PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Biologicals
2.2. Animals
2.3. Metal Oxide Nanoparticle Fabrication
2.3.1. Synthesis of Manganese Oxide (MnO) and Iron Oxide (Fe3O4) Nanoparticles
2.3.2. Polymer Encapsulation of Metal Oxide NPs
2.4. Nanoparticle Characterization
2.4.1. X-ray Diffraction (XRD)
2.4.2. Electron Microscopy
2.4.3. Dynamic Light Scattering (DLS) and Zeta Potential (ζ-Potential)
2.4.4. Fourier Transform InfraRed Spectroscopy (FTIR)
2.4.5. ThermoGravimetric Analysis (TGA)
2.4.6. Metal Content and Encapsulation Efficiency Assessment
2.5. Neutrophil Extracellular Trap (NET) Assay
2.5.1. Neutrophil Isolation from Murine Bone Marrow
2.5.2. NET Assay Dosing Calculation
2.5.3. Ex Vivo NET Assay
2.6. Characterization of Neutrophil dsDNA Release, Reactive Oxygen Species Production, and Cytokine Release
2.7. Scanning Electron Microscopy of NETs
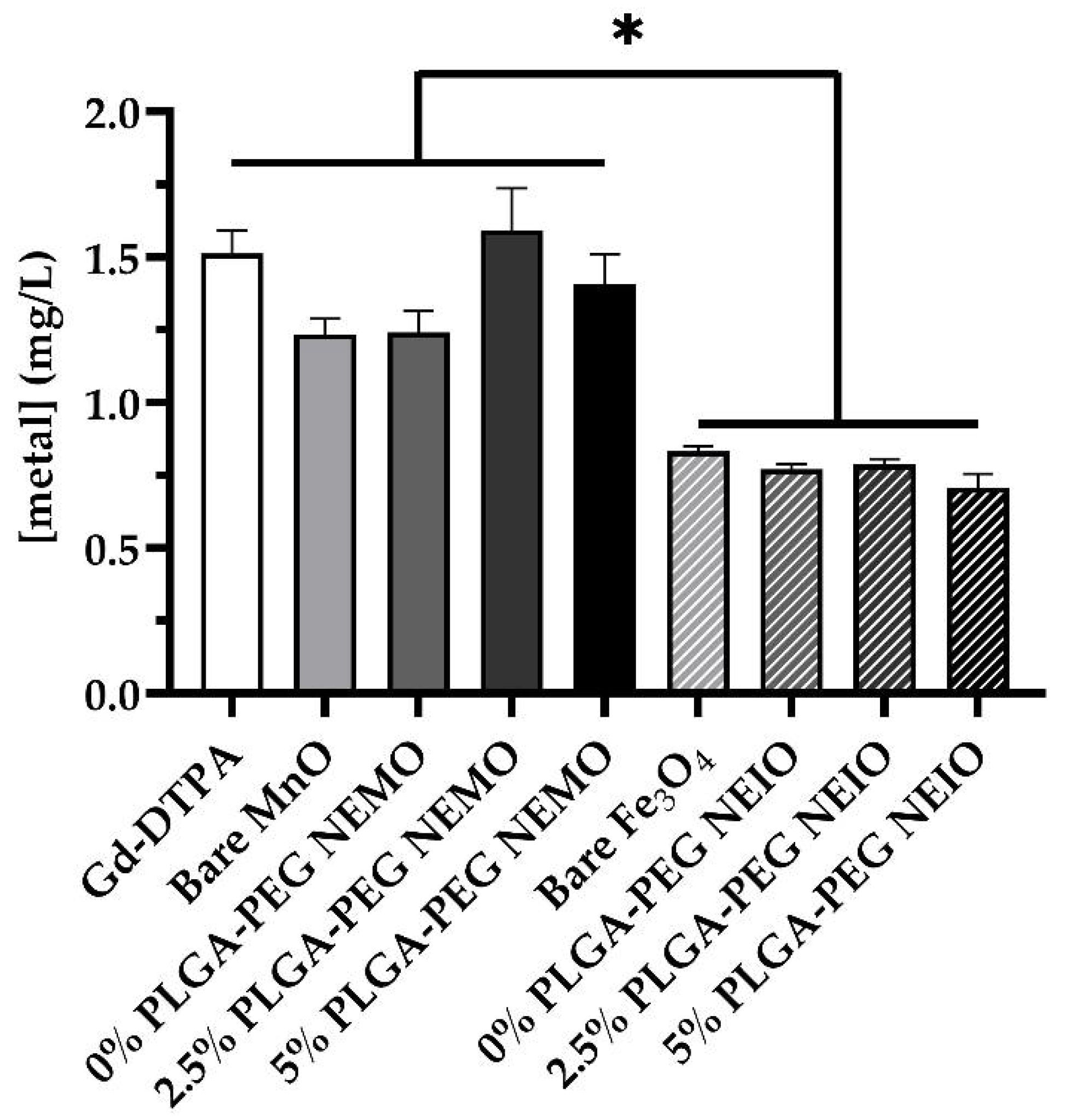
2.8. Neutrophil Metal Content
2.9. Statistical Analysis
3. Results
3.1. Synthesized MnO and Fe3O4 Bare NPs Displayed Small Sizes with Intended Oxidation State
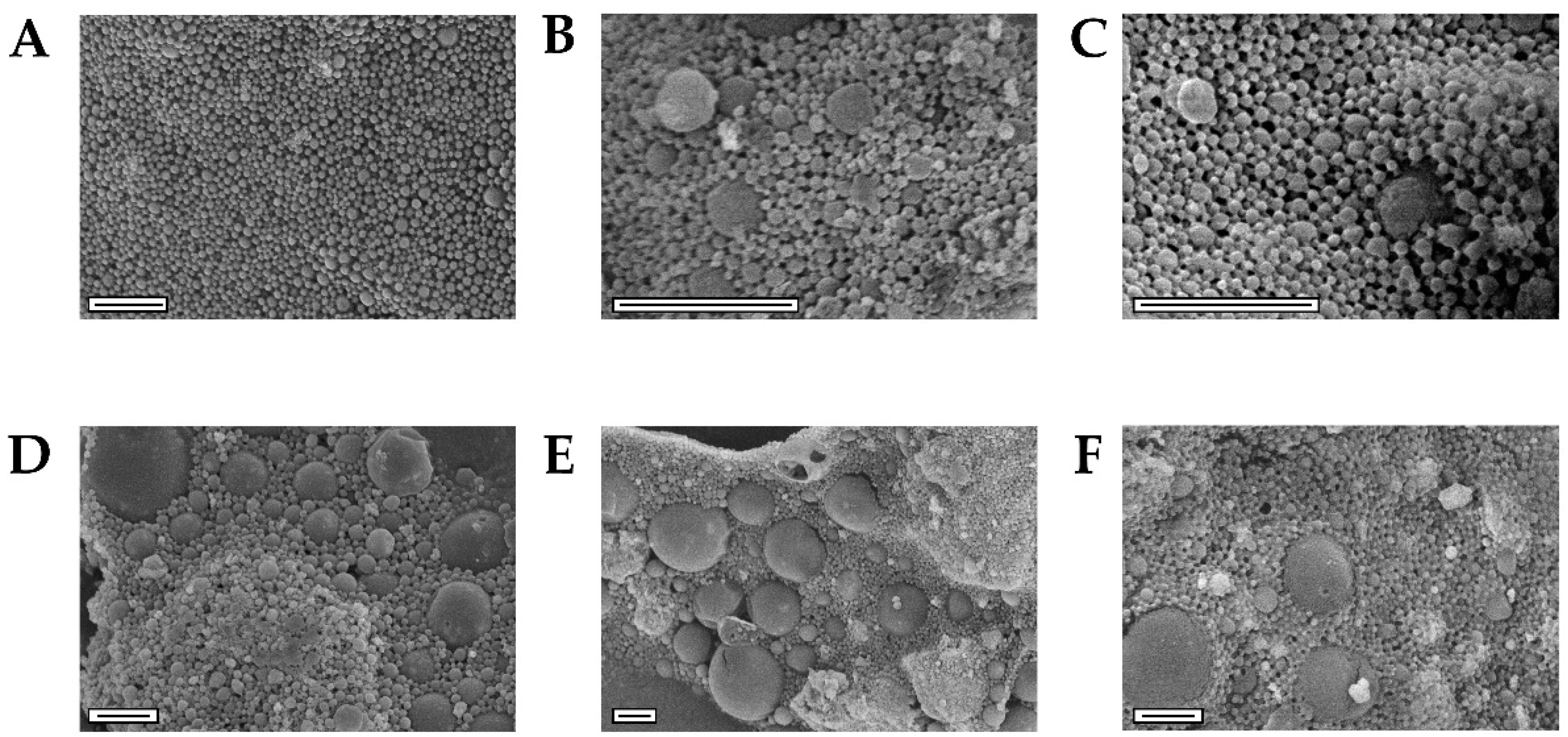
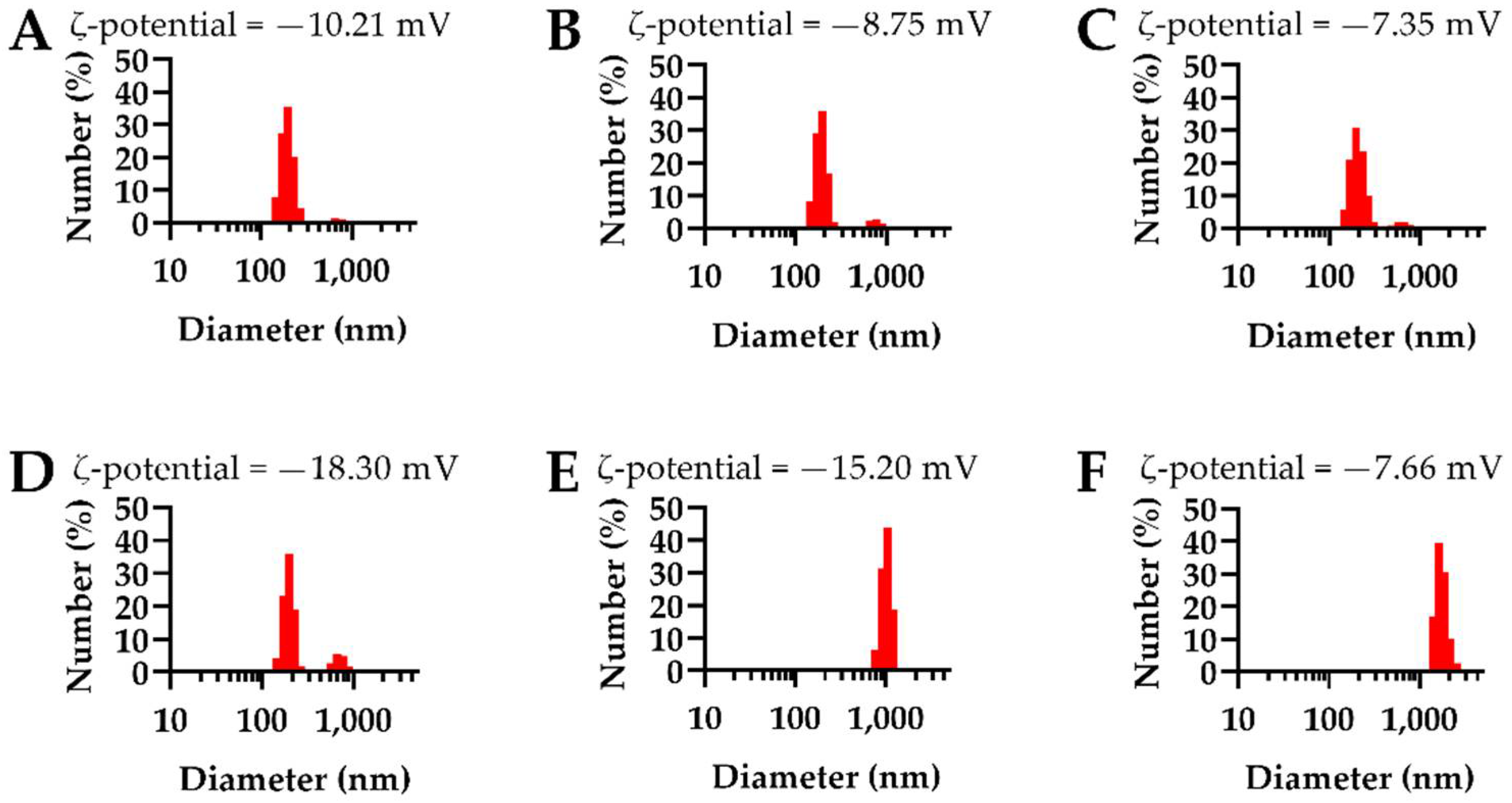
3.2. NEIO Particles Displayed Larger Particle Size and Aggregation While NEMO Particles Displayed Smaller and More Consistent Particle Size
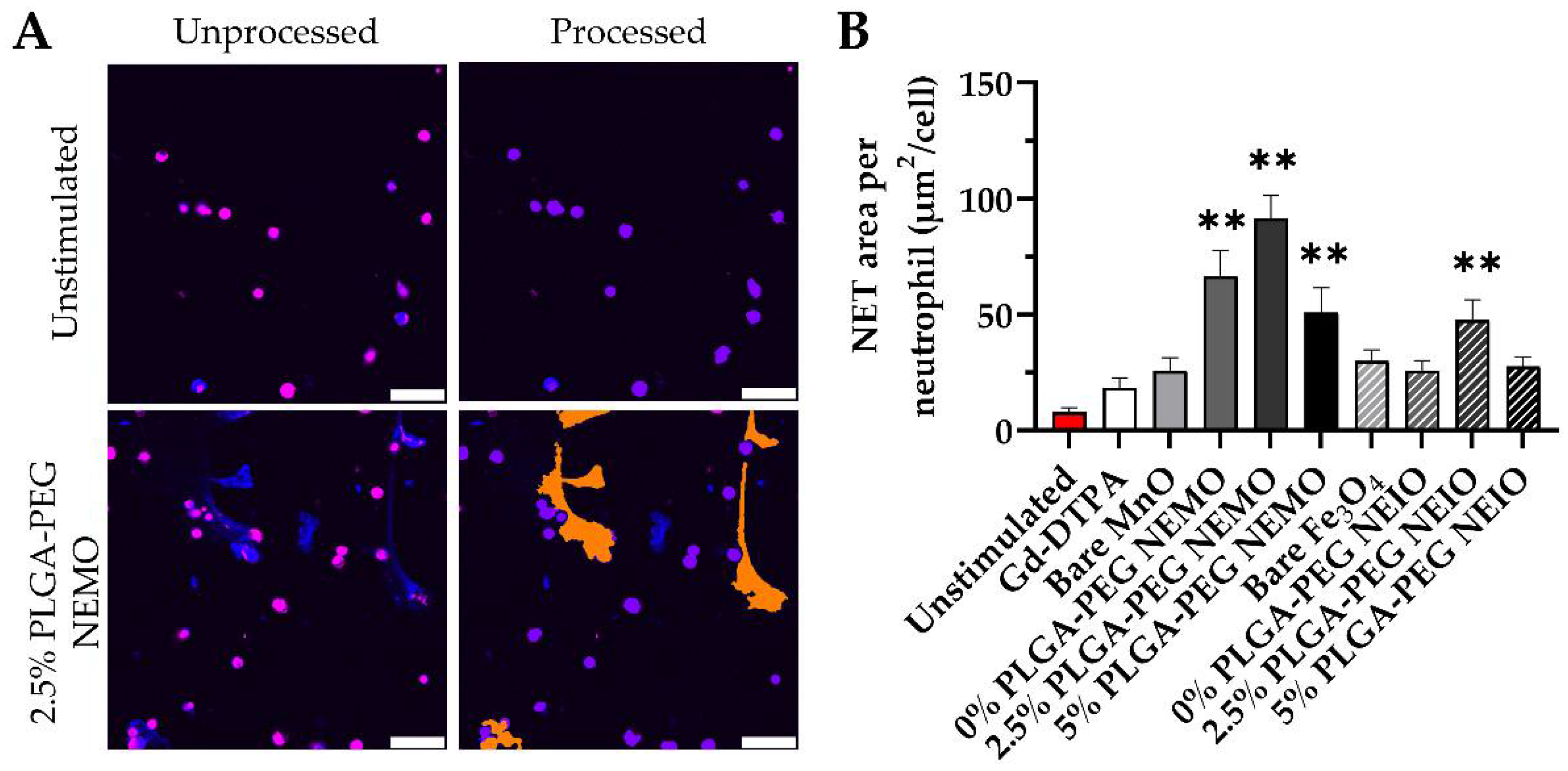
3.3. Ex Vivo Fluorescent Imaging Reveals That NEMO Particles Elicit the Highest NETosis
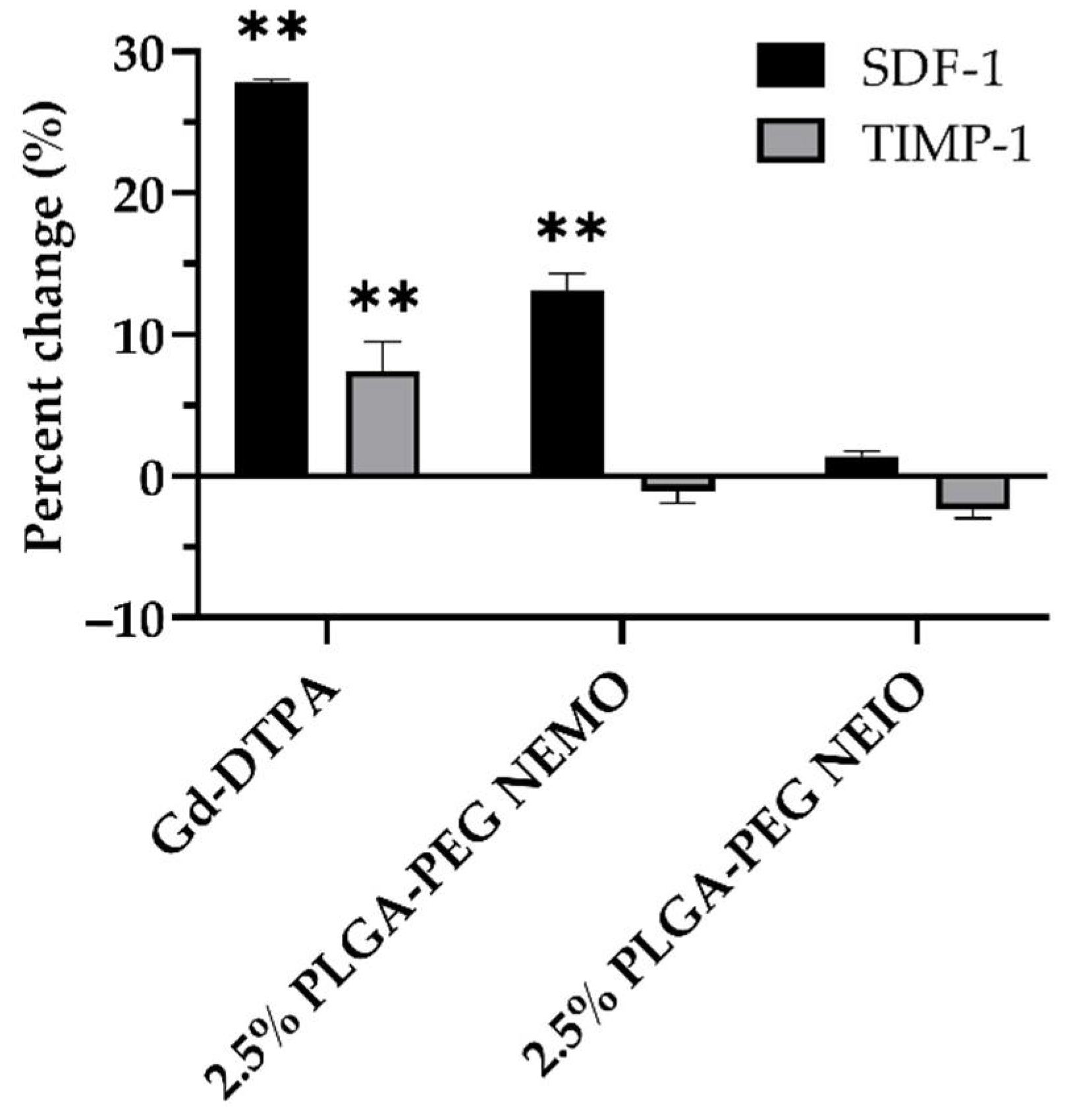
3.4. MRI Contrast Agents Provoke Non-NETotic Cell-Free dsDNA Release, Extracellular ROS, and Altered Cytokine Expression
3.5. MRI Contrast Agents Yield Differential Leukocyte Phagocytosis Dependent on Formulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Limitations of Mammograms|How Often Are Mammograms Wrong? Available online: https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/mammograms/limitations-of-mammograms.html (accessed on 1 December 2021).
- Durand, M.A.; Friedewald, S.M.; Plecha, D.M.; Copit, D.S.; Barke, L.D.; Rose, S.L.; Hayes, M.K.; Greer, L.N.; Dabbous, F.M.; Conant, E.F. False-Negative Rates of Breast Cancer Screening with and without Digital Breast Tomosynthesis. Radiology 2021, 298, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Clinicopathological Features of Breast Cancer without Mammographic Findings Suggesting Malignancy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33285381/ (accessed on 1 December 2021).
