A Mediated Enzymatic Electrochemical Sensor Using Paper-Based Laser-Induced Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
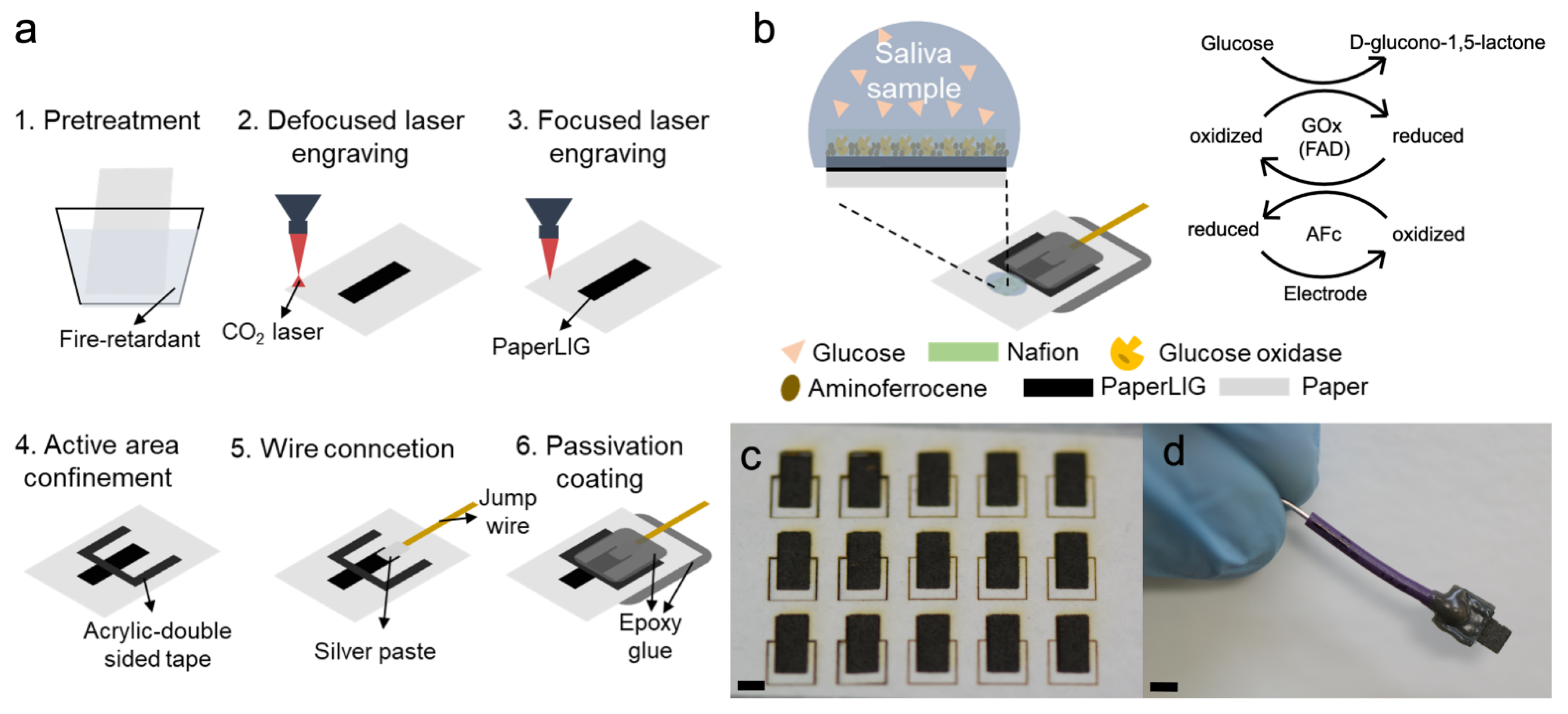
2.2. Fabrication of PaperLIG Electrode
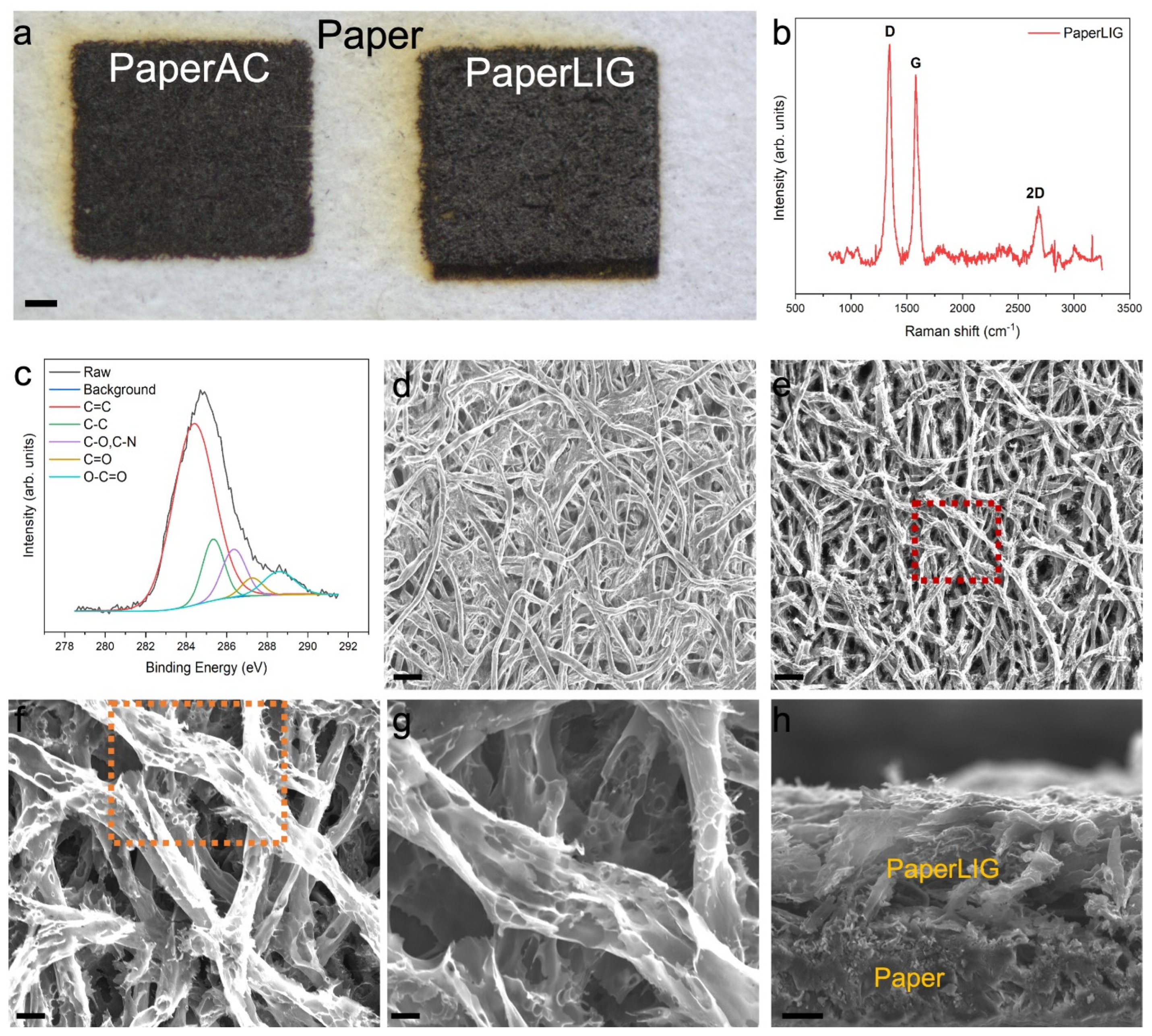
2.3. Characterization of the PaperLIG Material
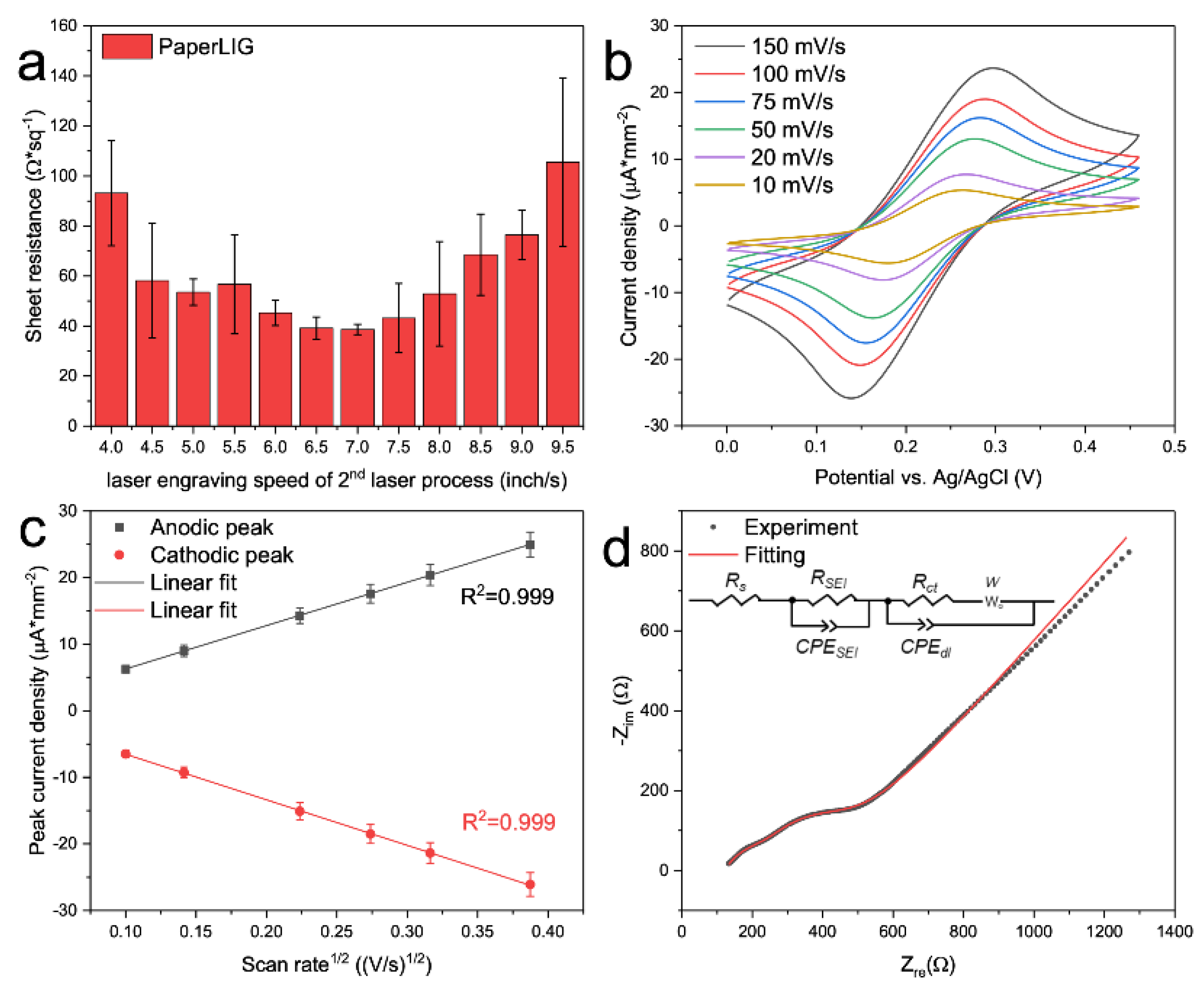
2.4. Electrochemical Measurement of the PaperLIG Electrode
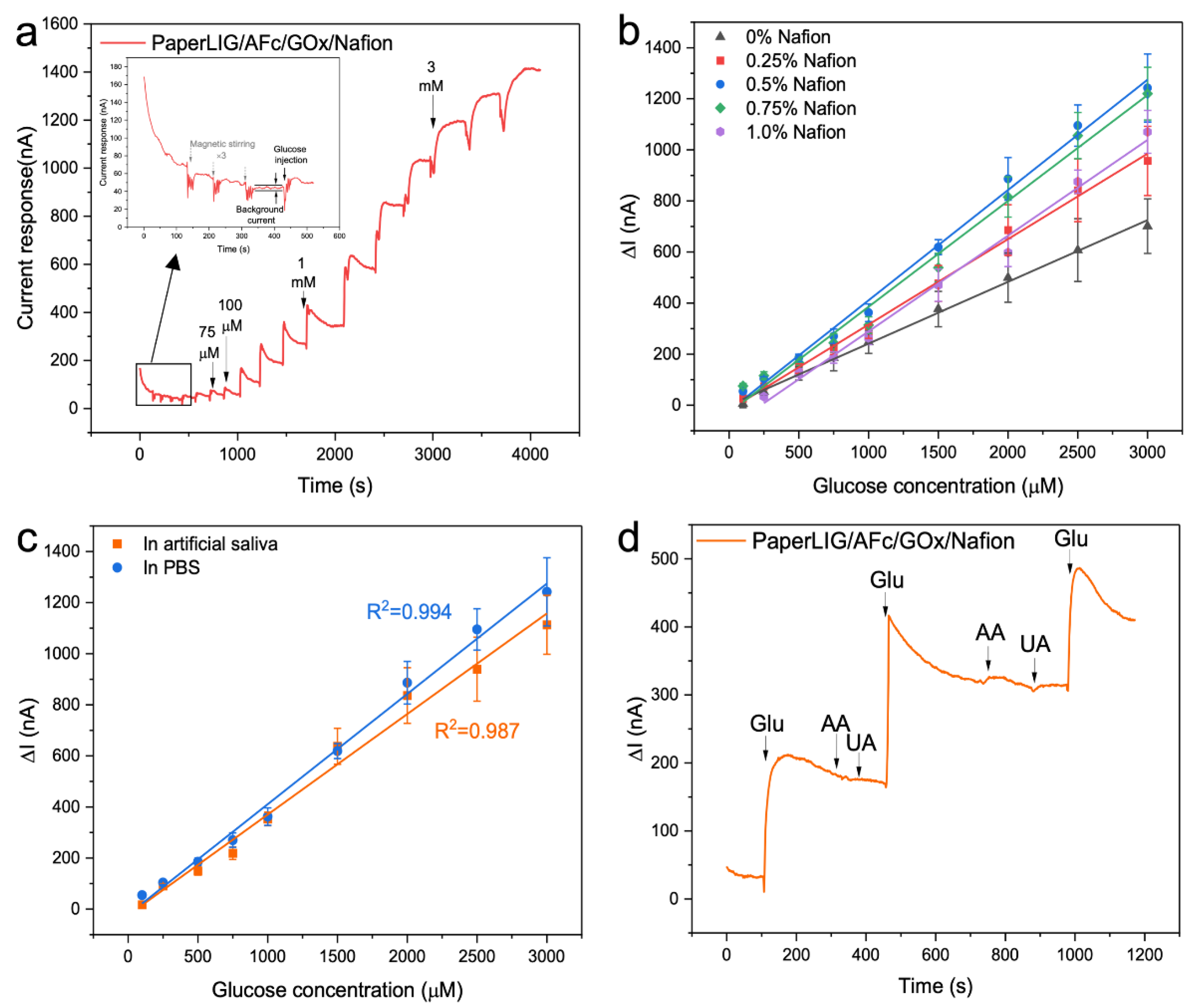
2.5. Preparation and Evaluation of Enzymatic Glucose Biosensor
3. Results
3.1. Fabrication and Characterization of PaperLIG Material
3.2. Characterization of PaperLIG Electrode
3.3. Development of PaperLIG-Based Glucose Biosensor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Taylor, P.J.; Thompson, C.H.; Brinkworth, G.D. Effectiveness and Acceptability of Continuous Glucose Monitoring for Type 2 Diabetes Management: A Narrative Review. J. Diabetes Investig. 2018, 9, 713–725. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.-M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a Magic Biofluid Available for Multilevel Assessment and a Mirror of General Health—A Systematic Review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Padmashree, S.; Jayalekshmi, R. Correlation of Salivary Glucose, Blood Glucose and Oral Candidal Carriage in the Saliva of Type 2 Diabetics: A Case-Control Study. Contemp. Clin. Dent. 2014, 5, 312–317. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, M.T.; Sunitha, J.; Dawar, G.; Sinha, N.; Rallan, N.S. Correlation of Salivary Glucose Level with Blood Glucose Level in Diabetes Mellitus. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 334–339. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors 2022, 12, 467. [Google Scholar] [CrossRef]
