Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis
Abstract
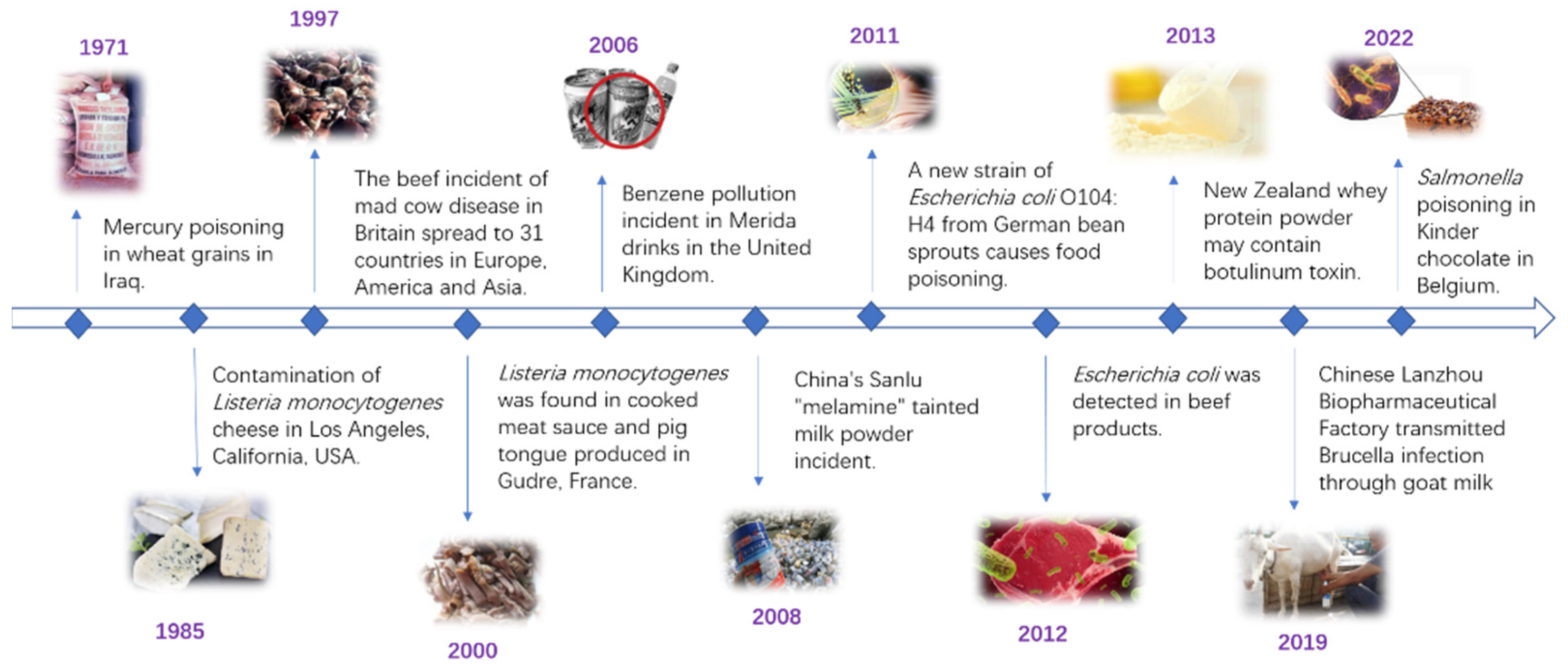
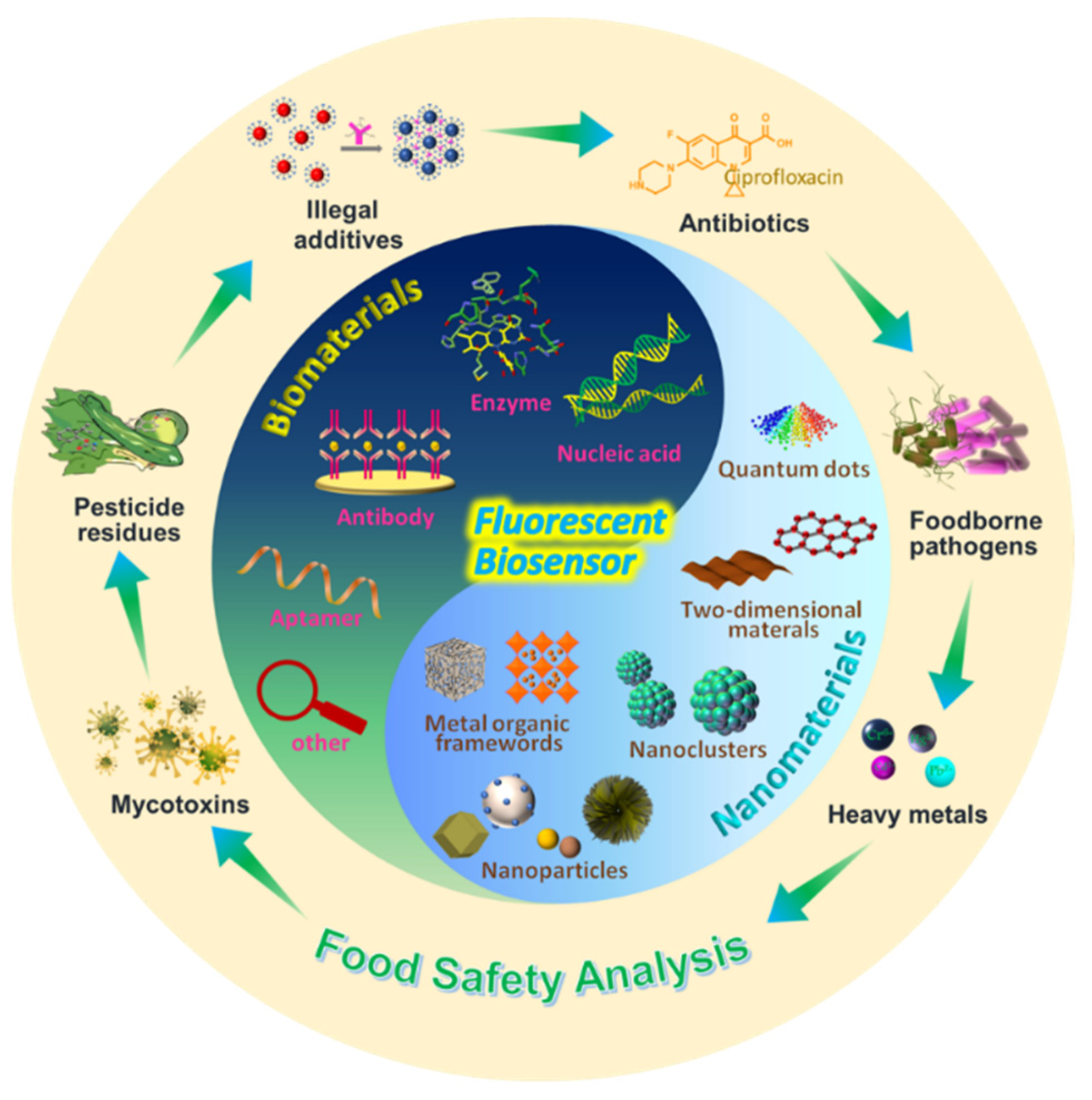
:1. Introduction
2. Biomaterials Used in Food Safety Analysis
2.1. Aptamers
2.2. Antibodies
2.3. Enzymes
2.4. Nucleic Acids
2.5. Others
3. Nanomaterials in Food Safety Analysis
3.1. Nanoparticles
3.2. Nanoclusters
3.3. Two-Dimensional Materials
3.4. Metal-Organic Frameworks
3.5. Others
4. Application of Fluorescent Biosensors for Food Safety Analysis
4.1. Mycotoxins
4.2. Heavy Metals
4.3. Antibiotics
4.4. Foodborne Pathogens
4.5. Other Illegal Additives
5. Conclusions and Perspectives
- (1)
- With the development of bio- and nanomaterials, the development of inexpensive, easy-to-synthesize, and eco-friendly materials remains a topic for future research.