- Supplemental MRI Screening for Women with Extremely Dense Breast Tissue|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1903986 (accessed on 1 December 2021).
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Green, L.A.; Karow, J.A.; Toman, J.E.; Lostumbo, A.; Xie, K. Review of Breast Augmentation and Reconstruction for the Radiologist with Emphasis on MRI. Clin. Imaging 2018, 47, 101–117. [Google Scholar] [CrossRef]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A Review of Various Modalities in Breast Imaging: Technical Aspects and Clinical Outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.D.; Lee, W.-M.; Roubidoux, M.A.; Goodsitt, M.M.; Lashbrook, C.; Davis, C.E.; Kripfgans, O.D.; Carson, P.L. Preliminary Clinical Experience with a Combined Automated Breast Ultrasound and Digital Breast Tomosynthesis System. Ultrasound Med. Biol. 2018, 44, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Pierre, V.C.; Allen, M.J.; Caravan, P. Contrast Agents for MRI: 30+ Years and Where Are We Going? J. Biol. Inorg. Chem. 2014, 19, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Kitajima, K.; Maeda, T.; Watanabe, S.; Ueno, Y.; Sugimura, K. Recent Topics Related to Nephrogenic Systemic Fibrosis Associated with Gadolinium-based Contrast Agents. Int. J. Urol. 2012, 19, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Choi, J.W.; Moon, W.-J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-Based Contrast Agent Toxicity: A Review of Known and Proposed Mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Khongorzul, E.; Nakajima, T.; Kameo, S.; Koyama, H.; Tsushima, Y.; Koibuchi, N. Effects of Gadolinium-Based Contrast Agents on Thyroid Hormone Receptor Action and Thyroid Hormone-Induced Cerebellar Purkinje Cell Morphogenesis. Front. Endocrinol. 2016, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairinisa, M.A.; Ariyani, W.; Tsushima, Y.; Koibuchi, N. Effects of Gadolinium Deposits in the Cerebellum: Reviewing the Literature from In Vitro Laboratory Studies to In Vivo Human Investigations. Int. J. Environ. Res. Public Health 2021, 18, 7214. