Ultrasensitive Determination of Glial-Fibrillary-Acidic-Protein (GFAP) in Human Serum-Matrix with a Label-Free Impedimetric Immunosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipments
2.3. Standard and Sample Preparation
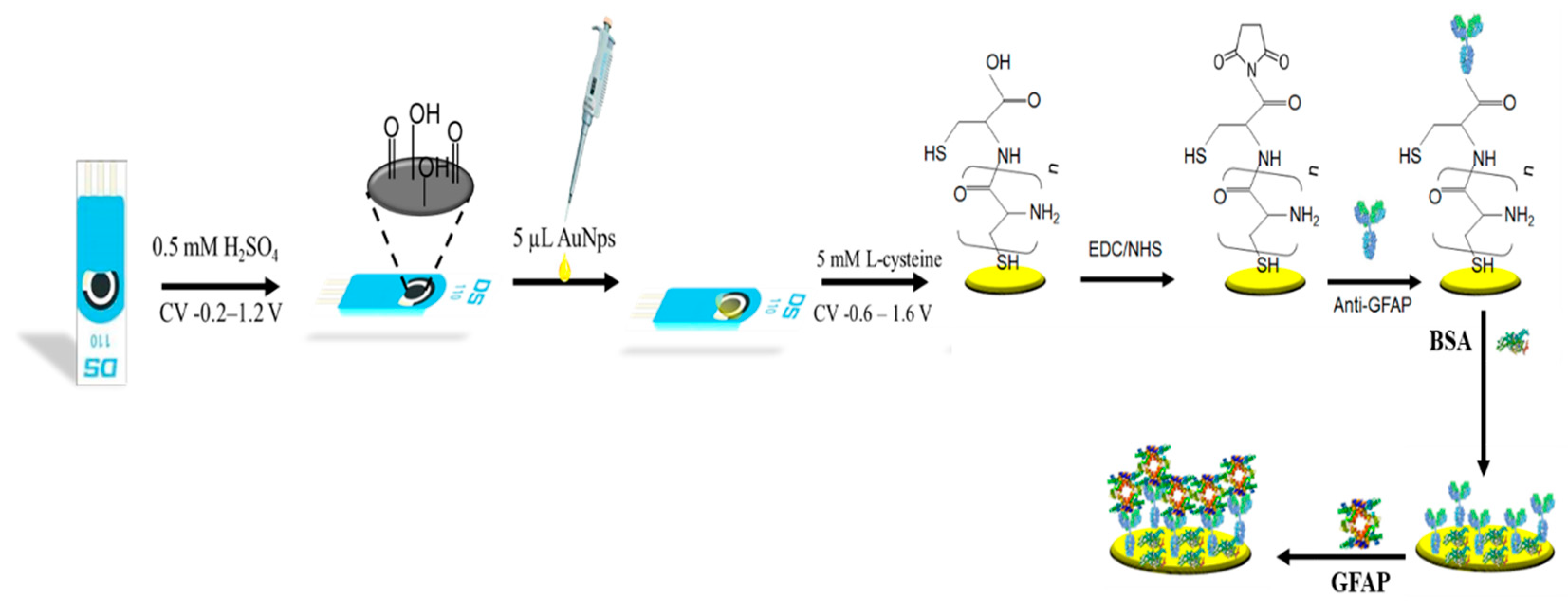
2.3.1. Preparation of Gold Nanoparticles Modified SPCE
2.3.2. Preparation of the Synthetic Human Serum Sample
2.3.3. Design of the Immunosensor
3. Results
3.1. Electrochemical Characterization of the Immunosensor
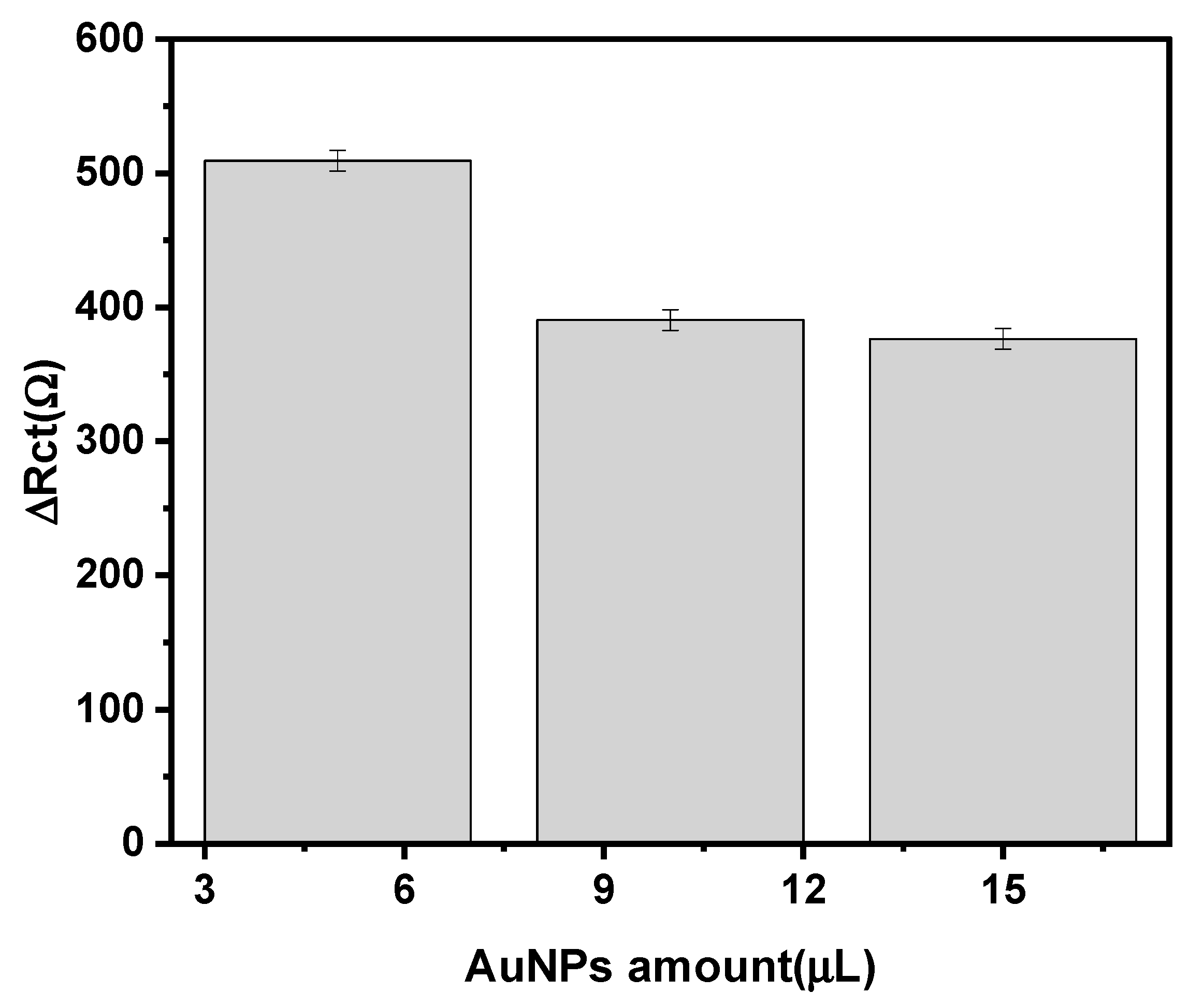
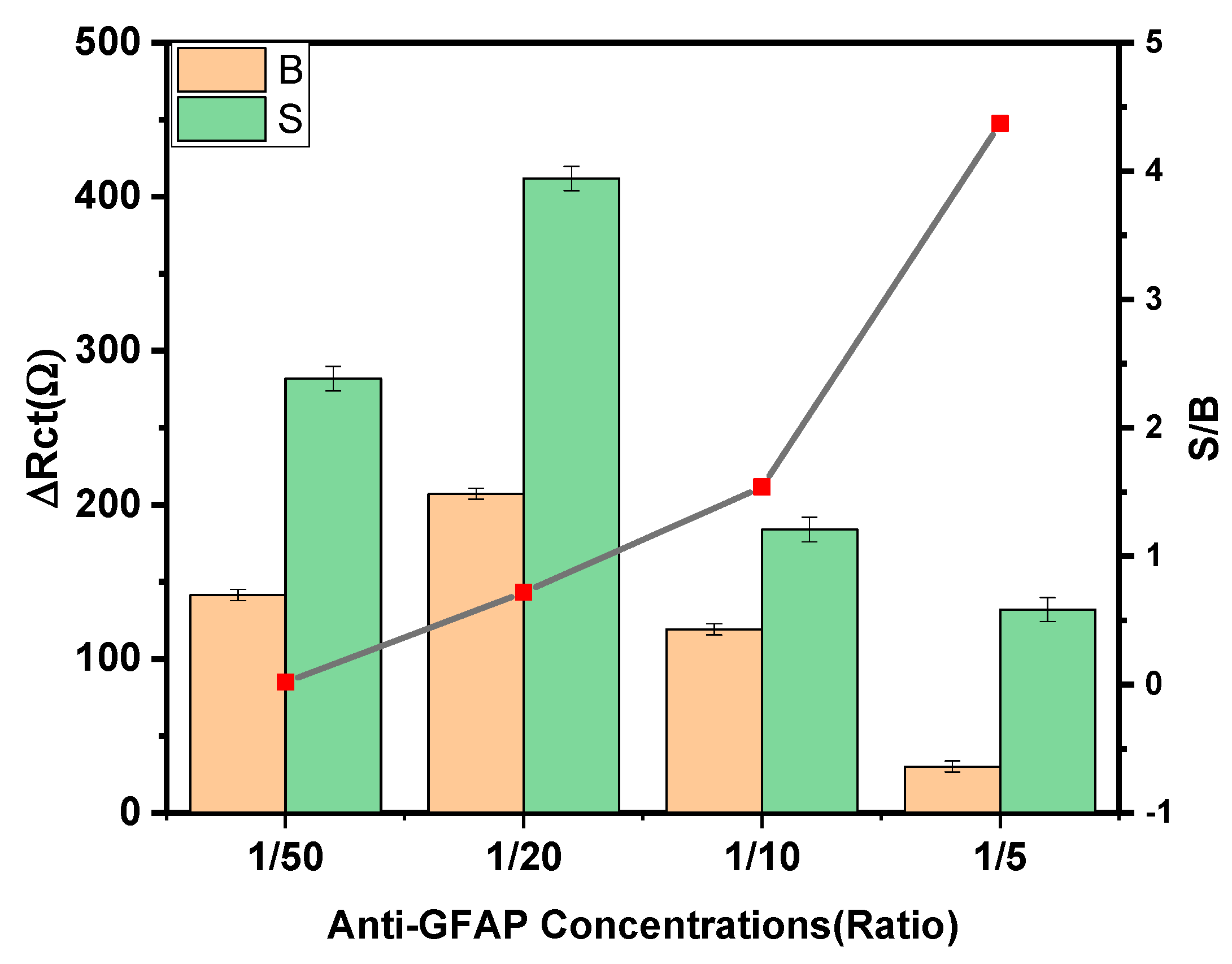
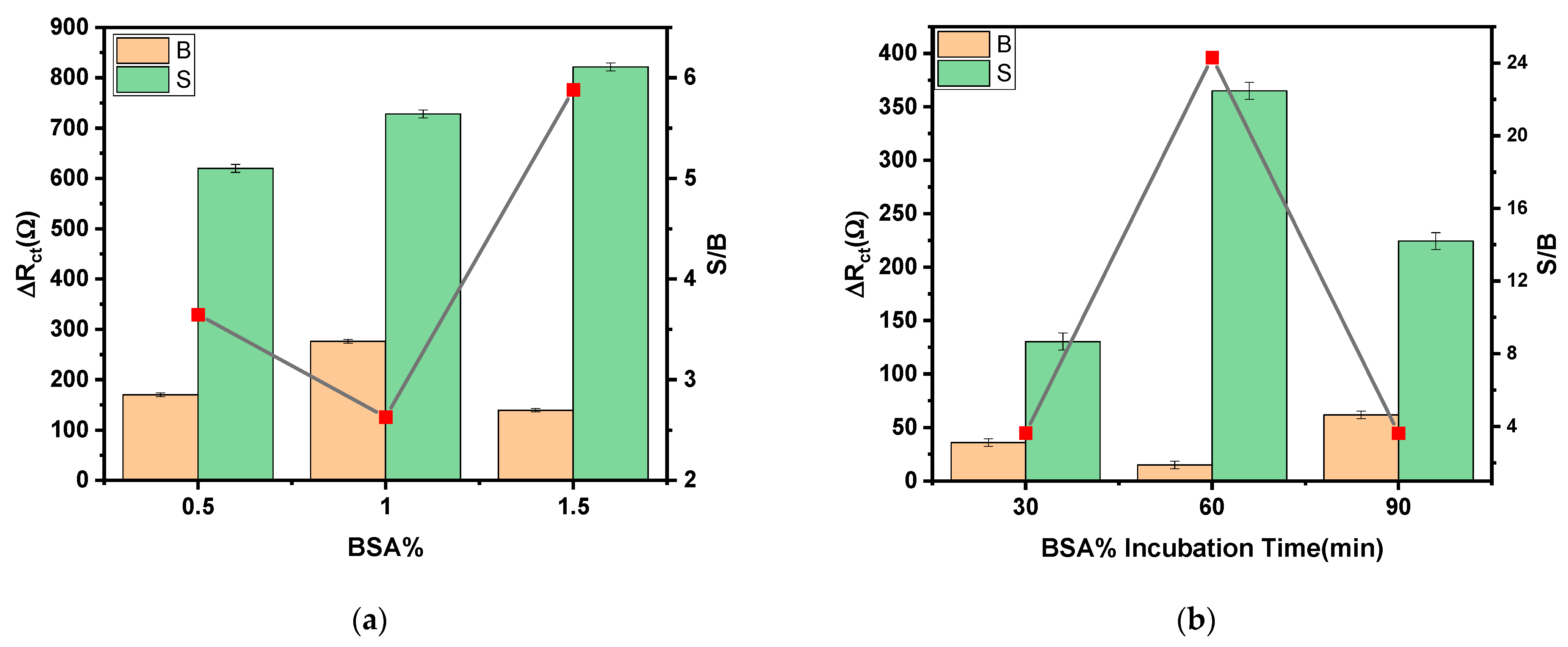
3.2. Optimization of the Experimental Variables Involved in the Preparation of the Immunosensor
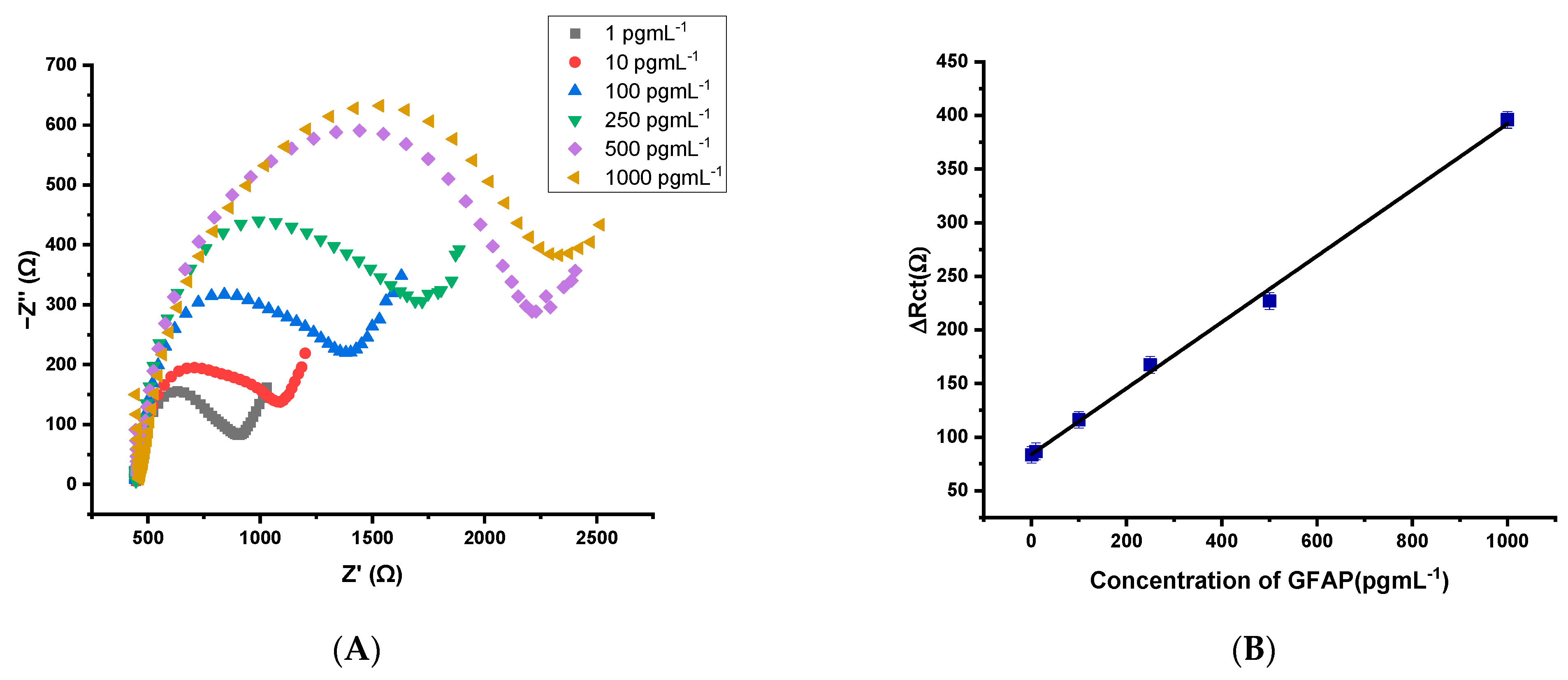
3.3. Analytical Performances for the Designed GFAP Biosensor
3.4. Application of the GFAP Immunosensor in a Synthetic Human Serum Sample
3.5. Selectivity Performance of the Immunosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vissers, J.L.; Mersch, M.E.; Rosmalen, C.F.; van Heumen, M.J.; van Geel, W.J.; Lamers, K.J.; Rosmalen, F.M.; Swinkels, L.M.; Thomsen, J.; Herrmann, M. Rapid immunoassay for the determination of glial fibrillary acidic protein (GFAP) in serum. Clin. Chim. Acta 2006, 366, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lumpkins, K.M.; Bochicchio, G.V.; Keledjian, K.; Simard, J.M.; McCunn, M.; Scalea, T. Glial Fibrillary Acidic Protein is Highly Correlated With Brain Injury. J. Trauma: Inj. Infect. Crit. Care 2008, 65, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res. Ther. 2021, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Simani, L.; Elmi, M.; Asadollahi, M. Serum GFAP level: A novel adjunctive diagnostic test in differentiate epileptic seizures from psychogenic attacks. Seizure 2018, 61, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Khetani, S.; Kollath, V.O.; Kundra, V.; Nguyen, M.D.; Debert, C.; Sen, A.; Karan, K.; Sanati-Nezhad, A. Polyethylenimine Modified Graphene-Oxide Electrochemical Immunosensor for the Detection of Glial Fibrillary Acidic Protein in Central Nervous System Injury. ACS Sensors 2018, 3, 844–851. [Google Scholar] [CrossRef]
- Petzold, A.; Keir, G.; Green, A.; Giovannoni, G.; Thompson, E. An ELISA for glial fibrillary acidic protein. J. Immunol. Methods 2004, 287, 169–177. [Google Scholar] [CrossRef]
- Jović, M.; Prim, D.; Saini, E.; Pfeifer, M.E. Towards a Point-of-Care (POC) Diagnostic Platform for the Multiplex Electrochemiluminescent (ECL) Sensing of Mild Traumatic Brain Injury (mTBI) Biomarkers. Biosensors 2022, 12, 172. [Google Scholar] [CrossRef]