- Moonla, C.; Lee, D.H.; Rokaya, D.; Rasitanon, N.; Kathayat, G.; Lee, W.-Y.; Kim, J.; Jeerapan, I. Review—Lab-in-a-Mouth and Advanced Point-of-Care Sensing Systems: Detecting Bioinformation from the Oral Cavity and Saliva. ECS Sens. Plus 2022, 1, 021603. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical Sensors Targeting Salivary Biomarkers: A Comprehensive Review. TrAC Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-Induced Graphene-Based Wearable Epidermal Ion-Selective Sensors for Noninvasive Multiplexed Sweat Analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liu, P.; Guo, X. Laser-Induced Graphene Based Flexible Electronic Devices. Biosensors 2022, 12, 55. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella Enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Vanegas, D.C.; Patiño, L.; Mendez, C.; de Oliveira, D.A.; Torres, A.M.; Gomes, C.L.; McLamore, E.S. Laser Scribed Graphene Biosensor for Detection of Biogenic Amines in Food Samples Using Locally Sourced Materials. Biosensors 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Nah, J.; Kim, H.; Ko, S.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.; Park, J.Y. A Chemically Modified Laser-Induced Porous Graphene Based Flexible and Ultrasensitive Electrochemical Biosensor for Sweat Glucose Detection. Sens. Actuators B Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Dai, X.; Wang, Y.; Sun, M.; Zhou, C.; Du, H.; Rao, H. Novel Flexible Bifunctional Amperometric Biosensor Based on Laser Engraved Porous Graphene Array Electrodes: Highly Sensitive Electrochemical Determination of Hydrogen Peroxide and Glucose. J. Hazard. Mater. 2021, 402, 123774. [Google Scholar] [CrossRef]
- Bauer, M.; Wunderlich, L.; Weinzierl, F.; Lei, Y.; Duerkop, A.; Alshareef, H.N.; Baeumner, A.J. Electrochemical Multi-Analyte Point-of-Care Perspiration Sensors Using on-Chip Three-Dimensional Graphene Electrodes. Anal. Bioanal. Chem. 2021, 413, 763–777. [Google Scholar] [CrossRef]
