Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Fe2O3 Magnetic Nanospheres
2.3. Carboxylation of MWCNTs
2.4. Synthesis of Molecularly Imprinted Polymers
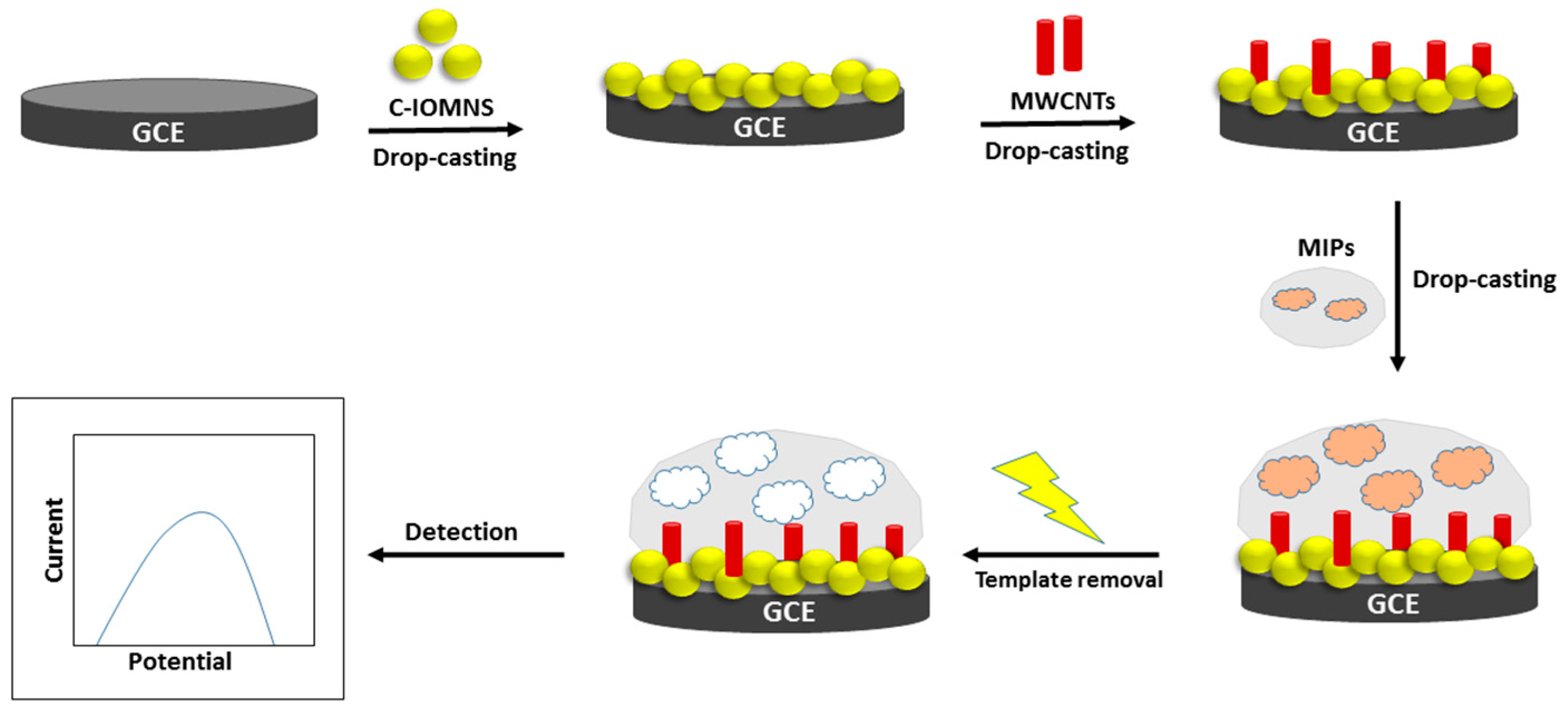
2.5. Fabrication of C-IO-MNSs/cMWCNTs/MIPs onto GCE
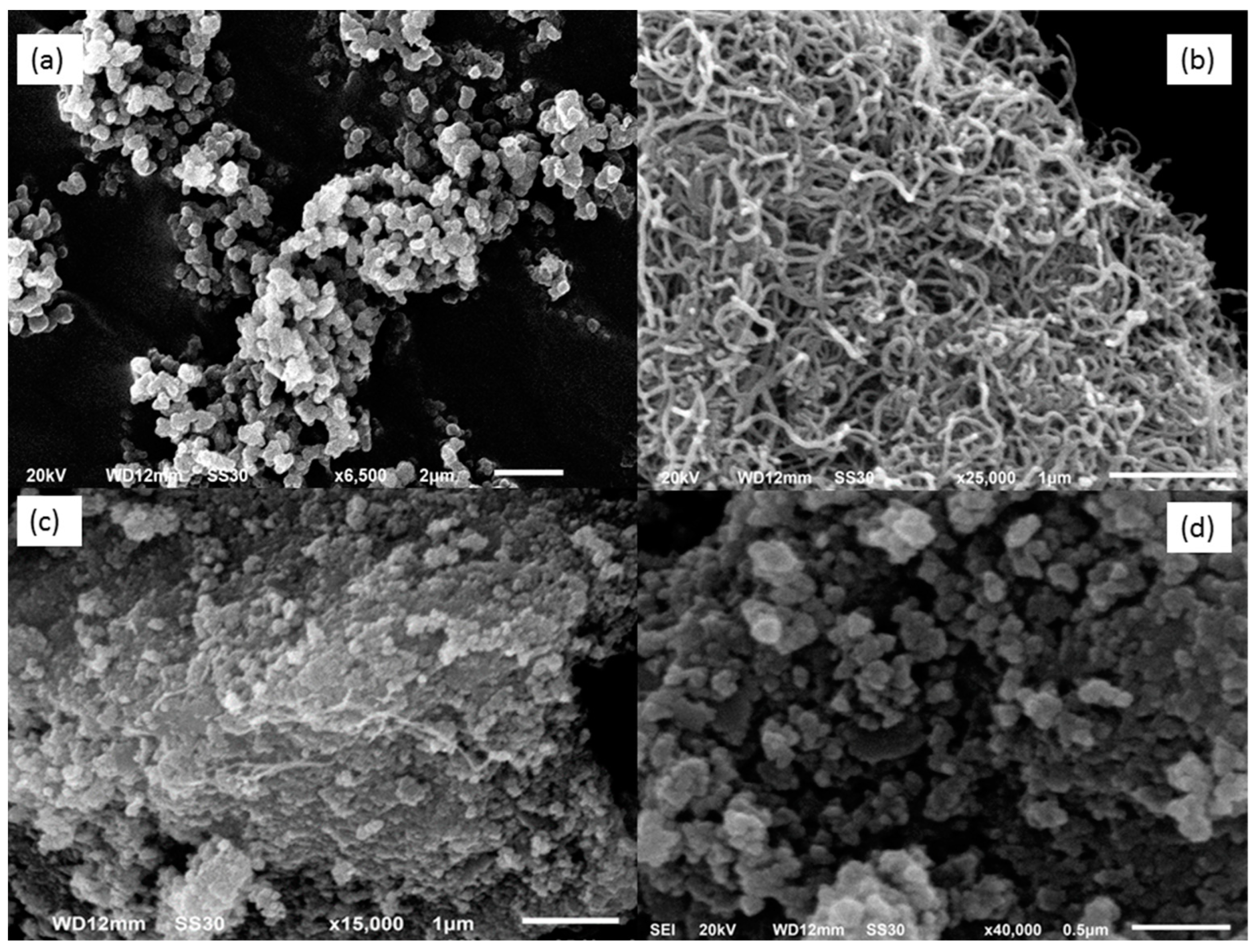
2.6. Characterization of Modified Electrodes
2.7. Cross-Reactivity and Real Sample Application
3. Results and Discussions
3.1. Fabrication and Characterization of C-IO-MNS/cMWCNT/sol-gel-MIP/GCE
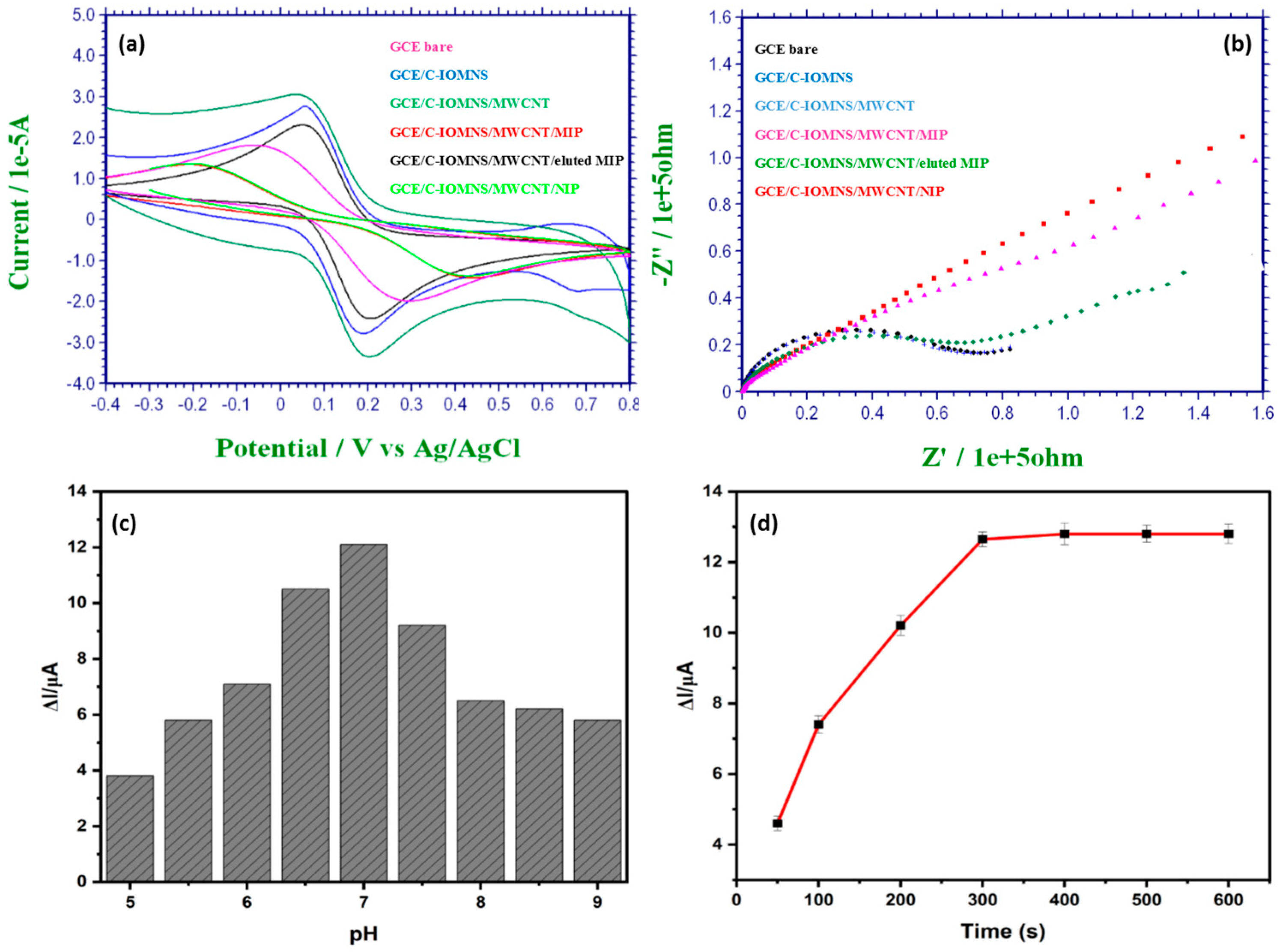
3.2. Electrochemical Characterization of Fabricated C-IO-MNS/MWCNT/sol-gel-MIP electrode
3.3. Optimization of C-IO-MNS/cMWCNT/sol-gel-MIP/GCE sensor