- Missler, U.; Wiesmann, M.; Wittmann, G.; Magerkurth, O.; Hagenström, H. Measurement of Glial Fibrillary Acidic Protein in Human Blood: Analytical Method and Preliminary Clinical Results. Clin. Chem. 1999, 45, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Joseph, J. A novel time-resolved fluorescent lateral flow immunoassay for quantitative detection of the trauma brain injury biomarker-glial fibrillary acidic protein. Sens. Diagn. 2021, 1, 193–197. [Google Scholar] [CrossRef]
- Timilsina, S.S.; Ramasamy, M.; Durr, N.; Ahmad, R.; Jolly, P.; Ingber, D.E. Biofabrication of Multiplexed Electrochemical Immunosensors for Simultaneous Detection of Clinical Biomarkers in Complex Fluids. Adv. Heal. Mater. 2022, 11, 2200589. [Google Scholar] [CrossRef]
- Salahandish, R.; Haghayegh, F.; Khetani, S.; Hassani, M.; Nezhad, A.S. Immuno-affinity Potent Strip with Pre-Embedded Intermixed PEDOT:PSS Conductive Polymers and Graphene Nanosheets for Bio-Ready Electrochemical Biosensing of Central Nervous System Injury Biomarkers. ACS Appl. Mater. Interfaces 2022, 14, 28651–28662. [Google Scholar] [CrossRef] [PubMed]