- (2)
- Many nanomaterial-based biosensors have been successfully developed; however, their detection performance must be improved. Some suffer from limitations, such as low stability, poor repeatability, and a weak anti-interference ability. Therefore, there is an urgent need to develop efficient methods for fluorescence biosensing.
- (3)
- High-performance aptamers have been screened. Aptamer screening has accelerated the development of new aptasensors. Although numerous aptasensors have been developed, not all types of analyte have been investigated. Biomaterial analogs should be developed and integrated into biosensors to increase the number of aptamers.
- (4)
- Currently, newly developed biosensors are still in their early stages. Future research in this area should focus on real-time monitoring or on-site analysis, for example, the investigation of portable devices for food safety analysis.
- (5)
- Although nano- and biomaterials have advantages in terms of performance, their synthesis conditions must be optimized. In addition to full purification and the removal of impurities, multiple fluorescences should be used instead of a single fluorescence.
- (6)
- Increased practical applications. Currently, most fluorescent systems are in the experimental stage. The practical applications of nanomaterials-based fluorescent biosensors in complex matrices remain a great challenge. Adopting machine learning and microfluidic systems into fluorescence biosensors may meet the criteria of cheap real-time detection in complex matrices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzwolak, W. Assessment of HACCP plans in standardized food safety management systems—The case of small-sized Polish food businesses. Food Control 2019, 106, 106716. [Google Scholar] [CrossRef]
- Hermann, C.A.; Duerkop, A.; Baeumner, A.J. Food Safety Analysis Enabled through Biological and Synthetic Materials: A Critical Review of Current Trends. Anal. Chem. 2019, 91, 569–587. [Google Scholar] [CrossRef]
- Wang, Y.; Lau, K.; Lau, F. Clenbuterol Food Poisoning from Snake Meat Consumption: An Outbreak of 13 Cases. Hong Kong J. Emergency Med. 2017, 22, 46–49. [Google Scholar] [CrossRef]
- Schisler, D.O.; Mabee, M.S.; Hahn, C.W. Rapid Identification of Important Beer Microorganisms Using Gas Chromatography. J. Am. Soc. Brew. Chem. 2018, 37, 69–76. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Gonzalez Pereyra, M.L.; Monge, M.P.; Cavaglieri, L.R.; Chiacchiera, S.M. Validation of a liquid chromatography/tandem mass spectrometry method for the detection of aflatoxin B1 residues in broiler liver. Rev. Argent Microbiol 2018, 50, 157–164. [Google Scholar] [CrossRef]
- Sporl, J.; Speer, K.; Jira, W. Simultaneous Mass Spectrometric Detection of Proteins of Ten Oilseed Species in Meat Products. Foods 2022, 11, 2155. [Google Scholar] [CrossRef]
- Azizan, N.I.; Mokhtar, N.F.K.; Arshad, S.; Sharin, S.N.; Mohamad, N.; Mustafa, S.; Hashim, A.M. Detection of Lard Adulteration in Wheat Biscuits Using Chemometrics-Assisted GCMS and Random Forest. Food Anal. Methods 2021, 14, 2276–2287. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, B.; Sheng, P.; Liao, X.; Shi, L.; Fang, L.; Zhou, L.; Kong, W. Aptasensors for mycotoxins in foods: Recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 21, 2032–2073. [Google Scholar] [CrossRef]
- Thakur, M.S.; Ragavan, K.V. Biosensors in food processing. J. Food Sci. Technol. 2012, 50, 625–641. [Google Scholar] [CrossRef] [Green Version]
- Wegari Dera, M.; Bogale Teseme, W. Review on the Application of Food Nanotechnology in Food Processing. Am. J. Eng. Tech. Manag. 2020, 5, 12. [Google Scholar] [CrossRef]
- Alhadrami, H.; Chinnappan, R.; Eissa, S.; Rahamn, A.; Zourob, M. High affinity truncated DNA aptamers for the development of fluorescence based progesterone biosensors. Anal. Biochem. 2017, 525, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Pan, M.; Xie, X.; Liu, K.; Yang, J.; Wang, S. Aptamer-Based Fluorescent Biosensor for the Rapid and Sensitive Detection of Allergens in Food Matrices. Foods 2021, 10, 2598. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Qing, T.; Xiao, J.; Qian, C.; Juan, Y. Rapid Visualized Detection of Escherichia coli O157:H7 by DNA Hydrogel Based on Rolling Circle Amplification. Chin. J. Anal. Chem. 2021, 49, 377–386. [Google Scholar]
- Li, Y.; Liu, X.; Lin, Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012, 132, 1549–1554. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kaya, S.I.; Ozkan, S.A. Recent advances of enzyme biosensors for pesticide detection in foods. J. Food Meas. Charact. 2021, 15, 4582–4595. [Google Scholar] [CrossRef]
- Patra, I.; Kammoud, K.; Qaim, Z.; Mamadoliev, I.; Jawad, M.; Hammid, A.T.; Karim, Y.; Yasin, G. Perspectives and Trends in Advanced MXenes-Based Optical Biosensors for the Recognition of Food Contaminants. Crit. Rev. Anal. Chem. 2022; online ahead of print. [Google Scholar]