- Cella, J.A. Degradation and Stability of Polyimides. Polym. Degrad. Stab. 1992, 36, 99–110. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent Organic Pollutants Carried by Synthetic Polymers in the Ocean Environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Kulyk, B.; Silva, B.F.R.; Carvalho, A.F.; Silvestre, S.; Fernandes, A.J.S.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene from Paper for Mechanical Sensing. ACS Appl. Mater. Interfaces 2021, 13, 10210–10221. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, M.; Kim, B.G.; Kim, Y.H. Electronic Functionality Encoded Laser-Induced Graphene for Paper Electronics. ACS Appl. Nano Mater. 2020, 3, 6899–6904. [Google Scholar] [CrossRef]
- Settu, K.; Chiu, P.-T.; Huang, Y.-M. Laser-Induced Graphene-Based Enzymatic Biosensor for Glucose Detection. Polymers 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, B.; Liu, B. Amperometric Glucose Sensor with Ferrocene as an Electron Transfer Mediator. Biosens. Bioelectron. 1992, 7, 215–222. [Google Scholar] [CrossRef]
- El-Desoky, H.S.; Koleeb, A.I.; Bassuiny, R.I.; Mohamed, T.M. Development of an Effective and Economic Biosensor for Diabetic Blood Monitoring Based on MWCNTs, Artificial Redox Mediator Ferrocene, Nafion Polymer and a Local Extracted and Purified Glucose Oxidase Enzyme from Penicillium Notatum F-158 Fungus. J. Electrochem. Soc. 2021, 168, 127502. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chiang, Y.-H.; Chiang, H.-Y. A Label-Free Electrochemical Impedimetric Immunosensor with Biotinylated-Antibody for SARS-CoV-2 Nucleoprotein Detection in Saliva. Biosensors 2022, 12, 265. [Google Scholar] [CrossRef]
- EL Sharif, H.F.; Dennison, S.R.; Tully, M.; Crossley, S.; Mwangi, W.; Bailey, D.; Graham, S.P.; Reddy, S.M. Evaluation of Electropolymerized Molecularly Imprinted Polymers (E-MIPs) on Disposable Electrodes for Detection of SARS-CoV-2 in Saliva. Anal. Chim. Acta 2022, 1206, 339777. [Google Scholar] [CrossRef] [PubMed]