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association between MRI Exposure during Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Martinez de la Torre, C.; Grossman, J.H.; Bobko, A.A.; Bennewitz, M.F. Tuning the Size and Composition of Manganese Oxide Nanoparticles through Varying Temperature Ramp and Aging Time. PLoS ONE 2020, 15, e0239034. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of Sub-100 Nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mori, A.; Huang, L. Role of Liposome Size and RES Blockade in Controlling Biodistribution and Tumor Uptake of GM1-Containing Liposomes. Biochim. Biophys. Acta 1992, 1104, 95–101. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the Optimal Size of Anticancer Nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 2015, 9, 12425–12435. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Black, K.C.L.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for in Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape Effects of Filaments versus Spherical Particles in Flow and Drug Delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-D.; Do, T.-O. Size- and Shape-Controlled Synthesis of Monodisperse Metal Oxide and Mixed Oxide Nanocrystals. Nanocrystal 2011, 66, 55–84. [Google Scholar] [CrossRef] [Green Version]
- Arvizo, R.R.; Miranda, O.R.; Moyano, D.F.; Walden, C.A.; Giri, K.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Reid, J.M.; Mukherjee, P. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nanoparticles. PLoS ONE 2011, 6, e24374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Iancu, S.D.; Albu, C.; Chiriac, L.; Moldovan, R.; Stefancu, A.; Moisoiu, V.; Coman, V.; Szabo, L.; Leopold, N.; Bálint, Z. Assessment of Gold-Coated Iron Oxide Nanoparticles as Negative T2 Contrast Agent in Small Animal MRI Studies. Int. J. Nanomed. 2020, 15, 4811–4824. [Google Scholar] [CrossRef]
- Chhour, P.; Gallo, N.; Cheheltani, R.; Williams, D.; Al-Zaki, A.; Paik, T.; Nichol, J.L.; Tian, Z.; Naha, P.C.; Witschey, W.R.; et al. Nanodisco Balls: Control over Surface versus Core Loading of Diagnostically Active Nanocrystals into Polymer Nanoparticles. ACS Nano 2014, 8, 9143–9153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese Oxide-Based Multifunctionalized Mesoporous Silica Nanoparticles for PH-Responsive MRI, Ultrasonography and Circumvention of MDR in Cancer Cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef]