- Schmitz, F.R.W.; Valerio, A.; Oliveira, D.; Hotza, D. An overview and future prospects on aptamers for food safety. Appl. Microbiol. Biotechnol. 2020, 104, 6929–6939. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.; Wang, K.; Chen, X.; Fu, L.; Wang, Y. Screening and Identification of Specific Aptamers for Shellfish Allergen Tropomyosin with Capillary Electrophoresis-SELEX. Food Anal. Methods 2022, 15, 1535–1544. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, J.; Sun, Y.; Mo, L.; Liu, B.; Pan, X.; Liu, Z.; Tan, W. Aptamer-Based Logic Computing Reaction on Living Cells to Enable Non-Antibody Immune Checkpoint Blockade Therapy. J. Am. Chem. Soc. 2021, 143, 8391–8401. [Google Scholar] [CrossRef]
- Xuan, W.; Xia, Y.; Li, T.; Wang, L.; Liu, Y.; Tan, W. Molecular Self-Assembly of Bioorthogonal Aptamer-Prodrug Conjugate Micelles for Hydrogen Peroxide and pH-Independent Cancer Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 937–944. [Google Scholar] [CrossRef]
- Cheng, E.L.; Cardle, I.; Kacherovsky, N.; Bansia, H.; Wang, T.; Zhou, Y.; Raman, J.; Yen, A.; Gutierrez, D.; Salipante, S.J.; et al. Discovery of a Transferrin Receptor 1-Binding Aptamer and Its Application in Cancer Cell Depletion for Adoptive T-Cell Therapy Manufacturing. J. Am. Chem. Soc. 2022, 144, 13851–13864. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Hu, H.; Huang, Y.; Yang, X.; Wang, Q.; Chen, X. Improving the detection limit of Salmonella colorimetry using long ssDNA of asymmetric-PCR and non-functionalized AuNPs. Anal. Biochem. 2021, 626, 114229. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- El-Moghazy, A.Y.; Wisuthiphaet, N.; Yang, X.; Sun, G.; Nitin, N. Electrochemical biosensor based on genetically engineered bacteriophage T7 for rapid detection of Escherichia coli on fresh produce. Food Control 2022, 135, 108811. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Lee, J.; Gehring, A.G.; Capobianco, J.A. Flow-Through Electrochemical Biosensor for the Detection of Listeria monocytogenes Using Oligonucleotides. Sensors 2021, 21, 3754. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Fathi, F.; Jalili, R.; Shoeibie, S.; Dastmalchi, S.; Khataee, A.; Rashidi, M.R. SPR enhanced DNA biosensor for sensitive detection of donkey meat adulteration. Food Chem. 2020, 331, 127163. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lyu, S.; Gu, G.; Bolten, S. Selection of aptamers targeted to food-borne pathogenic bacteria Vibrio parahaemolyticus. Food Sci. Nutr. 2020, 8, 3835–3842. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Yang, C.; Hayat, A.; Marty, J.L. Aptamers: A Promising Tool for Ochratoxin A Detection in Food Analysis. Toxins 2013, 5, 1988–2008. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiu, E. Detection of pathogenic bacteria by magneto-immunoassays: A review. J. Biomed. Sci. 2021, 35, 277–283. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Fotina, H.; Zhang, H.; Chen, J. Advances in Antibody Preparation Techniques for Immunoassays of Total Aflatoxin in Food. Molecules 2020, 25, 4113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Sun, Y.; Beier, R.; Lei, H.; Gee, S.; Hammock, B.D.; Wang, H.; Wang, Z.; Sun, X.; Shen, Y.; et al. Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. TrAC Trends Anal. Chem. 2017, 88, 25–40. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, B.; Zhao, Q.; Wang, S.; Zhang, Y. Preparation of a Broad-Spectrum Heterocyclic Aromatic Amines (HAAs) Antibody and Its Application in Detection of Eight HAAs in Heat Processed Meat. J. Agric. Food Chem. 2020, 68, 15501–15508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Q.; Huang, H.; Li, M.; Yan, S.; Li, Y.; Chang, Z. Sensitive chemical sensor array based on nanozymes for discrimination of metal ions and teas. Luminescence 2020, 35, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Niu, X.; Li, X.; He, Y.; Song, H.; Peng, Y.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. A smartphone-integrated ready-to-use paper-based sensor with mesoporous carbon-dispersed Pd nanoparticles as a highly active peroxidase mimic for H2O2 detection. Sens. Actuators B 2018, 265, 412–420. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Luo, M.; Wang, Y.; Li, P. Nanozyme-assisted technique for dual mode detection of organophosphorus pesticide. Ecotoxicol. Environ. Saf. 2019, 179, 17–23. [Google Scholar] [CrossRef]
- Huang, L.; Chen, K.; Zhang, W.; Zhu, W.; Liu, X.; Wang, J.; Wang, R.; Hu, N.; Suo, Y.; Wang, J. ssDNA-tailorable oxidase-mimicking activity of spinel MnCo2O4 for sensitive biomolecular detection in food sample. Sens. Actuators B 2018, 269, 79–87. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Luo, J.; Tian, Y.; Zhou, N. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. [Google Scholar] [CrossRef]
