Hydrogen Peroxide and Hypochlorite Responsive Fluorescent Nanoprobes for Sensitive Cancer Cell Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Synthesis of Ce6@Lum-AuNPs
2.3. Ce6 Loading Capacity Calculation
2.4. FL Properties of the Ce6@Lum-AuNPs
2.5. Specificity of the Ce6@Lum-AuNPs for H2O2 and ClO−
2.6. Cell Culture
2.7. In Vitro Cytotoxicity Test
2.8. In Vitro Bioluminescence Imaging of Cancer Cells
3. Results and Discussion
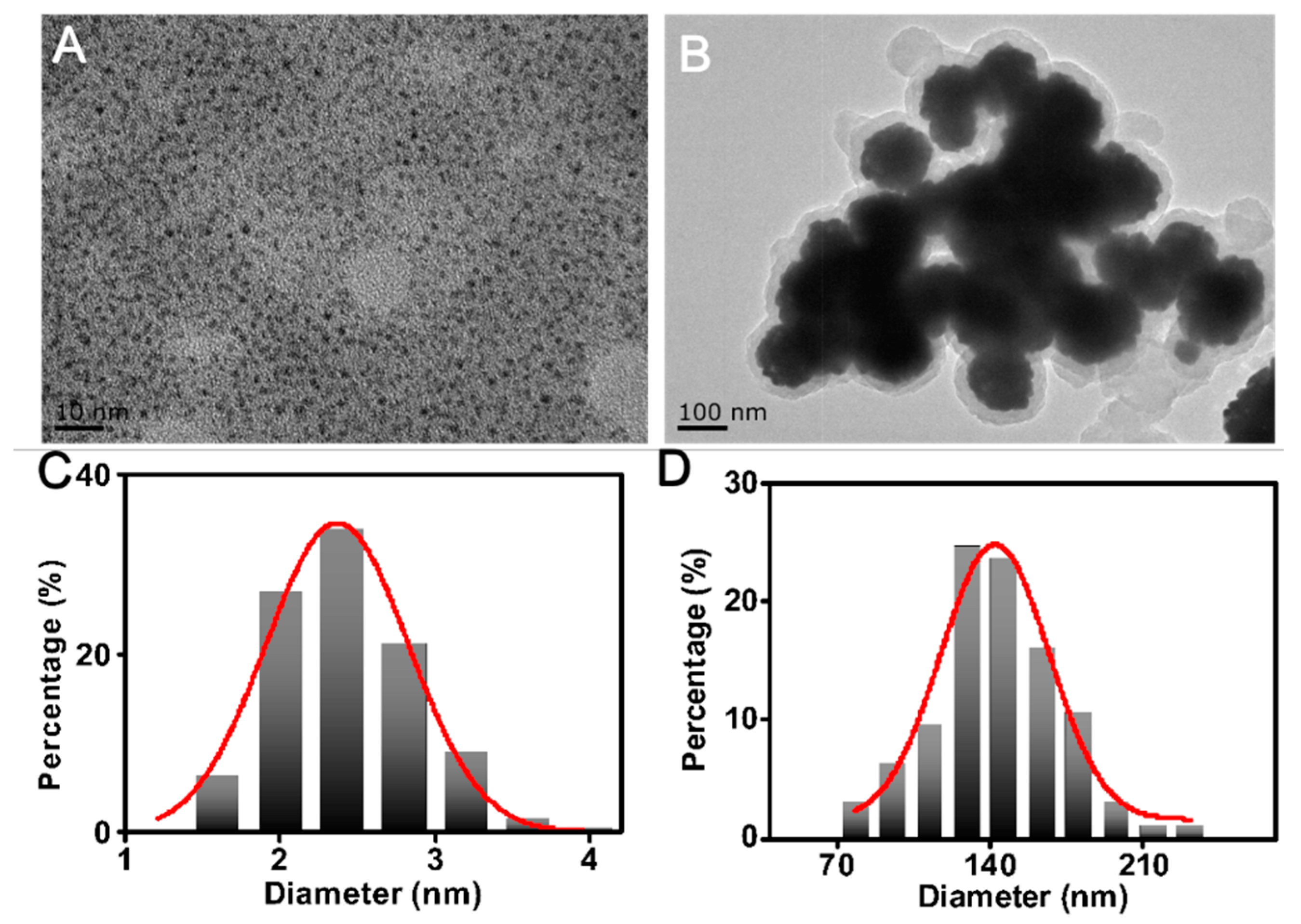
3.1. Synthesis and Characterization of Ce6@Lum-AuNPs
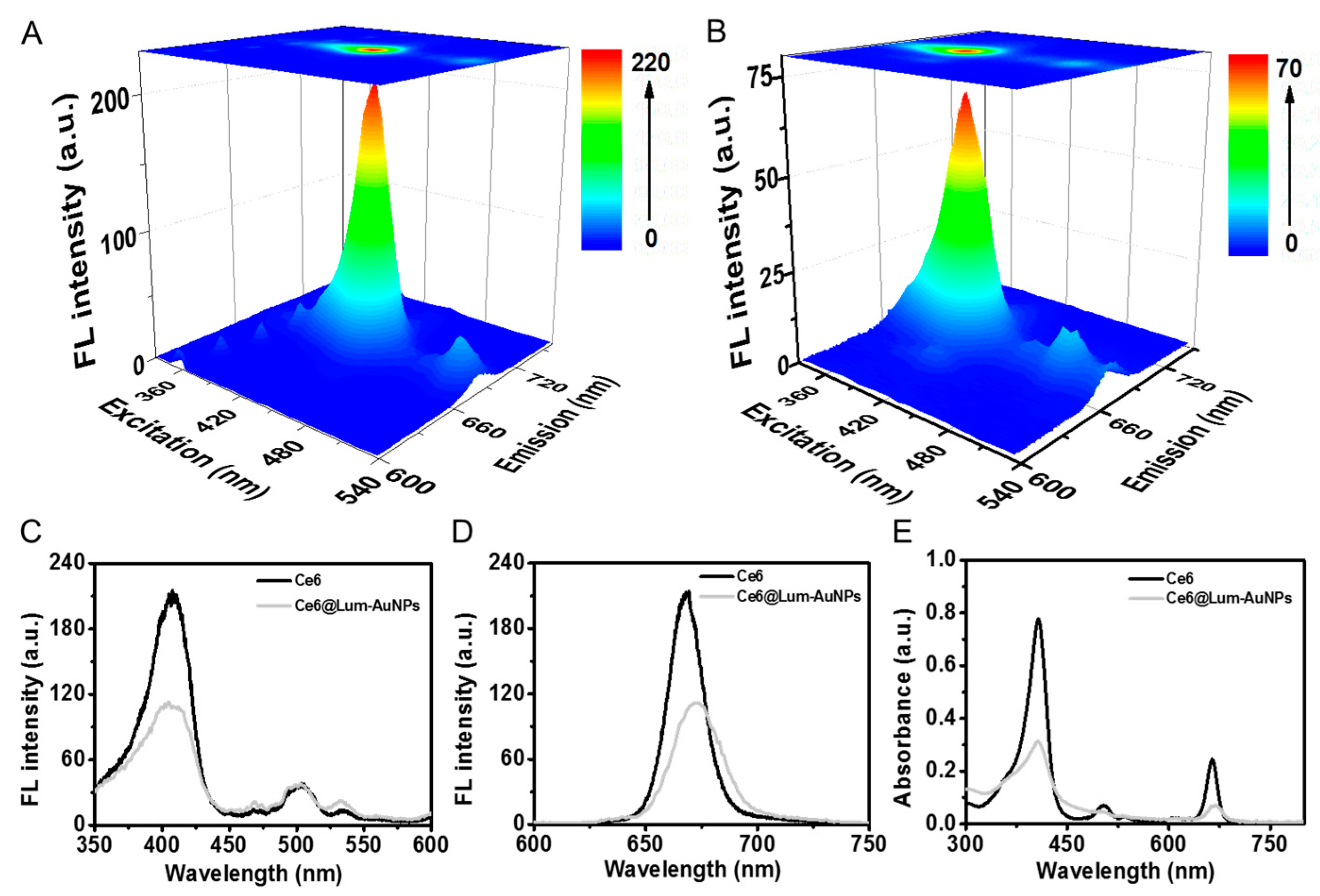
3.2. FL Spectroscopy Characterization of Ce6@Lum-AuNPs
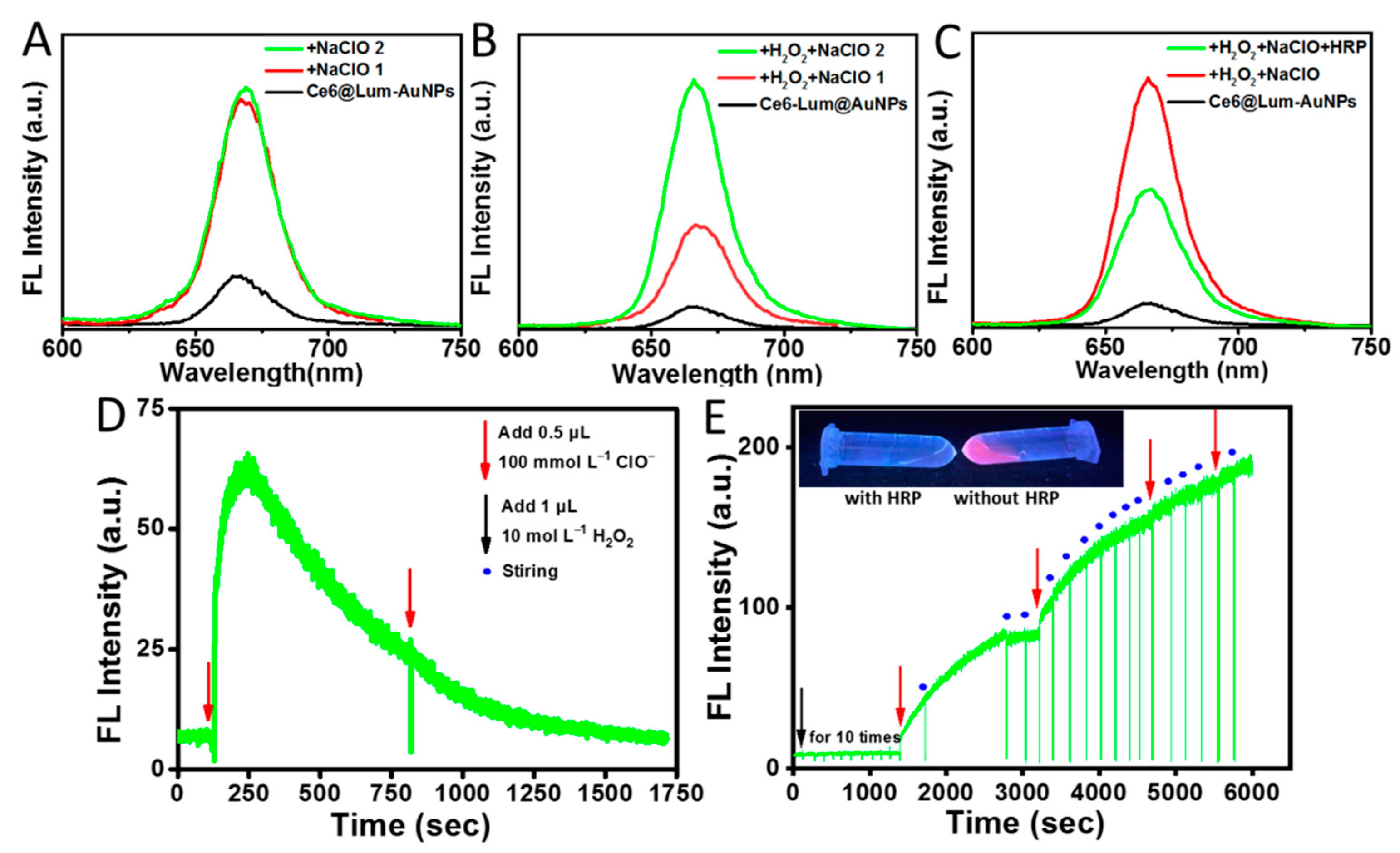
3.3. Specificity of the Ce6@Lum-AuNPs for H2O2 and ClO−
3.4. Cytocompatibility Evaluation In Vitro
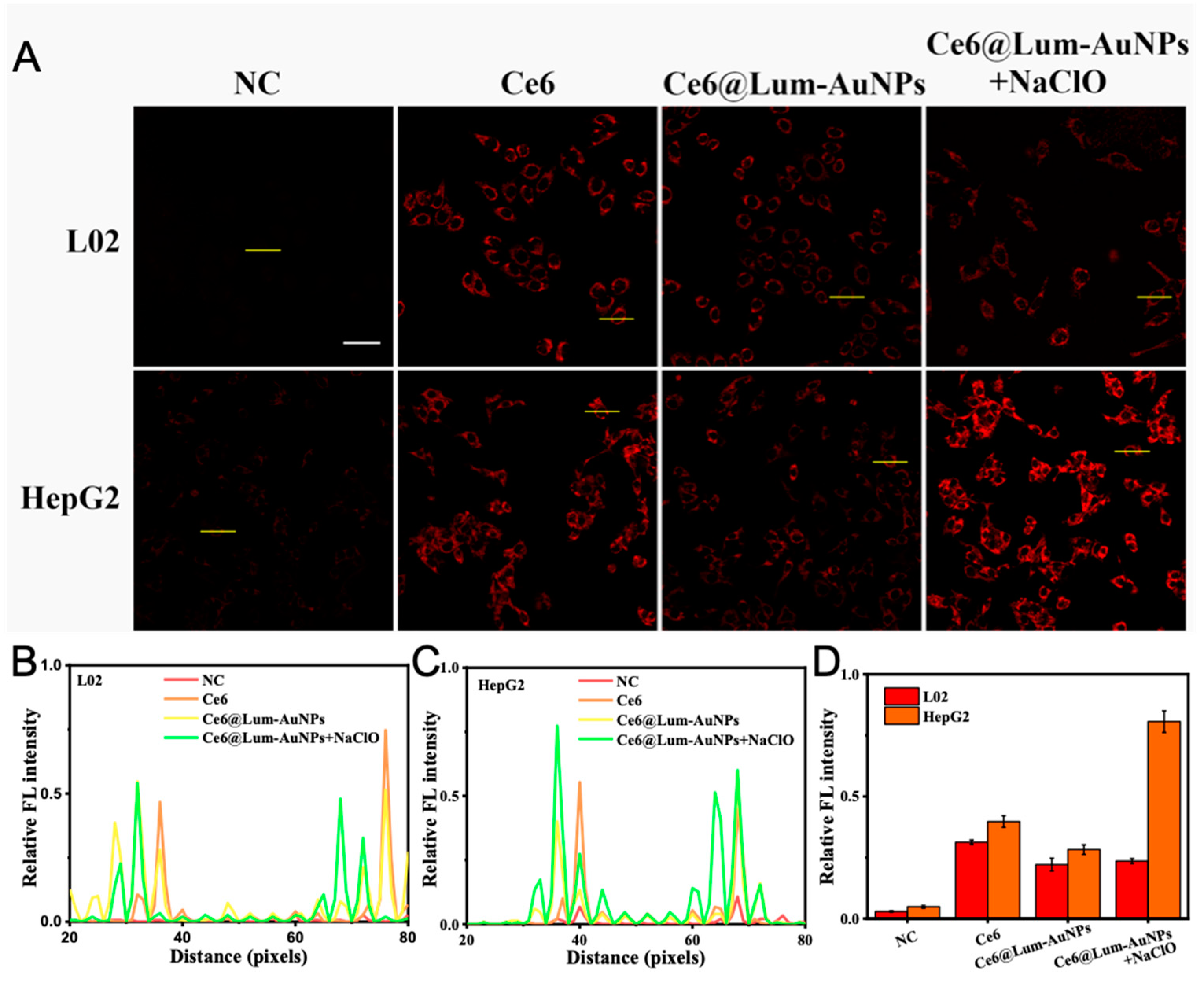
3.5. High-Resolution FL Imaging of Cells In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Roy, I.; Yang, C.H.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; An, H.J.; Zhang, D.L.; Tao, H.; Dou, Y.; Li, X.; Zhang, J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci. Adv. 2019, 5, eaat2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. 