- Staley, B.F.; Xu, F.; Cowie, S.J.; Barlaz, M.A.; Hater, G.R. Release of Trace Organic Compounds during the Decomposition of Municipal Solid Waste Components. Environ. Sci. Technol. 2006, 40, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An Overview of Paper and Paper Based Food Packaging Materials: Health Safety and Environmental Concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of Sustainable and Environment-Friendly Flame Retardants Used in Plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-Induced Graphene Fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Minhas-Khan, A.; Nambi, S.; Grau, G. Low-Resistance Laser-Induced Graphitic Carbon by Maximizing Energy Delivery and Pulse Overlap. Carbon 2021, 181, 310–322. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Santos, N.F.; Pereira, S.O.; Moreira, A.; Girão, A.V.; Carvalho, A.F.; Fernandes, A.J.S.; Costa, F.M. IR and UV Laser-Induced Graphene: Application as Dopamine Electrochemical Sensors. Adv. Mater. Technol. 2021, 6, 2100007. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, F.; Yue, R.; Du, Y. Electrochemically Fabricated Flower-like Graphene as a Highly Efficient Pt Electrocatalyst Support for Methanol Oxidation. RSC Adv. 2014, 4, 12105–12108. [Google Scholar] [CrossRef]
- de Lima, F.; Maia, G. Oxidized/Reduced Graphene Nanoribbons Facilitate Charge Transfer to the Fe(CN)63−/Fe(CN)64− Redox Couple and towards Oxygen Reduction. Nanoscale 2015, 7, 6193–6207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, F.; Fortunato, G.V.; Maia, G. A Remarkably Simple Characterization of Glassy Carbon-Supported Films of Graphite, Graphene Oxide, and Chemically Converted Graphene Using Fe(CN)3−6/Fe(CN)4−6 and O2 as Redox Probes. RSC Adv. 2013, 3, 9550–9560. [Google Scholar] [CrossRef]