- Salahandish, R.; Hassani, M.; Zare, A.; Haghayegh, F.; Sanati-Nezhad, A. Autonomous electrochemical biosensing of glial fibrillary acidic protein for point-of-care detection of central nervous system injuries. Lab. Chip 2022, 22, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, G.; Gamella, M.; Unal, M.A.; Gucuyener, K.; Montero-Calle, A.; Barderas, R.; Pingarrón, J.M.; Campuzano, S.; Ozkan, S.A. Assisting dementia diagnosis through the electrochemical immunosensing of glial fibrillary acidic protein. Talanta 2022, 246, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehmandoust, M.; Erk, E.E.; Soylak, M.; Erk, N.; Karimi, F. Metal–Organic Framework Based Electrochemical Immunosensor for Label-Free Detection of Glial Fibrillary Acidic Protein as a Biomarker. Ind. Eng. Chem. Res. 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Bott-Neto, J.L.; Machado, S.A.S.; Oliveira, O.N. Label-Free Electrochemical Immunosensor Made with Tree-like Gold Dendrites for Monitoring 25-Hydroxyvitamin D3 Metabolite. ACS Appl. Mater. Interfaces 2022, 14, 31455–31462. [Google Scholar] [CrossRef]
- Mendes, R.; Carvalhal, R.; Kubota, L. Effects of different self-assembled monolayers on enzyme immobilization procedures in peroxidase-based biosensor development. J. Electroanal. Chem. 2008, 612, 164–172. [Google Scholar] [CrossRef]