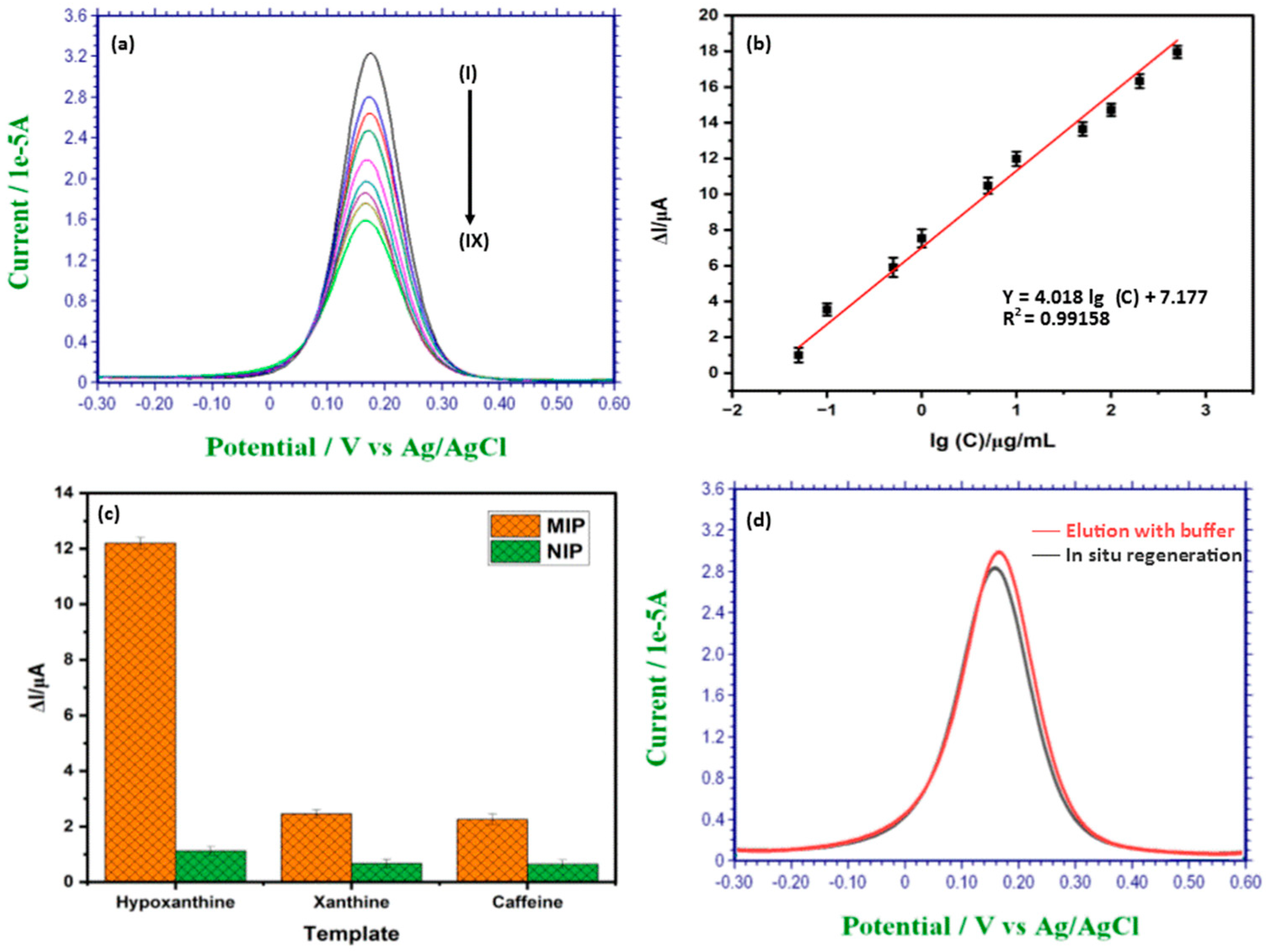
3.4. Detection of Hypoxanthine Using C-IO-MNS/cMWCNT/sol-gel-MIP/GCE
3.5. Selectivity Study of the MIP Sensor
3.6. Regeneration of the MIP Sensor
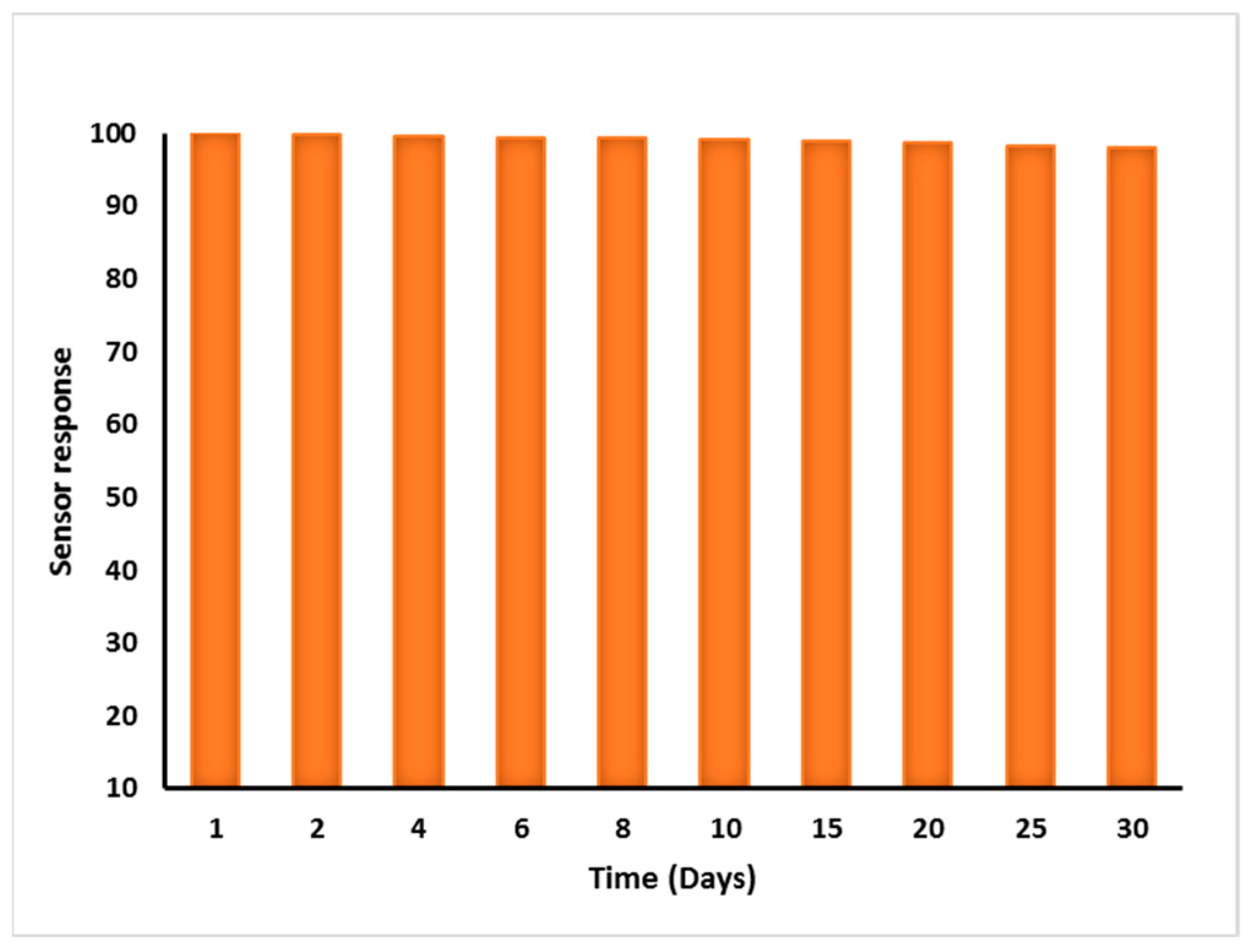
3.7. Repeatability, Reproducibility, and Stability of MIP Sensor
3.8. Application of MIP Sensor for Hypoxanthine Analysis in Meat Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, D.; Pandey, C.M.; Kumar, D. Highly Sensitive Enzymatic Biosensor Based on Polyaniline-Wrapped Titanium Dioxide Nanohybrid for Fish Freshness Detection. Appl. Biochem. Biotechnol. 2022, 194, 3765–3778. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnsoongnoen, S.; Jaroensuk, N. The grades and freshness assessment of eggs based on density detection using machine vision and weighing sensor. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Verma, N. Fibre-optic biosensor for the detection of xanthine for the evaluation of meat freshness. J. Phys. Conf. Ser. 2020, 1531, 12098. [Google Scholar] [CrossRef]
- Daniel, A.P.; Veeck, A.P.L.; Klein, B.; Ferreira, L.F.; Cunha, M.A.; Parodi, T.V.; Zeppenfeld, C.C.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; et al. Using the Essential Oil of Aloysia triphylla (L’Her.) Britton to Sedate Silver Catfish (Rhamdia quelen) during Transport Improved the Chemical and Sensory Qualities of the Fish during Storage in Ice. J. Food Sci. 2014, 79, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zou, L.; Zhang, Y.; Zhang, G.; Li, X.; Li, M.; Ye, H.; Liu, F.; Pan, H.; Yang, Y. Determination of purine contents in different parts of pork and beef by high performance liquid chromatography. Food Chem. 2015, 170, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Paper-Based Enzyme Biosensor for One-Step Detection of Hypoxanthine in Fresh and Degraded Fish. ACS Sensors. 2020, 5, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Dervisevic, E.; Şenel, M. Recent progress in nanomaterial-based electrochemical and optical sensors for hypoxanthine and xanthine. A review. Microchim. Acta. 2019, 186, 749. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Sundhoro, M.; Agnihotra, S.R.; Amberger, B.; Augustus, K.; Khan, N.D.; Barnes, A.; BelBruno, J.; Mendecki, L. An electrochemical molecularly imprinted polymer sensor for rapid and selective food allergen detection. Food Chem. 2021, 344, 128648. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.S.; Jung, J. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef] [PubMed]
- Lungu, A.; Sandu, I.; Boscornea, C.; Tomas, S.; Mihailciuc, C. Electrochemical study of curcumin and bisdemethoxycurcumin on activated glassy carbon electrode. Rev. Roum. Chim. 2010, 53, 109–115. [Google Scholar]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C. 2016, 67, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Minni, S.; Neelam, S. Electrochemical preparation of—Fe3O4/MWCNT—Polyaniline nanocomposite film for development of urea biosensor and its application in milk sample. J. Food Meas. Charact. 2019. [Google Scholar] [CrossRef]
- Garg, D.; Verma, N.; Monika. Preparation and Characterization of sol-gel molecularly imprinted polymers for the selective adsorption of hypoxanthine. 2022.