2020 Cancer statistics, 2020 Ca-a Cancer. J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, S.; Cao, W.; Cai, K.; Liang, X.J. Biomedical nanomaterials for imaging-guided cancer therapy. Nanoscale 2012, 4, 6135–6149. [Google Scholar] [CrossRef]
- Wu, X.; Chang, S.; Sun, X.; Guo, Z.; Li, Y.; Tang, J.; Shen, Y.; Shi, J.; Tian, H.; Zhu, W. Constructing NIR silica-cyanine hybrid nanocomposite for bioimaging in vivo: A breakthrough in photo-stability and bright fluorescence with large Stokes shift. Chem. Sci. 2013, 4, 1221–1228. [Google Scholar] [CrossRef]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Hu, R.; Toh, H.T.; Liu, X.; Lin, G.M.; Yin, F.; Yoon, H.S.; Yong, K.T. Assembling Mn: ZnSe quantum dots-siRNA nanoplexes for gene silencing in tumor cells. Biomater. Sci. 2015, 3, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Zhang, Y.Y.; Ye, J.; Chen, D.H.; Rehman, F.U.; Li, Q.W.; Chen, Y.; Jiang, H.; Wang, X.M. Facile synthesis of fluorescent Au/Ce nanoclusters for high-sensitive bioimaging. J. Nanobiotechnol. 