- Arya, S.K.; Pui, T.S.; Wong, C.C.; Kumar, S.; Rahman, A.R.A. Effects of the Electrode Size and Modification Protocol on a Label-Free Electrochemical Biosensor. Langmuir 2013, 29, 6770–6777. [Google Scholar] [CrossRef]
- Arya, S.K.; Wang, K.Y.; Wong, C.C.; Rahman, A.R.A. Anti-EpCAM modified LC-SPDP monolayer on gold microelectrode based electrochemical biosensor for MCF-7 cells detection. Biosens. Bioelectron. 2013, 41, 446–451. [Google Scholar] [CrossRef]
- Arya, S.K.; Solanki, P.R.; Datta, M.; Malhotra, B.D. Recent advances in self-assembled monolayers based biomolecular electronic devices. Biosens. Bioelectron. 2009, 24, 2810–2817. [Google Scholar] [CrossRef]
- Arya, S.K.; Prusty, A.K.; Singh, S.; Solanki, P.R.; Pandey, M.K.; Datta, M.; Malhotra, B.D. Cholesterol biosensor based on N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane self-assembled monolayer. Anal. Biochem. 2007, 363, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.M.; Singh, R.; Sumana, G.; Pandey, M.K.; Malhotra, B.D. Electrochemical genosensor based on modified octadecanethiol self-assembled monolayer for Escherichia coli detection. Sens. Actuators B Chem. 2011, 151, 333–340. [Google Scholar] [CrossRef]