- Behrent, A.; Griesche, C.; Sippel, P.; Baeumner, A.J. Process-Property Correlations in Laser-Induced Graphene Electrodes for Electrochemical Sensing. Microchim. Acta 2021, 188, 159. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, B.; Pereira, S.O.; Fernandes, A.J.S.; Fortunato, E.; Costa, F.M.; Santos, N.F. Laser-Induced Graphene from Paper for Non-Enzymatic Uric Acid Electrochemical Sensing in Urine. Carbon 2022, 197, 253–263. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, T.; Silvestre, S.; Coelho, J.; Marques, A.C.; Martins, R.; Sales, M.G.F.; Fortunato, E. Laser-Induced Graphene on Paper toward Efficient Fabrication of Flexible, Planar Electrodes for Electrochemical Sensing. Adv. Mater. Interfaces 2021, 8, 2101502. [Google Scholar] [CrossRef]
- Vaillancourt, M.; Wei Chen, J.; Fortier, G.; Bélanger, D. Electrochemical and Enzymatic Studies of Electron Transfer Mediation by Ferrocene Derivatives with Nafion-Glucose Oxidase Electrodes. Electroanalysis 1999, 11, 23–31. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Ju, H.-X.; Chen, H.-Y. A Miniaturized Glucose Biosensor Based on the Coimmobilization of Glucose Oxidase and Ferrocene Perchlorate in Nafion at a Microdisk Platinum Electrode. Sens. Actuators B Chem. 1997, 40, 89–94. [Google Scholar] [CrossRef]
- Bertoncello, P.; Peruffo, M.; Li, F.; Unwin, P.R. Functional Electrochemically-Active Ultra-Thin Nafion Films. Colloids Surf. Physicochem. Eng. Asp. 2008, 321, 222–226. [Google Scholar] [CrossRef]
- García-Carmona, L.; Martín, A.; Sempionatto, J.R.; Moreto, J.R.; González, M.C.; Wang, J.; Escarpa, A. Pacifier Biosensor: Toward Noninvasive Saliva Biomarker Monitoring. Anal. Chem. 2019, 91, 13883–13891. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of Direct Electron Transfer and Enzyme Activity of Glucose Oxidase on Graphene Surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Rafighi, P.; Tavahodi, M.; Haghighi, B. Fabrication of a Third-Generation Glucose Biosensor Using Graphene-Polyethyleneimine-Gold Nanoparticles Hybrid. Sens. Actuators B Chem. 2016, 232, 454–461. [Google Scholar] [CrossRef]