- Park, M.; Tsai, S.-L.; Chen, W. Microbial Biosensors: Engineered Microorganisms as the Sensing Machinery. Sensors 2013, 13, 5777–5795. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, W.; Cheng, Y. Aptamer labeled nanozyme-based ELISA for ampicillin residue detection in milk. Chem. Pap. 2022, 76, 3077–3085. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, X.; Li, Q.; Chen, F.; Chen, Y.R.; Deng, J.; Han, D.; Hao, C.; Huang, F.; Huang, Y. Nucleic Acids Analysis. Sci. China Chem. 2021, 64, 171–203. [Google Scholar] [CrossRef]
- Xia, X.; Yang, H.; Cao, J.; Zhang, J.; He, Q.; Deng, R. Isothermal nucleic acid amplification for food safety analysis. TrAC Trends Anal. Chem. 2022, 153, 116641. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Zheng, Z.; Zhou, G.; Ji, X.; Wang, H.; He, Z. A new colorimetric platform for ultrasensitive detection of protein and cancer cells based on the assembly of nucleic acids and proteins. Anal. Chim. Acta 2015, 880, 1–7. [Google Scholar] [CrossRef]
- Biwu, L.; Juewen, L. Interface-Driven Hybrid Materials Based on DNA-Functionalized Gold Nanoparticles. Matter 2019, 1, 825–847. [Google Scholar]
- Lee, J.; Wang, Z.; Liu, J.; Lu, Y. Highly Sensitive and Selective Colorimetric Sensors for Uranyl (UO22+): Development and Comparison of Labeled and Label-Free DNAzyme-Gold Nanoparticle Systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef] [Green Version]
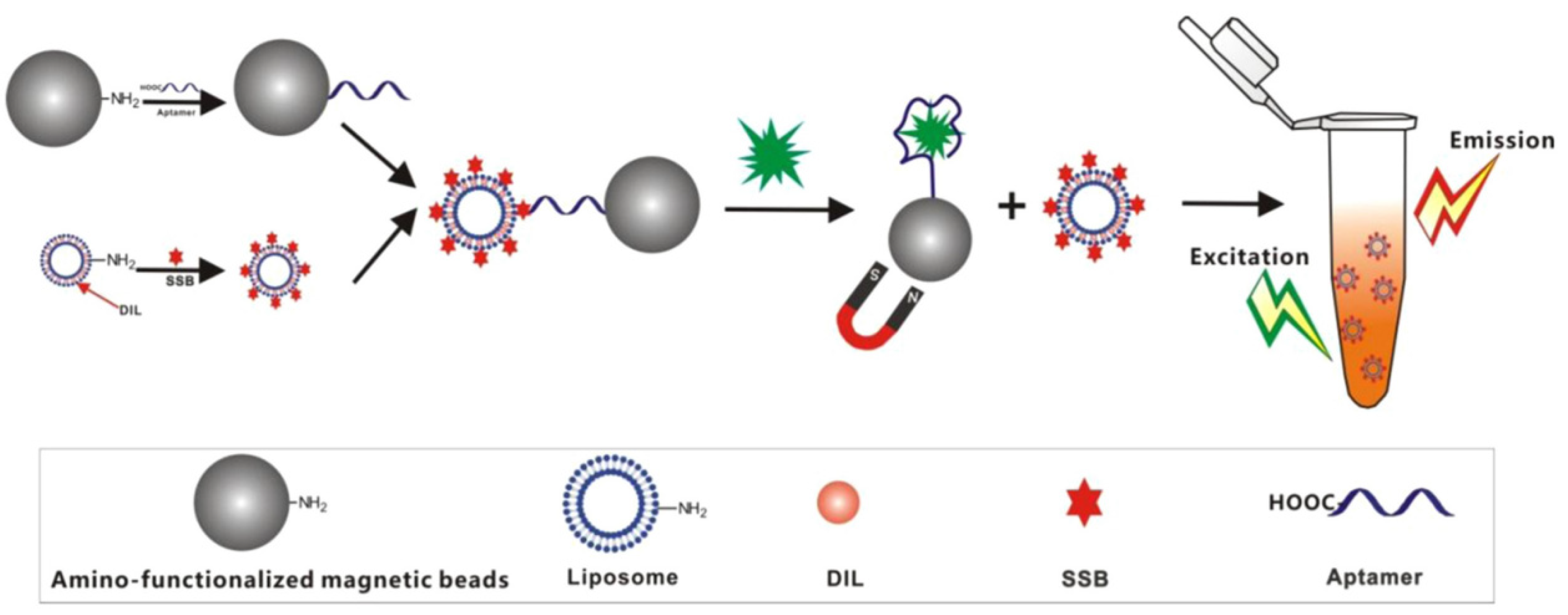
- Miao, Y.-B.; Ren, H.-X.; Gan, N.; Cao, Y.; Li, T.; Chen, Y. Fluorescent aptasensor for chloramphenicol detection using DIL-encapsulated liposome as nanotracer. Biosens. Bioelectron. 2016, 81, 454–459. [Google Scholar] [CrossRef]
- Zhou, J.; Han, H.; Liu, J. Nucleobase, nucleoside, nucleotide, and oligonucleotide coordinated metal ions for sensing and biomedicine applications. Nano Res. 2022, 15, 71–84. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Wang, W.; Lu, Z.; Han, H.; Liu, J. Kanamycin Adsorption on Gold Nanoparticles Dominates Its Label-Free Col-orimetric Sensing with Its Aptamer. Langmuir 2020, 36, 11490–11498. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef]
- Biella, S.; Prati, L.; Rossi, M. Selective Oxidation of D-Glucose on Gold Catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bragg, L.M.; Servos Mark, R.; Juewen, L. Gold nanoparticles as dehydrogenase mimicking nanozymes for estradiol degradation. Chin. Chem. Lett. 2019, 30, 1655–1658. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Li, J.; Chen, L.; Wang, N.; Yu, S.; Li, G.; Xiong, L.; Ju, H. Colorimetric Detection of Nucleic Acids through Triplex-Hybridization Chain Reaction and DNA-Controlled Growth of Platinum Nanoparticles on Graphene Oxide. Anal. Chem. 2020, 92, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, J.; Zeng, T.; Zhang, Z.; Chen, J.; Wong, G.; Qiu, X.; Liu, W.; Gao George, F.; Bi, Y. Ultrasensitive Ebola Virus Detection Based on Electroluminescent Nanospheres and Immunomagnetic Separation. Anal. Chem. 2017, 89, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Guo, W.; Qin, X.; Zhao, J.; Pei, M.; Ding, F. A sensitive electrochemical aptasensor for highly specific detection of streptomycin based on the porous carbon nanorods and multifunctional graphene nanocomposites for signal amplification. Sens. Actuators B 2017, 241, 151–159. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, T.; Li, J.; Jin, Q.; Xiao, R.; Wang, S.; Wang, C. Difunctional immunochromatographic assay based on magnetic quantum dot for ultrasensitive and simultaneous detection of multiple mycotoxins in foods. Sens. Actuators B 2022, 359, 131528. [Google Scholar] [CrossRef]
- Bai, Y.; Shu, T.; Su, L.; Zhang, X. Fluorescent Gold Nanoclusters for Biosensor and Bioimaging Application. Crystals 2020, 10, 357. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Yang, Q.; Li, H.; Li, F. pH and Redox Dual-Response Disulfide Bond-Functionalized Red-Emitting Gold Nanoclusters for Monitoring the Contamination of Organophosphorus Pesticides in Foods. Anal. Chem. 2021, 93, 7362–7368. [Google Scholar] [CrossRef] [PubMed]
- Obliosca, J.M.; Liu, C.; Yeh, H.C. Fluorescent silver nanoclusters as DNA probes. Nanoscale 2013, 5, 8443–8461. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Pebdeni, A.; Mousavizadegan, M.; Hosseini, M. Sensitive detection of S. aureus using aptamer- and vancomycin -copper nanoclusters as dual recognition strategy. Food Chem. 2021, 361, 130137. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, Y.; Zhang, L.; Liu, K. Synthesis, properties, and applications of large-scale two-dimensional materials by polymer-assisted deposition. J. Semicond. 2019, 40, 061003. [Google Scholar] [CrossRef]