- Koyun, O. Poly(L-Cysteine) Modified Pencil Graphite Electrode for Determination of Sunset Yellow in Food and Beverage Samples by Differential Pulse Voltammetry. Int. J. Electrochem. Sci. 2018, 13, 159–174. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Serafín, V.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Asadpour-Zeynali, K. Ultrasensitive determination of receptor tyrosine kinase with a label-free electrochemical immunosensor using graphene quantum dots-modified screen-printed electrodes. Anal. Chim. Acta 2018, 1011, 28–34. [Google Scholar] [CrossRef]
- Honda, M.; Tsuruta, R.; Kaneko, T.; Kasaoka, S.; Yagi, T.; Todani, M.; Fujita, M.; Izumi, T.; Maekawa, T. Serum Glial Fibrillary Acidic Protein Is a Highly Specific Biomarker for Traumatic Brain Injury in Humans Compared With S-100B and Neuron-Specific Enolase. J. Trauma Inj. Infect. Crit. Care 2010, 69, 104–109. [Google Scholar] [CrossRef]
- Wang, T. Electrochemical Quantitative Detection of Glial Fibrillary Acidic Protein Based on Molecularly Imprinted Polymer Sensor. Int. J. Electrochem. Sci. 2017, 12, 7341–7350. [Google Scholar] [CrossRef]
- Sayad, A.; Uddin, S.M.; Yao, S.; Wilson, H.; Chan, J.; Zhao, H.; Donnan, G.; Davis, S.; Skafidas, E.; Yan, B.; et al. A magnetoimpedance biosensor microfluidic platform for detection of glial fibrillary acidic protein in blood for acute stroke classification. Biosens. Bioelectron. 2022, 211, 114410. [Google Scholar] [CrossRef]


