-on-a-disc: A simple and versatile tool for quantification of antibiotic treatment efficacy. Anal. Chem. 2020, 92, 13871–13879. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-based oxygen (O2) sensors for on-chip monitoring of cell, tissue and organ metabolism. Biosensors 2021, 12, 6. [Google Scholar] [CrossRef]
- Piccinno, E.; Monteduro, A.G.; Dituri, F.; Rizzato, S.; Giannelli, G.; Maruccio, G. Validation of a Lab-on-Chip Assay for Measuring Sorafenib Effectiveness on HCC Cell Proliferation. Int. J. Mol. Sci. 2021, 22, 13090. [Google Scholar] [CrossRef]
- Walczak, R.; Śniadek, P.; Dziuban, J.A.; Kluger, J.; Soyta, A.C. Supravital fluorometric apoptosis detection in a single mouse embryo using lab-on-a-chip. Lab Chip 2011, 11, 3263–3268. [Google Scholar] [CrossRef]
- Madison, A.C.; Royal, M.W.; Vigneault, F.; Chen, L.; Griffin, P.B.; Horowitz, M.; Church, G.M.; Fair, R.B. Scalable device for automated microbial electroporation in a digital microfluidic platform. ACS Synth. Biol. 2017, 6, 1701–1709. [Google Scholar] [CrossRef]
- Fekete, N.; Béland, A.V.; Campbell, K.; Clark, S.L.; Hoesli, C.A. Bags versus flasks: A comparison of cell culture systems for the production of dendritic cell–based immunotherapies. Transfusion 2018, 58, 1800–1813. [Google Scholar] [CrossRef] [Green Version]
- Rothbauer, M.; Zirath, H.; Ertl, P. Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip 2018, 18, 249–270. [Google Scholar] [CrossRef] [Green Version]
- Baruffaldi, D.; Palmara, G.; Pirri, C.; Frascella, F. 3D cell culture: Recent development in materials with tunable stiffness. ACS Appl. Bio Mater. 2021, 4, 2233–2250. [Google Scholar] [CrossRef]
- Podwin, A.; Lizanets, D.; Przystupski, D.; Kubicki, W.; Śniadek, P.; Kulbacka, J.; Wymysłowski, A.; Walczak, R.; Dziuban, J.A. Lab-on-chip platform for culturing and dynamic evaluation of cells development. Micromachines 2020, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Mandenius, C.F. Conceptual design of micro-bioreactors and organ-on-chips for studies of cell cultures. Bioengineering 2018, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Kratz, S.R.A.; Höll, G.; Schuller, P.; Ertl, P.; Rothbauer, M. Latest trends in biosensing for microphysiological organs-on-a-chip and body-on-a-chip systems. Biosensors 2019, 9, 110. [Google Scholar] [CrossRef]
- Tajeddin, A.; Mustafaoglu, N. Design and fabrication of organ-on-chips: Promises and challenges. Micromachines 2021, 12, 1443. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovecchio, N.; Costantini, F.; Nascetti, A.; de Cesare, G.; Caputo, D. Thin-Film-Based Multifunctional System for Optical Detection and Thermal Treatment of Biological Samples. Biosensors 2022, 12, 969. https://doi.org/10.3390/bios12110969
Lovecchio N, Costantini F, Nascetti A, de Cesare G, Caputo D. Thin-Film-Based Multifunctional System for Optical Detection and Thermal Treatment of Biological Samples. Biosensors. 2022; 12(11):969. https://doi.org/10.3390/bios12110969
Chicago/Turabian StyleLovecchio, Nicola, Francesca Costantini, Augusto Nascetti, Giampiero de Cesare, and Domenico Caputo. 2022. "Thin-Film-Based Multifunctional System for Optical Detection and Thermal Treatment of Biological Samples" Biosensors 12, no. 11: 969. https://doi.org/10.3390/bios12110969
APA StyleLovecchio, N., Costantini, F., Nascetti, A., de Cesare, G., & Caputo, D. (2022). Thin-Film-Based Multifunctional System for Optical Detection and Thermal Treatment of Biological Samples. Biosensors, 12(11), 969. https://doi.org/10.3390/bios12110969