2015, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Du, P.; Eisenberg, R. Energy upconversion sensitized by a platinum(II) terpyridyl acetylide complex. Chem. Sci. 2010, 1, 502–506. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, X.A.; Wu, X.M.; Jiang, L.P.; Burda, C.; Zhu, J.J. Rapid sonochemical synthesis of highly luminescent non-toxic AuNCs and Au@AgNCs and Cu(II) sensing. Chem. Commun. 2011, 47, 4237–4239. [Google Scholar] [CrossRef]
- Bilecka, I.; Elser, P.; Niederberger, M. Kinetic and thermodynamic aspects in the microwave-assisted synthesis of ZnO nanoparticles in benzyl alcohol. ACS Nano 2009, 3, 467–477. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Sun, X.P. Microwave-assisted rapid synthesis of Ag nanoparticles/graphene nanosheet composites and their application for hydrogen peroxide detection. J. Nanoparticle Res. 2011, 13, 4539–4548. [Google Scholar] [CrossRef]
- Lai, X.; Jiang, H.; Wang, X. Biodegradable Metal Organic Frameworks for Multimodal Imaging and Targeting Theranostics. Biosensors 2021, 11, 299. [Google Scholar] [CrossRef]
- Dong, X.; Ye, J.; Chen, Y.; Tanziela, T.; Jiang, H.; Wang, X. Intelligent peptide-nanorods against drug-resistant bacterial infection and promote wound healing by mild-temperature photothermal therapy. Chem. Eng. J. 2022, 432, 134061. [Google Scholar] [CrossRef]
- Cai, W.; Yin, L.; Jiang, H.; Weizmann, Y.; Wang, X. Intelligent Bio-Responsive Fluorescent Au–shRNA Complexes for Regulated Autophagy and Effective Cancer Bioimaging and Therapeutics. Biosensors 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; You, Q.; Yang, L.; Li, P.; Lu, Q.; Wang, S.; Tan, F.; Ji, Y.; Li, N. Rodlike MSN@Au Nanohybrid-Modified Supermolecular Photosensitizer for NIRF/MSOT/CT/MR Quadmodal Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 6777–6788. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Song, X.; Liang, C.; Gong, H.; Chen, Q.; Wang, C.; Liu, Z. Photosensitizer-Conjugated Albumin-Polypyrrole Nanoparticles for Imaging-Guided In Vivo Photodynamic/Photothermal Therapy. Small 2015, 11, 3932–3941. [Google Scholar] [CrossRef]