- Garg, M.; Gupta, A.; Sharma, A.; Singh, S. Advancements in 2D Materials Based Biosensors for Oxidative Stress Biomarkers. ACS Appl. Bio Mater. 2021, 4, 5944–5960. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, L.; Li, Z.; Lu, Z.; Yin, W.; Nie, A.; Ding, F.; Wang, B.; Han, H. Versatile Electrochemiluminescence Assays for PEDV Antibody Based on Rolling Circle Amplification and Ru-DNA Nanotags. Anal. Chem. 2018, 90, 7415–7421. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Z.; Liu, R.; Wu, R.; Zhang, J. DNA-encoded MXene-Pt nanozyme for enhanced colorimetric sensing of mercury ions. Chem. Eng. J. 2022, 442, 136072. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, S.; Shu, F.; Liu, Z. An MnO2 nanosheet as a label-free nanoplatform for homogeneous biosensing. Chem. Commun. 2014, 50, 1095–1097. [Google Scholar] [CrossRef]
- Xi, Q.; Zhou, D.-M.; Kan, Y.-Y.; Ge, J.; Wu, Z.-K.; Yu, R.-Q.; Jiang, J.-Q. Highly Sensitive and Selective Strategy for MicroRNA Detection Based on WS2 Nanosheet Mediated Fluorescence Quenching and Duplex-Specific Nuclease Signal Amplification. Anal. Chem. 2014, 86, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.; Wang, W.; Tan, X.; Lu, Z.; Han, H. Metal-organic frameworks-based sensitive electrochemiluminescence biosensing. Biosens. Bioelectron. 2020, 164, 112332. [Google Scholar] [CrossRef]
- Mortada, B.; Matar, T.; Sakaya, A.; Atallah, H.; Kara Ali, Z.; Karam, P.; Hmadeh, M. Postmetalated Zirconium Metal Organic Frameworks as a Highly Potent Bactericide. Inorg. Chem. 2017, 56, 4739–4744. [Google Scholar] [CrossRef]
- Safaei, S.; Kazemian, H.; Junk, P.C. Dual functional MOF as a selective fluorescent naked-eye detector and effective sorbent for mercury ion. J. Solid State Chem. 2021, 300, 122267. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, G.; Gou, D.; Luo, P.; Yao, Y.; Chen, H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super P. Biosens. Bioelectron. 2019, 142, 111486. [Google Scholar] [CrossRef]
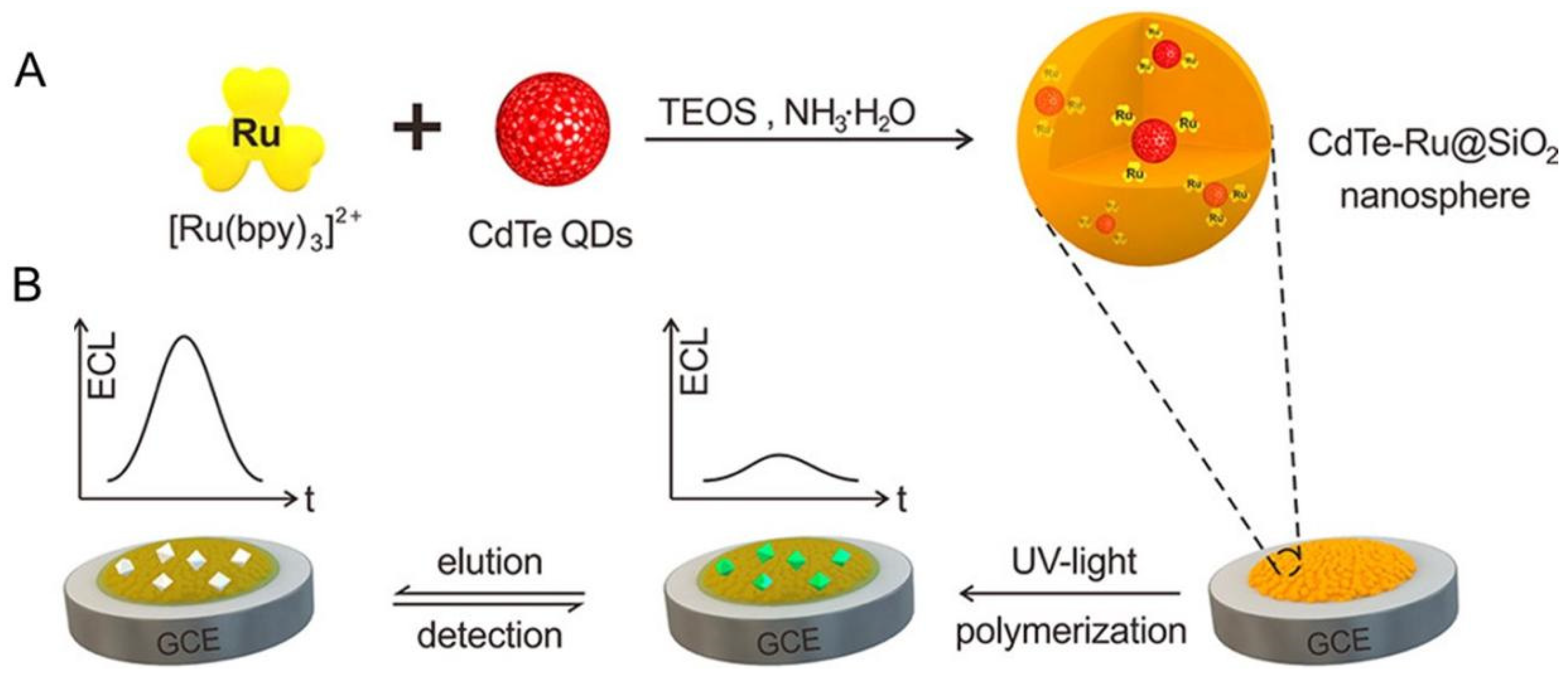
- Chen, M.-M.; Wang, Y.; Cheng, S.-B.; Wen, W.; Zhang, X.; Wang, S.; Huang, W.-H. Construction of Highly Efficient Resonance Energy Transfer Platform Inside a Nanosphere for Ultrasensitive Electrochemiluminescence Detection. Anal. Chem. 2018, 90, 5075–5081. [Google Scholar] [CrossRef]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly Imprinted Polymer as an Antibody Substitution in Pseudo-immunoassays for Chemical Contaminants in Food and Environmental Samples. J. Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, N.; Guclu, G.; Kelebek, H.; Capanoglu, E.; Selli, S. Application of Molecularly Imprinted Polymers for the Detection of Volatile and Off-Odor Compounds in Food Matrices. ACS Omega 2022, 7, 15258–15266. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, T.; Ye, L.; Wang, Y.; Xie, C. Core-shell magnetic molecularly imprinted polymer nanoparticles for the extraction of triazophos residues from vegetables. Microchim. Acta 2017, 184, 1011–1019. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, M.; Zhang, X.; Cao, J.; She, Y.; Cao, Z.; Wang, J.; Abd El-Aty, A.M. Acetylcholinesterase Immobilized on Magnetic Mesoporous Silica Nanoparticles Coupled with Fluorescence Analysis for Rapid Detection of Carbamate Pesticides. ACS Appl. Nano Mater. 2022, 5, 1327–1338. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Jing, X.; Miao, H.; Zhao, Y. SERS-Active Composites with Au–Ag Janus Nanoparticles/Perovskite in Immunoassays for Staphylococcus aureus Enterotoxins. ACS Appl. Mater. Interfaces. 2022, 14, 3293–3301. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Miao, H.; Jing, X. “Add on” Dual-Modal Optical Immunoassay by Plasmonic Metal NP-Semiconductor Composites. Anal. Chem. 2021, 93, 3250–3257. [Google Scholar] [CrossRef]
- Ma, H.; Li, M.; Yu, T.; Zhang, H.; Xiong, M.; Li, F. Magnetic ZIF-8-Based Mimic Multi-enzyme System as a Colorimetric Biosensor for Detection of Aryloxyphenoxypropionate Herbicides. ACS Appl. Mater. Interfaces 2021, 13, 44329–44338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Liu, J.; Su, Z. Bio-inspired Nanoenzyme Synthesis and Its Application in A Portable Immunoassay for Food Allergy Proteins. J. Agric. Food Chem. 2021, 69, 14751–14760. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Kasimir, M.; Behrens, M.; Schulz, M.; Kuchenbuch, H.; Focke, C.; Humpf, H.-U. Intestinal Metabolism of α- and β-Glucosylated Modified Mycotoxins T-2 and HT-2 Toxin in the Pig Cecum Model. J. Agric. Food Chem. 2020, 68, 5455–5461. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Zhao, S.; Niazi, S.; Mohsin, A.; Shoaib, M.; Duan, N.; Wu, S.; Wang, Z. Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sens. Actuators B 2018, 277, 328–335. [Google Scholar] [CrossRef]
- Goryacheva, O.A.; Beloglazova, N.V.; Goryacheva, I.Y.; Saeger, S.D. Homogenous FRET-based fluorescent immunoassay for deoxynivalenol detection by controlling the distance of donor-acceptor couple. Talanta 2021, 225, 121973. [Google Scholar] [CrossRef]