- Van Schooneveld, M.M.; Cormode, D.P.; Koole, R.; van Wijngaarden, J.T.; Calcagno, C.; Skajaa, T.; Hilhorst, J.; Hart, D.C.; Fayad, Z.A.; Mulder, W.J.M.; et al. A Fluorescent, Paramagnetic and PEGylated Gold/Silica Nanoparticle for MRI, CT and Fluorescence Imaging. Contrast Media Mol. Imaging 2010, 5, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Alt, K.; Ju, Y.; Gunawan, S.T.; Braunger, J.A.; Wang, T.-Y.; Dai, Y.; Dai, Q.; Richardson, J.J.; Guo, J.; et al. Ligand-Functionalized Poly(Ethylene Glycol) Particles for Tumor Targeting and Intracellular Uptake. Biomacromolecules 2019, 20, 3592–3600. [Google Scholar] [CrossRef] [PubMed]
- Capolla, S.; Garrovo, C.; Zorzet, S.; Lorenzon, A.; Rampazzo, E.; Spretz, R.; Pozzato, G.; Núñez, L.; Tripodo, C.; Macor, P.; et al. Targeted Tumor Imaging of Anti-CD20-Polymeric Nanoparticles Developed for the Diagnosis of B-Cell Malignancies. Int. J. Nanomed. 2015, 10, 4099–4109. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted Delivery of MicroRNA-29b by Transferrin-Conjugated Anionic Lipopolyplex Nanoparticles: A Novel Therapeutic Strategy in Acute Myeloid Leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.; Banerjee, S. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Uz, M.; Bulmus, V.; Alsoy Altinkaya, S. Effect of PEG Grafting Density and Hydrodynamic Volume on Gold Nanoparticle–Cell Interactions: An Investigation on Cell Cycle, Apoptosis, and DNA Damage. Langmuir 2016, 32, 5997–6009. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current Understanding of Interactions between Nanoparticles and the Immune System. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Gao, H. The Impact of Protein Corona on the Behavior and Targeting Capability of Nanoparticle-Based Delivery System. Int. J. Pharm. 2018, 552, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The Evolution of the Protein Corona around Nanoparticles: A Test Study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromen, C.A.; Kelley, W.J.; Fish, M.B.; Adili, R.; Noble, J.; Hoenerhoff, M.J.; Holinstat, M.; Eniola-Adefeso, O. Neutrophil–Particle Interactions in Blood Circulation Drive Particle Clearance and Alter Neutrophil Responses in Acute Inflammation. ACS Nano 2017, 11, 10797–10807. [Google Scholar] [CrossRef]