- Pereira, S.O.; Santos, N.F.; Carvalho, A.F.; Fernandes, A.J.S.; Costa, F.M. Electrochemical Response of Glucose Oxidase Adsorbed on Laser-Induced Graphene. Nanomaterials 2021, 11, 1893. [Google Scholar] [CrossRef]
- Lopez, T.B.G.; Palisoc, S.T.; Natividad, M.T. Highly Sensitive [Ru(Bpy)3]2+/Nafion® Modified Indium Tin Oxide-Based Sensor for Heavy Metal Detection. Sens. Bio-Sens. Res. 2017, 15, 34–40. [Google Scholar] [CrossRef]
- Baker, D.R.; Simmerman, R.F.; Sumner, J.J.; Bruce, B.D.; Lundgren, C.A. Photoelectrochemistry of Photosystem I Bound in Nafion. Langmuir 2014, 30, 13650–13655. [Google Scholar] [CrossRef]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Moore, E. A Sensitive Electrochemical Analysis for Cadmium and Lead Based on Nafion-Bismuth Film in a Water Sample. Sens. Bio-Sens. Res. 2021, 34, 100454. [Google Scholar] [CrossRef]
- Brown, R.S.; Luong, J.H.T. A Regenerable Pseudo-Reagentless Glucose Biosensor Based on Nafion Polymer and l,1′-Dimethylferricinium Mediator. Anal. Chim. Acta 1995, 310, 419–427. [Google Scholar] [CrossRef]
- Cánovas, R.; Parrilla, M.; Blondeau, P.; Andrade, F.J. A Novel Wireless Paper-Based Potentiometric Platform for Monitoring Glucose in Blood. Lab. Chip 2017, 17, 2500–2507. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Masuda, T.; Takai, M. A Modifiable, Spontaneously Formed Polymer Gel with Zwitterionic and N-Hydroxysuccinimide Moieties for an Enzymatic Biofuel Cell. ACS Appl. Polym. Mater. 2021, 3, 631–639. [Google Scholar] [CrossRef]
- Rishpon, J.; Gottesfeld, S.; Campbell, C.; Davey, J.; Zawodzinski, T.A. Amperometric Glucose Sensors Based on Glucose Oxidase Immobilized in Nafion. Electroanalysis 1994, 6, 17–21. [Google Scholar] [CrossRef]