- Peltomaa, R.; Benito-Peña, E.; Barderas, R.; Sauer, U.; Gonzalez Andrade, M.C. Moreno-Bondi, M. Microarray-Based Immunoassay with Synthetic Mimotopes for the Detection of Fumonisin B1. Anal. Chem. 2017, 89, 6216–6223. [Google Scholar] [CrossRef]
- Peltomaa, R.; Amaro-Torres, F.; Carrasco, S.; Orellana, G.; Benito-Peña, E.; Moreno-Bondi, M.C. Homogeneous Quenching Immunoassay for Fumonisin B1 Based on Gold Nanoparticles and an Epitope-Mimicking Yellow Fluorescent Protein. ACS Nano 2018, 12, 11333–11342. [Google Scholar] [CrossRef]
- Yan, J.-X.; Hu, W.-J.; You, K.-H.; Ma, Z.-E.; Xu, Y.; Li, Y.-P.; He, Q.-H. Biosynthetic Mycotoxin Conjugate Mimetics-Mediated Green Strategy for Multiplex Mycotoxin Immunochromatographic Assay. J. Agric. Food Chem. 2020, 68, 2193–2200. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, Z.; Tian, Y.; Yang, Z.; Cao, Z.; Zhang, L.; Qi, Y.; Chang, J.; Zhang, S.; Wang, H. Shape Coding Microhydrogel for a Real-Time Mycotoxin Detection System Based on Smartphones. ACS Appl. Mater. Interfaces. 2019, 11, 8584–8590. [Google Scholar] [CrossRef]
- Liu, R.; Li, W.; Cai, T.; Deng, Y.; Ding, Z.; Liu, Y.; Zhu, X.; Wang, X.; Liu, J.; Liang, B. TiO2 Nanolayer-Enhanced Fluorescence for Simultaneous Multiplex Mycotoxin Detection by Aptamer Microarrays on a Porous Silicon Surface. ACS Appl. Mater. Interfaces 2018, 10, 14447–14453. [Google Scholar] [CrossRef] [PubMed]
- Landa, S.D.T.; Bogireddy, N.K.R.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M.; Shen, H.; Che, G.; Qiao, Y.; Liu, B. Recyclable Multifunctional Magnetic Mesoporous Silica Nanocomposite for Ratiometric Detection, Rapid Adsorption, and Efficient Removal of Hg(II). ACS Sustain. Chem. Eng. 2018, 6, 1744–1752. [Google Scholar] [CrossRef]
- Pi, K.; Liu, J.; Cappellen, P.V. A DNA-based biosensor for aqueous Hg(II): Performance under variable pH, temperature and competing ligand composition. J. Hazard. Mater. 2020, 385, 121572. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Reddy, A.S.; Panda, A.; Sarkar, D.; Son, Y.; Yoon, M. Reversible Fluorescence Switching of Metal−Organic Framework Nanoparticles for Use as Security Ink and Detection of Pb2+ Ions in Aqueous Media. ACS Appl. Nano Mater. 2020, 3, 3684–3692. [Google Scholar] [CrossRef]
- Bain, D.; Maity, S.; Paramanik, B.; Patra, A. Core-Size Dependent Fluorescent Gold Nanoclusters and Ultrasensitive Detection of Pb2+ Ion. ACS Sustain. Chem. Eng. 2018, 6, 2334–2343. [Google Scholar] [CrossRef]
- Yang, C.; Yu, P.; Li, Y.; Wang, J.; Ma, X.; Liu, N.; Lv, T.; Zheng, H.; Wu, H.; Li, H.; et al. Platform Formed from ZIF-8 and DNAzyme: “Turn-On” Fluorescence Assay for Simple, High-Sensitivity, and High-Selectivity Detection of Pb2+. J. Agric. Food. Chem. 2022, 70, 9567–9576. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Peng, Y.; Yang, H.; Zhou, Y. Highly sensitive biosensor based on aptamer and hybridization chain reaction for detection of cadmium ions. Luminescence 2022, 37, 665–671. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, Y.; Zou, W.; Wang, C.; Tao, H.; Wu, Y. V6O13 Nanobelts for Simultaneous Detection of Cd(II) and Pb(II) in Water. ACS Appl. Nano Mater. 2021, 4, 4654–4664. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Liu, J. One Pot Generation of Blue and Red Carbon Dots in One Binary Solvent System for Dual Channel Detection of Cr3+ and Pb2+ Based on Ion Imprinted Fluorescence Polymers. ACS Sens. 2019, 4, 1917–1924. [Google Scholar] [CrossRef]
- Huang, P.-J.J.; Liu, J. Sensing Parts-per-Trillion Cd2+, Hg2+, and Pb2+ Collectively and Individually Using Phosphorothioate DNAzymes. Anal. Chem. 2014, 86, 5999–6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikumar, A.; Panneerselvam, P.; Morad, N. Metal–Polydopamine Framework as an Effective Fluorescent Quencher for Highly Sensitive Detection of Hg(II) and Ag(I) Ions through Exonuclease III Activity. ACS Appl. Mater. Interfaces 2018, 10, 20550–20558. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Thapa, R.; Modi, B.; Bhandari, S.; Timilsina, A.P.; Yadav, R.P.; Aryal, B.; Gautam, S.; Sharma, P.; Thapa, B.B. Technological Advancements for the Detection of Antibiotics in Food Products. Processes 2021, 9, 1500. [Google Scholar] [CrossRef]
- Barveen, N.R.; Wang, T.J.; Chang, Y.H. Photochemical synthesis of Au nanostars on PMMA films by ethanol action as flexible SERS substrates for in-situ detection of antibiotics on curved surfaces. Chem. Eng. J. 2022, 431, 134240. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Tajahmadi, S.; Rezakazemi, M.; Amini, M.; Kamkar, M.; Rojas, O.J.; Arjmand, M. Coordination chemistry of metal–organic frameworks: Detection, adsorption, and photodegradation of tetracycline antibiotics and beyond. Coord. Chem. Rev. 2022, 464, 214562. [Google Scholar] [CrossRef]