- Bai, J.; Zhang, X.; Peng, Y.; Hong, X.; Liu, Y.; Jiang, S.; Ning, B.; Gao, Z. Ultrasensitive sensing of diethylstilbestrol based on AuNPs/MWCNTs-CS composites coupling with sol-gel molecularly imprinted polymer as a recognition element of an electrochemical sensor. Sensors Actuators B. Chem. 2017, 238, 420–426. [Google Scholar] [CrossRef]
- Mooltongchun, M.; Teepoo, S. A Simple and Cost-effective Microfluidic Paper-Based Biosensor Analytical Device and its Application for Hypoxanthine Detection in Meat Samples. Food Anal. Methods. 2019, 12, 2690–2698. [Google Scholar] [CrossRef]
- Mustafa, F.; Othman, A.; Andreescu, S. Cerium oxide-based hypoxanthine biosensor for Fish spoilage monitoring. Sens. Actuators B. Chem. 2021, 332, 129435. [Google Scholar] [CrossRef]
- Erol, E.; Yildirim, E.; Cete, S. Construction of biosensor for hypoxanthine determination by immobilization of xanthine oxidase and uricase in polypyrrole-paratoluenesulfonate film. J. Solid State Electrochem. 2020, 24, 1695–1707. [Google Scholar] [CrossRef]
- Erna, K.H.; Xia, W.; Felicia, L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of Curcumin/Rice Starch Films for Sensitive Detection of Hypoxanthine in Chicken and Fish Meat Development of curcumin/rice starch films for sensitive detection of hypoxanthine in chicken and fish meat. Carbohydr. Polym. Technol. Appl. 2022, 3, 100189. [Google Scholar] [CrossRef]

| Working Electrode | Detection Method | LOD (µM) | Linear Range (µM) | Sample | Reference |
|---|---|---|---|---|---|
| XOD/HRP/µ-PAD | Colorimetric | 13 | 36–293 | Meat | [17] |
| XOD/NBT/Sol–gel/ CS | Colorimetric | 4.1 | 4–35 | Fish | [7] |
| XOD/U/PPy-pTS/Pt | Amperometric | 5 | 5–500 | Fish | [19] |
| XOD/CeNPs | Colorimetric | 89 | 300–597 | Fish | [18] |
| CR-RS films | Colorimetric | 38.63 | 1–100 | Chicken and Fish | [20] |
| MIP/ MWCNT/C-IO-MNS/GCE | DPV | 1.21 | 15–367 | Chicken, Pork, and Goat meat | This work |
| Meat Sample (n = 3) | Added Hypoxanthine (µg/mL) | Detected (µg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Chicken | Unspiked | 2.44 ± 0.05 | - | 2.0 |
| 5 | 6.91 ± 0.13 | 92.8 | 1.9 | |
| 10 | 12.21 ± 0.21 | 98.2 | 1.7 | |
| Pork | Unspiked | 6.67 ± 0.10 | 1.5 | |
| 5 | 11.43 ± 0.2 | 97.94 | 1.8 | |
| 10 | 16.50 ± 0.25 | 98.98 | 1.5 | |
| Goat meat | Unspiked | 5.89 ± 0.10 | 1.7 | |
| 5 | 10.29 ± 0.19 | 94.49 | 1.9 | |
| 10 | 15.66 ± 0.25 | 98.57 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, D.; Verma, N.; Monika. Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine. Biosensors 2022, 12, 1157. https://doi.org/10.3390/bios12121157
Garg D, Verma N, Monika. Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine. Biosensors. 2022; 12(12):1157. https://doi.org/10.3390/bios12121157
Chicago/Turabian StyleGarg, Diksha, Neelam Verma, and Monika. 2022. "Molecularly Imprinted Polymer-Based Electrochemical Sensor for Rapid and Selective Detection of Hypoxanthine" Biosensors 12, no. 12: 1157. https://doi.org/10.3390/bios12121157