- Garland, N. Laser-Induced Graphene for Scalable Flexible Biosensing. Ph.D. Dissertation, Iowa State University, Ames, IA, USA, 2021. [Google Scholar]
- Liu, Q.; Liu, Y.; Wu, F.; Cao, X.; Li, Z.; Alharbi, M.; Abbas, A.N.; Amer, M.R.; Zhou, C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano 2018, 12, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A Thin Film Polyethylene Terephthalate (PET) Electrochemical Sensor for Detection of Glucose in Sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A Flexible Non-Enzymatic Glucose Sensor Based on Copper Nanoparticles Anchored on Laser-Induced Graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Fiorito, P.A.; Torresi, S.I.C. Glucose Amperometric Biosensor Based on the Co-Immobilization of Glucose Oxidase (GOx) and Ferrocene in Poly(Pyrrole) Generated from Ethanol/Water Mixtures. J. Braz. Chem. Soc. 2001, 12, 729–733. [Google Scholar] [CrossRef]
- Brânzoi, F.; Brânzoi, V. Amperometric Urea Biosensor Based Metallic Substrate Modified with a Nancomposite Film; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1035-4. [Google Scholar]
- Su, D.; Feng, B.; Xu, P.; Zeng, Q.; Shan, B.; Song, Y. Covalent Organic Frameworks and Electron Mediator-Based Open Circuit Potential Biosensor for in Vivo Electrochemical Measurements. Anal. Methods 2018, 10, 4320–4328. [Google Scholar] [CrossRef]
- Song, Y.; Su, D.; Shen, Y.; Liu, H.; Wang, L. Design and Preparation of Open Circuit Potential Biosensor for in Vitro and in Vivo Glucose Monitoring. Anal. Bioanal. Chem. 2017, 409, 161–168. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Tue, P.T.; Kawai, K.; Chailapakul, O.; Takamura, Y. Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles. Sensors 2018, 18, 444. [Google Scholar] [CrossRef] [Green Version]