- Cheng, L.; Kamkaew, A.; Sun, H.; Jiang, D.; Valdovinos, H.F.; Gong, H.; England, C.G.; Goel, S.; Barnhart, T.E.; Cai, W. Dual-Modality Positron Emission Tomography/Optical Image-Guided Photodynamic Cancer Therapy with Chlorin e6-Containing Nanomicelles. ACS Nano 2016, 10, 7721–7730. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Luo, G.F.; Qiu, W.X.; Lei, Q.; Liu, L.H.; Wang, S.B.; Zhang, X.Z. Mesoporous silica-based versatile theranostic nanoplatform constructed by layer-by-layer assembly for excellent photodynamic/chemo therapy. Biomaterials 2017, 117, 54–65. [Google Scholar] [CrossRef]
- Gong, H.; Dong, Z.; Liu, Y.; Yin, S.; Cheng, L.; Xi, W.; Xiang, J.; Liu, K.; Li, Y.; Liu, Z. Engineering of Multifunctional Nano-Micelles for Combined Photothermal and Photodynamic Therapy Under the Guidance of Multimodal Imaging. Adv. Funct. Mater. 2014, 24, 6492–6502. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Y.; Miao, Q.; Qi, X.; Ding, D.; Chen, P.; Pu, K. Regulating Near-Infrared Photodynamic Properties of Semiconducting Polymer Nanotheranostics for Optimized Cancer Therapy. ACS Nano 2017, 11, 8998–9009. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.W.; Zheng, Y.K.; Wang, Y.H.; Guo, Z.C.; Jiang, H.; Wang, X.M. A Novel Turn-on Fluorescent Sensor for the Sensitive Detection of Glutathione via Gold Nanocluster Preparation Based on Controllable Ligand-Induced Etching. Analyst 2020, 145, 4265–4275. [Google Scholar] [CrossRef]
- Christine, C.W. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2014, 840, 730–738. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ye, J.; Lv, G.; Liu, W.; Jiang, H.; Liu, X.; Wang, X. Hydrogen Peroxide and Hypochlorite Responsive Fluorescent Nanoprobes for Sensitive Cancer Cell Imaging. Biosensors 2022, 12, 111. https://doi.org/10.3390/bios12020111
Chen Y, Ye J, Lv G, Liu W, Jiang H, Liu X, Wang X. Hydrogen Peroxide and Hypochlorite Responsive Fluorescent Nanoprobes for Sensitive Cancer Cell Imaging. Biosensors. 2022; 12(2):111. https://doi.org/10.3390/bios12020111
Chicago/Turabian StyleChen, Yun, Jing Ye, Gang Lv, Weiwei Liu, Hui Jiang, Xiaohui Liu, and Xuemei Wang. 2022. "Hydrogen Peroxide and Hypochlorite Responsive Fluorescent Nanoprobes for Sensitive Cancer Cell Imaging" Biosensors 12, no. 2: 111. https://doi.org/10.3390/bios12020111