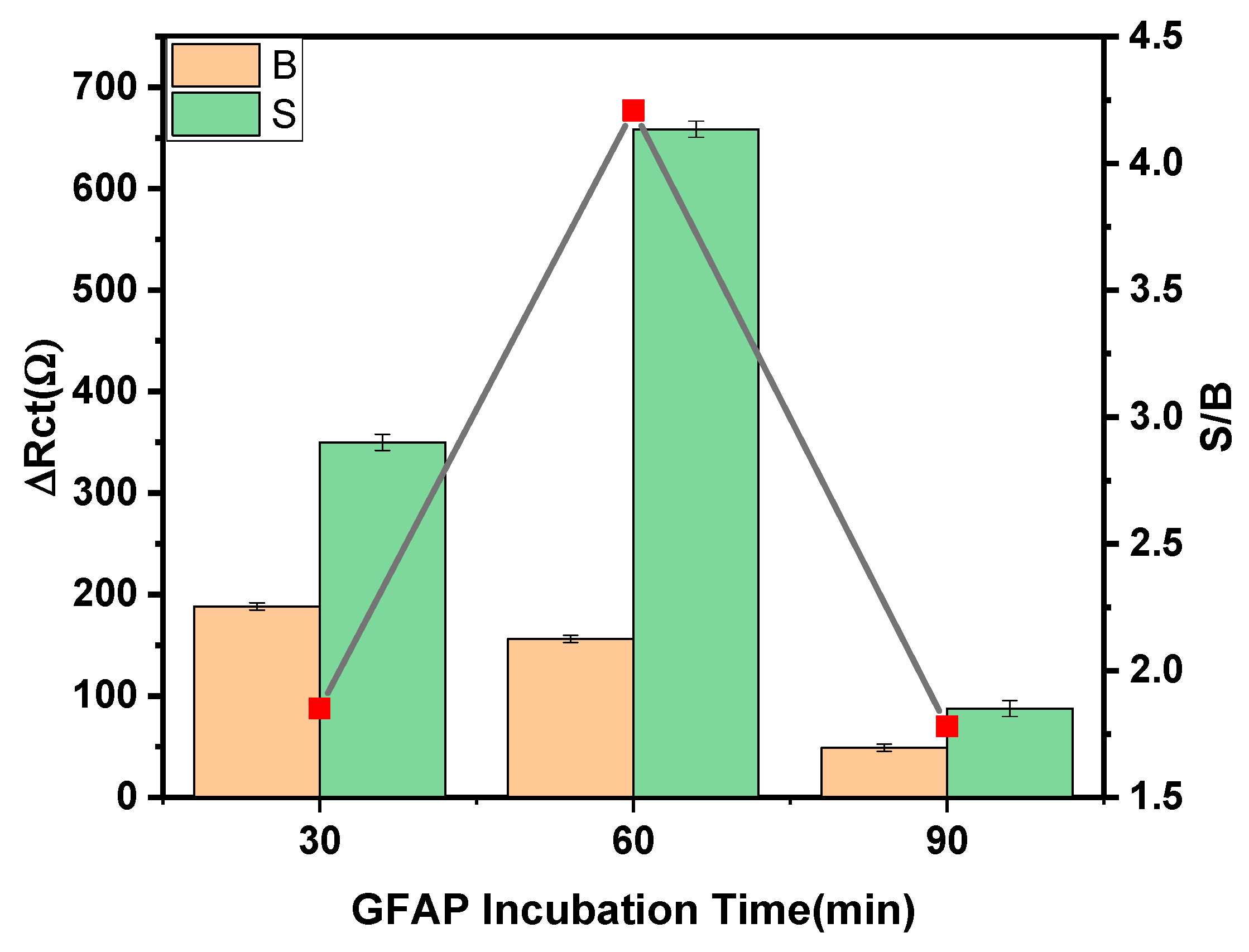


| Parameters | Checked Range | Selected Condition |
|---|---|---|
| AuNp Modifier Amount | 5–15 µL | 5 µL |
| Anti-GFAP concentration | 1–10 kDa | 10 kDa |
| Anti-GFAP incubation time | 30–90 min | 30 min |
| BSA% | 0.5–1.5% | 1.5% |
| BSA% incubation time | 30–90 min | 60 min |
| GFAP incubation time | 30–90 min | 60 min |
| Method | Signal | Detection | Bioreceptor | Redox Marker | LDR | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Tranducer | Technique | ||||||
| ELISA | - | Enzyme-linked immunosorbent assays | - | - | 0–14 ng/mL | 0.79 ng/mL | [27] |
| Enhanced lanthanide fluorescence immunoassay | - | DELFIA® 1234 fluorometer | - | - | 0.5–2 μg/mL | 0.2 μg/mL | [8] |
| MIP/MWCNT/SPCEs | SPCE | DPV | MIP | [Fe(CN)6] −3/−4 | 0.2–10 μg/mL | 0.04 μg/mL | [28] |
| PEI/GO/SPCE | graphene screen-printed electrode | EIS | GFAP antibody | [Fe(CN)6] −3/−4 | 1–100 pg/mL | N. M | [5] |
| Graphene@PEDOT:PSS/GIPEC electrode | Homemade three-PET electrode | EIS | GFAP antibody | [Fe(CN)6] −3/−4 | 1 pg/mL–10 ng/mL | 281.7 fg/mL | [11] |