- Jones, S.W.; Roberts, R.A.; Robbins, G.R.; Perry, J.L.; Kai, M.P.; Chen, K.; Bo, T.; Napier, M.E.; Ting, J.P.; DeSimone, J.M. Nanoparticle Clearance Is Governed by Th1/Th2 Immunity and Strain Background. J. Clin. Investig. 2013, 123, 3061–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumenko, V.; Nikitin, A.; Garanina, A.; Melnikov, P.; Vodopyanov, S.; Kapitanova, K.; Potashnikova, D.; Vishnevskiy, D.; Alieva, I.; Ilyasov, A. Neutrophil-Mediated Transport Is Crucial for Delivery of Short-Circulating Magnetic Nanoparticles to Tumors. Acta Biomater. 2020, 104, 176–187. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.H.; Cohen, Y.; Emili, A.; Chan, W.C.W. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Kelley, W.J.; Fromen, C.A.; Lopez-Cazares, G.; Eniola-Adefeso, O. PEGylation of Model Drug Carriers Enhances Phagocytosis by Primary Human Neutrophils. Acta Biomater. 2018, 79, 283–293. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat Med 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Sreejit, G.; Nagareddy, P.R.; Murphy, A.J. Attack of the NETs! NETosis Primes IL-1β-Mediated Inflammation in Diabetic Foot Ulcers. Clin. Sci. 2020, 134, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Schauer, C.; Hoffmann, M.; Herrmann, M. Why Does the Gout Attack Stop? A Roadmap for the Immune Pathogenesis of Gout. RMD Open 2015, 1, e000046. [Google Scholar] [CrossRef] [Green Version]
- Chatfield, S.M.; Grebe, K.; Whitehead, L.W.; Rogers, K.L.; Nebl, T.; Murphy, J.M.; Wicks, I.P. Monosodium Urate Crystals Generate Nuclease-Resistant Neutrophil Extracellular Traps via a Distinct Molecular Pathway. J. Immunol. 2018, 200, 1802–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.P.; Wallis, C. Dornase Alfa for Cystic Fibrosis. Cochrane Database Syst. Rev. 2010, 3, CD001127. [Google Scholar]
- Angelidou, I.; Chrysanthopoulou, A.; Mitsios, A.; Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Ritis, D.; Tsironidou, V.; Moschos, I.; Dalla, V. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef]
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil Extracellular Traps in Breast Cancer and beyond: Current Perspectives on NET Stimuli, Thrombosis and Metastasis, and Clinical Utility for Diagnosis and Treatment. Breast Cancer Res. 2019, 21, 145. [Google Scholar] [CrossRef] [Green Version]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. Available online: https://www.jci.org/articles/view/67484/pdf (accessed on 13 October 2021).