- Juska, V.B.; Juska, G. Copper-Nanostructure-Modified Laser-Scribed Electrodes Based on Graphitic Carbon for Electrochemical Detection of Dopamine and Glucose. J. Chem. Technol. Biotechnol. 2021, 96, 1086–1095. [Google Scholar] [CrossRef]
| Nafion Concentration (%) | LOD (µM) | Sensitivity (nA/µM) | Linear Range (mM) | R2 |
|---|---|---|---|---|
| 0 | 25–50 | 0.242 | 0.1–3 | 0.997 |
| 0.25 | 50–75 | 0.335 | 0.1–3 | 0.996 |
| 0.5 | 50–75 | 0.432 | 0.1–3 | 0.994 |
| 0.75 | 50–75 | 0.415 | 0.1–3 | 0.988 |
| 1 | 200–250 | 0.364 | 0.25–3 | 0.985 |
| Glucose Biosensor | Applied Potential (V) | LOD (µM) | Linear Range (mM) | Manufacturing Simplicity | Manufacturing Cost | Disposability * | Ref. |
|---|---|---|---|---|---|---|---|
| PET */Au/PB */GOx | +0.1 V | 2.7 | 0.02–1.11 | + | + | ++ | [64] |
| PET/SPCE */PB/GOx/Nafion | −0.2 V | 40 | 0.1–1.4 | +++ | ++ | ++ | [51] |
| PILIG/Cu NPs | +0.5 V | 0.39 | 1–6.0 | ++ | +++ | + | [65] |
| PILIG/Pt NPs/PPD/GOx | +0.6 V | 8 | 0.008–1 | ++ | +++ | + | [62] |
| PILIG/PB/Chitosan/GOx | −0.05 V | 13.7 ± 0.5 | <1.5 | ++ | +++ | + | [21] |
| PaperLIG/AFc/GOx/Nafion | −0.09 V | 50–75 | 0.1–3 | +++ | +++ | +++ | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Kasama, T.; Shin, J.; Huang, Y.; Miyake, R. A Mediated Enzymatic Electrochemical Sensor Using Paper-Based Laser-Induced Graphene. Biosensors 2022, 12, 995. https://doi.org/10.3390/bios12110995
Gao P, Kasama T, Shin J, Huang Y, Miyake R. A Mediated Enzymatic Electrochemical Sensor Using Paper-Based Laser-Induced Graphene. Biosensors. 2022; 12(11):995. https://doi.org/10.3390/bios12110995
Chicago/Turabian StyleGao, Panpan, Toshihiro Kasama, Jungchan Shin, Yixuan Huang, and Ryo Miyake. 2022. "A Mediated Enzymatic Electrochemical Sensor Using Paper-Based Laser-Induced Graphene" Biosensors 12, no. 11: 995. https://doi.org/10.3390/bios12110995