- Hong, C.; Zhang, X.; Ye, S.; Yang, H.; Huang, Z.; Yang, D.; Cai, R.; Tan, W. Aptamer-Pendant DNA Tetrahedron Nanostructure Probe for Ultrasensitive Detection of Tetracycline by Coupling Target-Triggered Rolling Circle Amplification. ACS Appl. Mater. Interfaces 2021, 13, 19695–19700. [Google Scholar] [CrossRef]
- Yang, K.; Jia, P.; Hou, J.; Bu, T.; Sun, X.; Liu, Y.; Wang, L. Innovative Dual-Emitting Ratiometric Fluorescence Sensor for Tetracyclines Detection Based on Boron Nitride Quantum Dots and Europium Ions. ACS Sustain. Chem. Eng. 2020, 8, 17185–17193. [Google Scholar] [CrossRef]
- Mousavizadegan, M.; Hosseini, M.; Sheikholeslami, M.N.; Hamidipanah, Y.; Ganjali, M.R. Smartphone image analysis-based fluorescence detection of tetracycline using machine learning. Food Chem. 2023, 403, 134364. [Google Scholar] [CrossRef]
- Arsand, J.B.; Jank, L.; Martins, M.T.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M.; Sirtori, C. Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry. Talanta 2016, 154, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Deng, S.; Yuan, Z.; Li, C.; Lu, Y.; He, Q.; Zhou, M.; Deng, R. Engineering Multivalence Aptamer Probes for Amplified and Label-Free Detection of Antibiotics in Aquatic Products. J. Agric. Food. Chem. 2020, 68, 2554–2561. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Du, C.; Li, Y.; Ma, X.; Yang, C.; Xu, W.; Sun, C. Structure-switching aptamer triggering signal amplification strategy for tobramycin detection based on hybridization chain reaction and fluorescence synergism. Talanta 2022, 243, 123318. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Ang, C.Y.W.; Thompson, H.C., Jr. Rapid method for the determination of ampicillin residues in animal muscle tissues by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 1997, 694, 401–407. [Google Scholar] [CrossRef]
- Chen, C.; Lei, H.; Liu, N.; Yan, H. An aptasensor for ampicillin detection in milk by fluorescence resonance energy transfer between upconversion nanoparticles and Au nanoparticles. Food Chem. 2022, 15, 100439. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Khataee, A.; Rashidi, M.R.; Razmjou, A. Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem. 2020, 314, 126172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Zhao, L.; Li, M.; Wen, Y. An fluorescence resonance energy transfer sensing platform based on signal amplification strategy of hybridization chain reaction and triplex DNA for the detection of Chloramphenicol in milk. Food Chem. 2021, 357, 129769. [Google Scholar] [CrossRef]
- Berendsen, B.; Pikkemaat, M.; Romkens, P.; Wegh, R.; van Sisseren, M.; Stolker, L.; Nielen, M. Occurrence of Chloramphenicol in Crops through Natural Production by Bacteria in Soil. J. Agric. Food. Chem. 2013, 61, 4004–4010. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhai, F.; Jia, M. Novel label-free fluorescence aptasensor for chloramphenicol detection based on a DNA four-arm junction-assisted signal amplification strategy. Food Chem. 2022, 366, 130648. [Google Scholar] [CrossRef]
- Mandal, S.; Paul, D.; Saha, S.; Das, P. Multi-layer perceptron for detection of different class antibiotics from visual fluorescence response of a carbon nanoparticle-based multichannel array sensor. Sens. Actuators, B 2022, 360, 131660. [Google Scholar] [CrossRef]
- Sheikholeslami, M.N.; Hamidipanah, Y.; Salehnia, F.; Arshian, S.; Hosseini, M.; Ganjali, M.R. Multiplex Detection of Antibiotic Residues in Milk: Application of MCR-ALS on Excitation–Emission Matrix Fluorescence (EEMF) Data Sets. Anal. Chem. 2022, 94, 6206–6215. [Google Scholar] [CrossRef]
- Khateb, H.; Klos, G.; Meyer, R.L.; Sutherland, D.S. Development of a Label-Free LSPR-Apta Sensor for Staphylococcus aureus Detection. ACS Appl. Bio Mater. 2020, 3, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Peng, J.; Han, J.; Zhang, G.; Huang, Y.; Duan, M.; Liu, D.; Xiong, Y.; Xia, S.; Lai, W. A novel method based on fluorescent magnetic nanobeads for rapid detection of Escherichia coli O157:H7. Food Chem. 2019, 276, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Zhang, P.; Yan, Z.; Zhou, Y.; Du, Y.; Qu, C.; Song, Y.; Zhou, D.; Qu, S.; et al. Cell-based fluorescent microsphere incorporated with carbon dots as a sensitive immunosensor for the rapid detection of Escherichia coli O157 in milk. Biosens. Bioelectron. 2021, 179, 113057. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Haruna, S.; Ali, S.; Xu, J.; Zhang, Y.; Lü, P.; Li, H.; Chen, Q. A sensitive and accurate fluorescent genosensor for Staphylococcus aureus detection. Sens. Actuators B 2022, 355, 131311. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Liao, Z.; Sun, Y.; Cheng, Q.; Song, Y.; Song, E.; Tan, W. Magnetism-Resolved Separation and Fluorescence Quantification for Near-Simultaneous Detection of Multiple Pathogens. Anal. Chem. 2018, 90, 9621–9628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Z.; Jin, M.; Du, P.; Chen, G.; Cui, X.; Zhang, Y.; Qin, G.; Yan, F.; Abd El-Aty, A.M. Fluorescence immunoassay for multiplex detection of organophosphate pesticides in agro-products based on signal amplification of gold nanoparticles and oligonucleotides. Food Chem. 2020, 326, 126813. [Google Scholar] [CrossRef]