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V. Neutrophil Extracellular Traps Produced during Inflammation Awaken Dormant Cancer Cells in Mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; De Rosa, V.; Iommelli, F.; Carriero, M.V.; Terlizzi, C.; Camerlingo, R.; Belli, S.; Fonti, R.; Di Minno, G.; Del Vecchio, S. Neutrophil Extracellular Traps as an Adhesion Substrate for Different Tumor Cells Expressing RGD-Binding Integrins. Int. J. Mol. Sci. 2018, 19, 2350. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016, 8, ra138–ra361. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.; Passam, F. Quantification of NETs-associated Markers by Flow Cytometry and Serum Assays in Patients with Thrombosis and Sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.; Posch, F.; Martinod, K.; Grilz, E.; Däullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D. Citrullinated Histone H3, a Biomarker of Neutrophil Extracellular Trap Formation, Predicts the Risk of Venous Thromboembolism in Cancer Patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.; Xu, A. Increased Neutrophil Elastase and Proteinase 3 and Augmented NETosis Are Closely Associated with β-Cell Autoimmunity in Patients with Type 1 Diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Papagoras, C.; Miltiades, P.; Angelidou, I.; Mitsios, A.; Kotsianidis, I.; Skendros, P.; Sivridis, E.; et al. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PLoS ONE 2016, 11, e0154484. [Google Scholar] [CrossRef]
- Boone, B.A.; Murthy, P.; Miller-Ocuin, J.; Doerfler, W.R.; Ellis, J.T.; Liang, X.; Ross, M.A.; Wallace, C.T.; Sperry, J.L.; Lotze, M.T.; et al. Chloroquine Reduces Hypercoagulability in Pancreatic Cancer through Inhibition of Neutrophil Extracellular Traps. BMC Cancer 2018, 18, 678. [Google Scholar] [CrossRef] [Green Version]
- Ma, A.; Kubes, P. Platelets, Neutrophils, and Neutrophil Extracellular Traps (NETs) in Sepsis. J. Thromb. Haemost. 2008, 6, 415–420. [Google Scholar] [CrossRef]
- Abdol Razak, N.; Elaskalani, O.; Metharom, P. Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis. Int. J. Mol. Sci. 2017, 18, 487. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil Extracellular Trap (NET) Impact on Deep Vein Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Rayes, R.F.; Mouhanna, J.G.; Nicolau, I.; Bourdeau, F.; Giannias, B.; Rousseau, S.; Quail, D.; Walsh, L.; Sangwan, V.; Bertos, N. Primary Tumors Induce Neutrophil Extracellular Traps with Targetable Metastasis-Promoting Effects. JCI Insight 2019, 4, e128008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartneck, M.; Keul, H.A.; Zwadlo-Klarwasser, G.; Groll, J. Phagocytosis Independent Extracellular Nanoparticle Clearance by Human Immune Cells. Nano Lett. 2010, 10, 59–63. [Google Scholar] [CrossRef]
- Kang, H.; Seo, J.; Yang, E.-J.; Choi, I.-H. Silver Nanoparticles Induce Neutrophil Extracellular Traps Via Activation of PAD and Neutrophil Elastase. Biomolecules 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Bilyy, R.; Unterweger, H.; Weigel, B.; Dumych, T.; Paryzhak, S.; Vovk, V.; Liao, Z.; Alexiou, C.; Herrmann, M.; Janko, C. Inert Coats of Magnetic Nanoparticles Prevent Formation of Occlusive Intravascular Co-Aggregates With Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Marion, T.N.; Liu, Y.; Zhang, L.; Cao, X.; Hu, H.; Zhao, Y.; Herrmann, M. Nanomaterial Exposure Induced Neutrophil Extracellular Traps: A New Target in Inflammation and Innate Immunity. J. Immunol. Res. 2019, 2019, 3560180. [Google Scholar] [CrossRef]
- Liz, R.; Simard, J.-C.; Leonardi, L.B.A.; Girard, D. Silver Nanoparticles Rapidly Induce Atypical Human Neutrophil Cell Death by a Process Involving Inflammatory Caspases and Reactive Oxygen Species and Induce Neutrophil Extracellular Traps Release upon Cell Adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef]
- Urner, M.; Schlicker, A.; Z’graggen, B.R.; Stepuk, A.; Booy, C.; Buehler, K.P.; Limbach, L.; Chmiel, C.; Stark, W.J.; Beck-Schimmer, B. Inflammatory Response of Lung Macrophages and Epithelial Cells after Exposure to Redox Active Nanoparticles: Effect of Solubility and Antioxidant Treatment. Environ. Sci. Technol. 2014, 48, 13960–13968. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.-L.; Hsu, C.-Y.; Aljuffali, I.A.; Chen, C.-H.; Chang, Y.-T.; Fang, J.-Y. Cationic Liposomes Evoke Proinflammatory Mediator Release and Neutrophil Extracellular Traps (NETs) toward Human Neutrophils. Colloids Surf. B Biointerfaces 2015, 128, 119–126. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Zhu, Y.; Lu, Y.; Chen, Q.; Fan, S.; Huang, Q.; Chen, X.; Xia, L.; Wei, Y. Gold Nanoparticles Synergize with Bacterial Lipopolysaccharide to Enhance Class A Scavenger Receptor Dependent Particle Uptake in Neutrophils and Augment Neutrophil Extracellular Traps Formation. Ecotoxicol. Environ. Saf. 2021, 211, 111900. [Google Scholar] [CrossRef]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch Jr, G.R. Novel Role of IL (Interleukin)-1β in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of Engineered Nanoparticles to Human Lung Epithelial Cells: Influence of Chemical Composition and Catalytic Activity on Oxidative Stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, C.M.; Bennewitz, M.F. Manganese Oxide Nanoparticle Synthesis by Thermal Decomposition of Manganese(II) Acetylacetonate. J. Vis. Exp. 2020, e61572. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Bennewitz, M.F.; Lobo, T.L.; Nkansah, M.K.; Ulas, G.; Brudvig, G.W.; Shapiro, E.M. Biocompatible and PH-Sensitive PLGA Encapsulated MnO Nanocrystals for Molecular and Cellular MRI. ACS Nano 2011, 5, 3438–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, I.-W.; Kutteri, D.A.; Gao, B.; Tian, H.; Hu, J. Methane Pyrolysis for Carbon Nanotubes and COx-Free H2 over Transition-Metal Catalysts. Energy Fuels 2019, 33, 197–205. [Google Scholar] [CrossRef]
- Silva, M.F.; Hechenleitner, A.A.W.; Irache, J.M.; de Oliveira, A.J.A.; Pineda, E.A.G. Study of Thermal Degradation of PLGA, PLGA Nanospheres and PLGA/Maghemite Superparamagnetic Nanospheres. Mat. Res. 2015, 18, 1400–1406. [Google Scholar] [CrossRef]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-Encapsulated Blend of PLGA-PEG Microspheres: In Vitro and in Vivo Study of the Effects of Localized/Targeted Drug Delivery on the Treatment of Triple-Negative Breast Cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef]
- Adib, A.A.; Nazemidashtarjandi, S.; Kelly, A.; Kruse, A.; Cimatu, K.; David, A.E.; Farnoud, A.M. Engineered Silica Nanoparticles Interact Differently with Lipid Monolayers Compared to Lipid Bilayers. Environ. Sci. Nano 2018, 5, 289–303. [Google Scholar] [CrossRef]
- Benoit, D.N.; Zhu, H.; Lilierose, M.H.; Verm, R.A.; Ali, N.; Morrison, A.N.; Fortner, J.D.; Avendano, C.; Colvin, V.L. Measuring the Grafting Density of Nanoparticles in Solution by Analytical Ultracentrifugation and Total Organic Carbon Analysis. Anal. Chem. 2012, 84, 9238–9245. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Jiang, Y.; Levy, C.N.; Hughes, S.M.; Zhang, H.; Hladik, F.; Woodrow, K.A. Optimization and Comparison of CD4-Targeting Lipid–Polymer Hybrid Nanoparticles Using Different Binding Ligands. J. Biomed. Mater. Res. Part A 2018, 106, 1177–1188. [Google Scholar] [CrossRef]
- Swamydas, M.; Lionakis, M.S. Isolation, Purification and Labeling of Mouse Bone Marrow Neutrophils for Functional Studies and Adoptive Transfer Experiments. J. Vis. Exp. 2013, e50586. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Schürholz, H.; Kirchin, M.A.; Bücker, A.; Fries, P. Safety and Adverse Effects during 24 Hours after Contrast-Enhanced MRI with Gadobenate Dimeglumine (MultiHance) in Children. Pediatr. Radiol. 2013, 43, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M.; Armstrong, M.R.; Barr, R.G.; Berger, B.L.; Czervionke, L.F.; Gonzalez, C.F.; Halford, H.H.; Kanal, E.; Kuhn, M.J.; Levin, J.M.; et al. A Clinical Comparison of the Safety and Efficacy of MultiHance (Gadobenate Dimeglumine) and Omniscan (Gadodiamide) in Magnetic Resonance Imaging in Patients with Central Nervous System Pathology. Investig. Radiol. 2001, 36, 65–71. [Google Scholar] [CrossRef] [PubMed]
- 000651—BALB/CJ. Available online: https://www.jax.org/strain/000651 (accessed on 1 December 2021).
- Mitruka, B.M.; Rawnsley, H.M. Clinical Biochemical and Hematological Reference Values in Normal Experimental Animals and Normal Humans; Masson Pub.: New York, NY, USA, 1981; ISBN 978-0-89352-163-9. [Google Scholar]
- Harkness, J.E.; Wagner, J.E. The Biology and Medicine of Rabbits and Rodents; Lea & Febiger: Philadelphia, PA, USA, 1989; ISBN 978-0-8121-1176-7. [Google Scholar]
- Christie, A.C. A Study of the Kultschitzky (Argentaffin) Cell with the Electron-Microscope, after Fixation by Osmium Tetroxide. J. Cell Sci. 1955, 96, 295–299. [Google Scholar] [CrossRef]
- Dwiranti, A.; Masri, F.; Rahmayenti, D.A.; Putrika, A. The Effects of Osmium Tetroxide Post-Fixation and Drying Steps on Leafy Liverwort Ultrastructure Study by Scanning Electron Microscopy. Microsc. Res. Tech. 2019, 82, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Kallman, F. The Properties and Effects of Osmium Tetroxide as a Tissue Fixative with Special Reference to Its Use for Electron Microscopy. Exp. Cell Res. 1953, 4, 127–141. [Google Scholar] [CrossRef]
- Braet, F.; De Zanger, R.; Wisse, E. Drying Cells for SEM, AFM and TEM by Hexamethyldisilazane: A Study on Hepatic Endothelial Cells. J. Microsc. 1997, 186, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Control of Size and Composition of Colloidal Nanocrystals of Manganese Oxide|Inorganic Chemistry. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.inorgchem.8b02124 (accessed on 2 December 2021).
- Belaïd, S.; Laurent, S.; Vermeech, M.; Vander Elst, L.; Perez-Morga, D.; Muller, R.N. A New Approach to Follow the Formation of Iron Oxide Nanoparticles Synthesized by Thermal Decomposition. Nanotechnology 2013, 24, 055705. [Google Scholar] [CrossRef]
- Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications|Chemical Reviews. Available online: https://pubs-acs-org.wvu.idm.oclc.org/doi/10.1021/cr068445e (accessed on 26 October 2021).