| automated PMMA layer microfluidic platform | Homemade screen-printed electrode | EIS | GFAP anti-body | [Fe(CN)6] −3/−4 | 10–1000 pg/mL | 3 pg/mL | [12] |
| Magnetoimpedance biosensor | Microfluidic chips | Magnetoimpedance | GFAP antibody | PBS | 0.01 .0 ng/mL. | 0.01 ng/mL | [29] |
| Anti-GFAP/L-cys/AuNPs/SPCE | SPCE | EIS | GFAP anti-body | [Fe(CN)6] −3/−4 | 1–1000 pg/mL | 0.051 pg/mL | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozcelikay, G.; Mollarasouli, F.; Unal, M.A.; Gucuyener, K.; Ozkan, S.A. Ultrasensitive Determination of Glial-Fibrillary-Acidic-Protein (GFAP) in Human Serum-Matrix with a Label-Free Impedimetric Immunosensor. Biosensors 2022, 12, 1165. https://doi.org/10.3390/bios12121165
Ozcelikay G, Mollarasouli F, Unal MA, Gucuyener K, Ozkan SA. Ultrasensitive Determination of Glial-Fibrillary-Acidic-Protein (GFAP) in Human Serum-Matrix with a Label-Free Impedimetric Immunosensor. Biosensors. 2022; 12(12):1165. https://doi.org/10.3390/bios12121165
Chicago/Turabian StyleOzcelikay, Goksu, Fariba Mollarasouli, Mehmet Altay Unal, Kıvılcım Gucuyener, and Sibel A. Ozkan. 2022. "Ultrasensitive Determination of Glial-Fibrillary-Acidic-Protein (GFAP) in Human Serum-Matrix with a Label-Free Impedimetric Immunosensor" Biosensors 12, no. 12: 1165. https://doi.org/10.3390/bios12121165