- Wang, L.; Haruna, S.A.; Ahmad, W.; Wu, J.; Chen, Q.; Ouyang, Q. Tunable multiplexed fluorescence biosensing platform for simultaneous and selective detection of paraquat and carbendazim pesticides. Food Chem. 2022, 388, 132950. [Google Scholar] [CrossRef]
- Amalraj, A.; Perumal, P. Dual-mode amplified fluorescence oligosensor mediated MOF-MoS2 for ultra-sensitive simultaneous detection of 17β-estradiol and chloramphenicol through catalytic target- recycling activity of exonuclease I. Microchem. J. 2022, 173, 106971. [Google Scholar] [CrossRef]

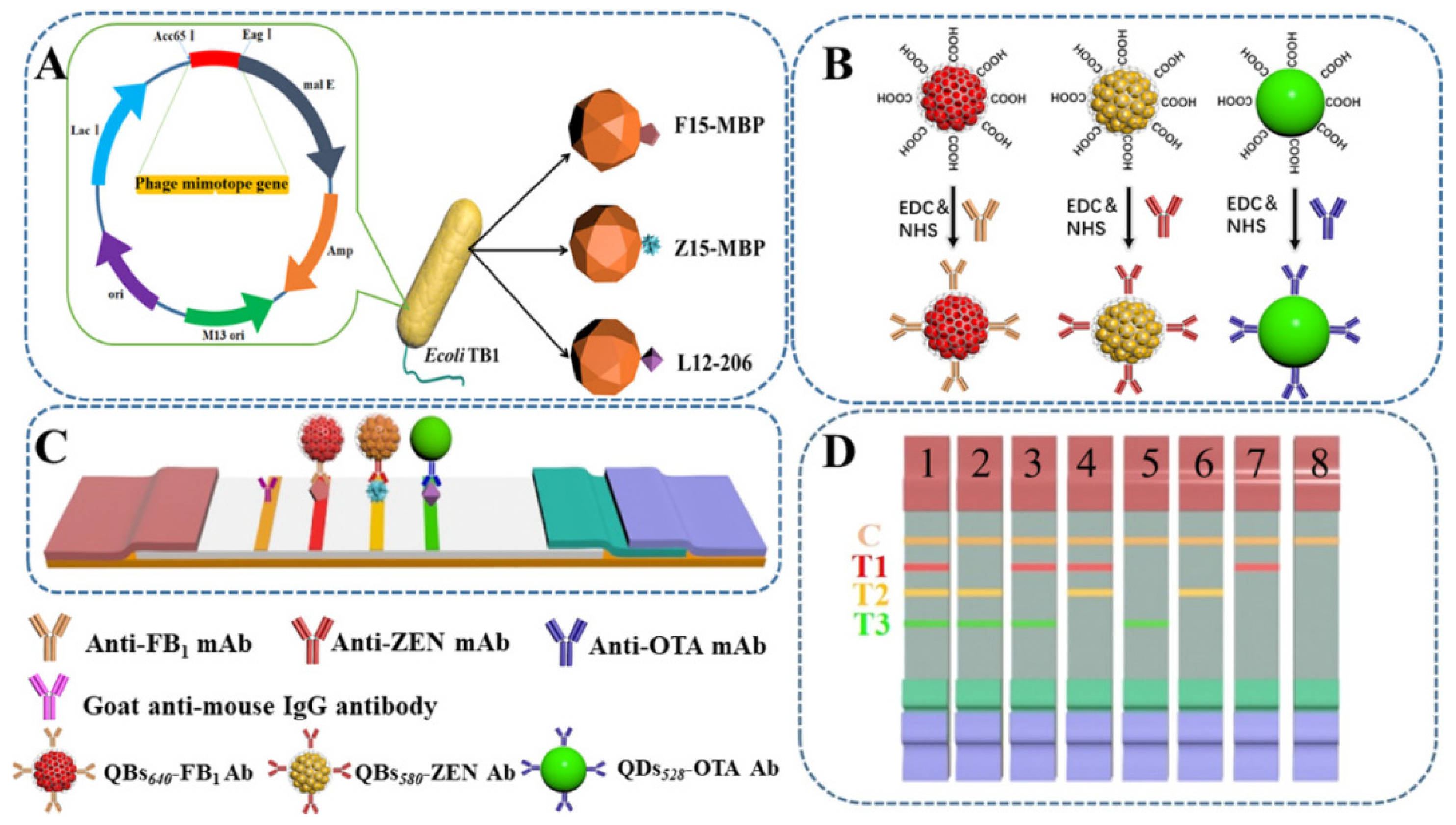

| Analytes | Nanomaterials | Linear Range | LOD | Food Matrix | Ref. |
|---|---|---|---|---|---|
| Mycotoxins | |||||
| T-2 toxin | AgNCs | 0.005–500 ng/mL | 0.93 pg/mL | maize and wheat | [86] |
| DON | QDs | 1–10 ng/ml | 28 μg/kg | wheat | [87] |
| FB1 | AuNPs | 7.3–22.6 ng/mL | 1.1 ng/mL | wheat | [89] |
| FB1, ZEN and OTA | QDs/QD | ------ | 0.25 ng/mL 3.0 ng/mL 0.5 ng/mL | ------ | [90] |
| OTA and AFB1 | hydrogel particles | 0.1–500 ng/mL, 0.1–200 ng/mL | 0.1 ng/mL | corn flour | [91] |
| OTA, AFB1, and FB1 | TiO2-Si | 0.1–10 ng/mL, 0.01–10 ng/mL, 0.001–10 ng/mL | 15.4 pg/mL 1.48 pg/mL 0.21 pg/mL | rice, corn, and wheat | [92] |
| Heavy metals | |||||
| Hg2+ | CdTe QDs | 0.7–900 nM | 2.5 nM | water | [94] |
| Hg2+ | DNA-hydrogel | ------ | 10 nM | water | [95] |
| Pb2+ | NH2-MIL-125(Ti) MOF | 0–11 nM | 7.7 pM | ------ | [96] |
| Pb2+ | AuNCs | 0–190 nM | 10 nM | pond water and river water | [97] |
| Pb2+ | ZIF-8 | 0.01–10.0 nM | 7.1 pM | water and fish | [98] |
| Cd2+ and Pb2+ | V6O13 nanobelts | 5–200 μg/L, 5–100 μg/L | 1.89 μg/L, 2.11 μg/L | water | [100] |
| Cr3+ and Pb2+ | CDs | 0.1–6.0 μM, 0.1–5.0 μM | 27 nM 34 nM | water | [101] |
| Cd2+, Hg2+ and Pb2+ | DNAzymes | ------ | 4.8 nM 2.0 nM 0.1 nM | ------ | [102] |
| Hg2+ and Ag+ | MPDA frame-works | 0–2 nM 1–3 nM | 1.3 pM 34 pM | water | [103] |
| Antibiotics | |||||
| TET | Apt-tet MBs | 0.001–10 ng/mL | 0.724 pg/mL | fish and honey | [108] |
| TET OTC DOX | BNQD-Eu3+ | ------ | 0.019 μM 0.104 μM 0.104 μM | milk and beef | [109] |
| TET | BSA-BMNCs | ------ | ------ | water and milk | [110] |
| KAN | aptamer probes | 0.1–75 nM | 0.097 nM | aquatic products | [112] |
| tobramycin | magnetic beads | 0.3–50 μM | 17.37 nM | ------ | [113] |
| AMP | UCNPs | 10–100 ng/mL | 3.9 ng/mL | milk | [115] |
| penicillin | CDs | 1–32 nM | 0.34 nM | milk | [116] |
| CAP | DNA four-arm junctions | 1.0 pg/mL–10 ng/mL | 0.72 pg/mL | milk and honey | [119] |
| AMP, CPFX, KAN, SMZ, TET, and TMP | carbon nanoparticle | ------ | ------ | poultry feeds | [120] |
| TET, AMP, and sulfacetamide | nanocluster | 50–5000 ng/mL 5–5000 ng/mL 50–5000 ng/mL | 3.5 ng/mL 1.4 ng/mL 7.6 ng/mL | milk | [121] |
| Foodborne pathogens | |||||
| Escherichia coli | CDs | 2.4 × 102–2.4 × 107 CFU/mL | 2.4 × 102 CFU/mL | milk | [124] |
| S. aureus | GOQDs | 1 × 10−17–1 × 10−11 mol/L | 0.98 × 10−17 mol/L | ------ | [125] |
| Escherichia coli, Salmonella typhimurium | apt-FMNPs | 40–108 CFU/mL 63–108 CFU/mL | 16 CFU/mL 25 CFU/mL | ------ | [126] |
| Other illegal additives | |||||
| Triazophos, parathion, chlorpyrifos | AuNPs | 0.01–20 μg/L, 0.05–50 μg/L, 0.5–1000 μg/L | 0.007 μg/L, 0.009 μg/L, 0.087 μg/L | rice, wheat, cucumber, cabbage, and apple | [127] |
| paraquat, carbendazim pesticides | UCNPs and BPNS | 1.0–1.0 × 105 ng/mL | 0.18 ng/mL, 0.45 ng/mL | ------ | [128] |
| CAP, 17β-estradiol | MOF-MoS2 | 0.9917–5 nM, 0–5 nM | 200 pM, 180 pM | milk, honey, and water | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Gui, Y.; Lv, X.; He, J.; Xie, F.; Li, J.; Cai, J. Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis. Biosensors 2022, 12, 1072. https://doi.org/10.3390/bios12121072
Zhou J, Gui Y, Lv X, He J, Xie F, Li J, Cai J. Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis. Biosensors. 2022; 12(12):1072. https://doi.org/10.3390/bios12121072
Chicago/Turabian StyleZhou, Jiaojiao, Yue Gui, Xuqin Lv, Jiangling He, Fang Xie, Jinjie Li, and Jie Cai. 2022. "Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis" Biosensors 12, no. 12: 1072. https://doi.org/10.3390/bios12121072
APA StyleZhou, J., Gui, Y., Lv, X., He, J., Xie, F., Li, J., & Cai, J. (2022). Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis. Biosensors, 12(12), 1072. https://doi.org/10.3390/bios12121072