- Perez De Berti, I.O.; Cagnoli, M.V.; Pecchi, G.; Alessandrini, J.L.; Stewart, S.J.; Bengoa, J.F.; Marchetti, S.G. Alternative Low-Cost Approach to the Synthesis of Magnetic Iron Oxide Nanoparticles by Thermal Decomposition of Organic Precursors. Nanotechnology 2013, 24, 175601. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, H.; Gong, X.; Xu, R.; Xiao, Y.; Dong, H.; Liu, X.; Liu, Y. A Simple Additive-Free Approach for the Synthesis of Uniform Manganese Monoxide Nanorods with Large Specific Surface Area. Nanoscale Res. Lett. 2013, 8, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.; Nan, K.; Nie, G.; Chen, H. Enhanced Anti-Tumor Efficacy by Co-Delivery of Doxorubicin and Paclitaxel with Amphiphilic Methoxy PEG-PLGA Copolymer Nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative Evaluation of Antibacterial Activity of Caffeic Acid Phenethyl Ester and PLGA Nanoparticle Formulation by Different Methods. Nanotechnology 2016, 27, 025103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Yu, D.; Zhang, W.; Mao, Z.; Gao, C. Influence of Bovine Serum Albumin Coated Poly(Lactic-Co-Glycolic Acid) Particles on Differentiation of Mesenchymal Stem Cells. RSC Adv. 2015, 5, 40924–40931. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Roy, M.; Lombi, E.; Donner, E.; Shon, H.K. Assessing the Aggregation Behaviour of Iron Oxide Nanoparticles under Relevant Environmental Conditions Using a Multi-Method Approach. Water Res. 2013, 47, 4585–4599. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation Effects on the Magnetic Properties of Iron Oxide Colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkov, D.V.; Gorn, N.L. Susceptibility of the Disordered System of Fine Magnetic Particles: A Langevin-Dynamics Study. J. Phys. Condens. Matter 2001, 13, 9369–9381. [Google Scholar] [CrossRef]
- Cabrera, D.; Coene, A.; Leliaert, J.; Artés-Ibáñez, E.J.; Dupré, L.; Telling, N.D.; Teran, F.J. Dynamical Magnetic Response of Iron Oxide Nanoparticles Inside Live Cells. ACS Nano 2018, 12, 2741–2752. [Google Scholar] [CrossRef]
- Van Haute, D.; Berlin, J.M. Challenges in Realizing Selectivity for Nanoparticle Biodistribution and Clearance: Lessons from Gold Nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Nanoparticles. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutscher, H.L.; Chao, P.; Deshmukh, M.; Singh, Y.; Hu, P.; Joseph, L.B.; Reimer, D.C.; Stein, S.; Laskin, D.L.; Sinko, P.J. Threshold Size for Optimal Passive Pulmonary Targeting and Retention of Rigid Microparticles in Rats. J. Control Release 2010, 143, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Wassel, R.A.; Grady, B.; Kopke, R.D.; Dormer, K.J. Dispersion of Super Paramagnetic Iron Oxide Nanoparticles in Poly(d,l-Lactide-Co-Glycolide) Microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 125–130. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. Preparation and Characterization of Sterile and Freeze-Dried Sub-200 Nm Nanoparticles. Int. J. Pharm. 2002, 233, 239–252. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Lim, K.; Hyun, Y.-M.; Lambert-Emo, K.; Capece, T.; Bae, S.; Miller, R.; Topham, D.J.; Kim, M. Neutrophil Trails Guide Influenza-Specific CD8+ T Cells in the Airways. Science 2015, 349, aaa4352. [Google Scholar] [CrossRef] [Green Version]
- Schoeps, B.; Eckfeld, C.; Prokopchuk, O.; Böttcher, J.P.; Häußler, D.; Steiger, K.; Demir, I.E.; Knolle, P.; Soehnlein, O.; Jenne, D.E. TIMP-1 Triggers Neutrophil Extracellular Trap Formation in Pancreatic Cancer. Cancer Res. 2021, 81, 3568–3579. [Google Scholar] [CrossRef]
- Garcia, J.; Liu, S.Z.; Louie, A.Y. Biological Effects of MRI Contrast Agents: Gadolinium Retention, Potential Mechanisms and a Role for Phosphorus. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170180. [Google Scholar] [CrossRef] [Green Version]
- Taoka, T.; Naganawa, S. Gadolinium-Based Contrast Media, Cerebrospinal Fluid and the Glymphatic System: Possible Mechanisms for the Deposition of Gadolinium in the Brain. Magn. Reson. Med. Sci. 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shida, Y.; Darko, S.; Nina, G.; Dagmar, S.; Xiaoliang, W.; Charaf, B.; Hans-Uwe, S. Untangling “NETosis” from NETs. Eur. J. Immunol. 2019, 49, 221–227. [Google Scholar]
- Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin? J. Cell. Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N. Tissue Inhibitor of Metalloproteinases (TIMP)-1 Creates a Premetastatic Niche in the Liver through SDF-1/CXCR4-dependent Neutrophil Recruitment in Mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Yang, L.; Li, J.; Wu, W.; Huang, M.; Lin, L.; Su, S. Tumor-Contacted Neutrophils Promote Metastasis by a CD90-TIMP-1 Juxtacrine–Paracrine Loop. Clin. Cancer Res. 2019, 25, 1957–1969. [Google Scholar] [CrossRef]
- Charzewski, Ł.; Krzyśko, K.A.; Lesyng, B. Structural Characterisation of Inhibitory and Non-Inhibitory MMP-9–TIMP-1 Complexes and Implications for Regulatory Mechanisms of MMP-9. Sci. Rep. 2021, 11, 13376. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Charindra, D.; Shrestha, M.; Umehara, H.; Ogawa, I.; Miyauchi, M.; Takata, T. Tissue Inhibitor of Metalloproteinase-1 Promotes Cell Proliferation through YAP/TAZ Activation in Cancer. Oncogene 2018, 37, 263–270. [Google Scholar] [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L. Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, D.; Caracciolo, G.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Anchordoquy, T.J.; Laganà, A. Surface Chemistry and Serum Type Both Determine the Nanoparticle–Protein Corona. J. Proteom. 2015, 119, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Erpenbeck, L.; Schön, M.P. Neutrophil Extracellular Traps: Protagonists of Cancer Progression? Oncogene 2017, 36, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Antoni, H.; Xia, W.; Masa, J.; Schuhmann, W.; Muhler, M. Tuning the Oxidation State of Manganese Oxide Nanoparticles on Oxygen- and Nitrogen-Functionalized Carbon Nanotubes for the Electrocatalytic Oxygen Evolution Reaction. Phys. Chem. Chem. Phys. 2017, 19, 18434–18442. [Google Scholar] [CrossRef] [PubMed]


| Metal Oxide Composition | MnO | Mn3O4 | Mn2O3 | Fe3O4 | Fe2O3 |
|---|---|---|---|---|---|
| MnO NPs | 72% | 21% | 8% | - | - |
| Fe3O4 NPs | - | - | - | 81% | 19% |
| Type of NP | Metal Loading (mg Metal Element/mg NP) | EE (%) | |
|---|---|---|---|
| Mn | Fe | ||
| Bare MnO | 0.63 | - | - |
| 0% PLGA-PEG NEMO | 0.28 | - | 38 |
| 2.5% PLGA-PEG NEMO | 0.23 | - | 51 |
| 5% PLGA-PEG NEMO | 0.29 | - | 61 |
| Bare Fe3O4 | - | 0.57 | - |
| 0% PLGA-PEG NEIO | - | 0.22 | 69 |
| 2.5% PLGA-PEG NEIO | - | 0.25 | 75 |
| 5% PLGA-PEG NEIO | - | 0.32 | 21 |
| Type of NP | Study Parameter | Key Findings |
|---|---|---|
| Silver [77] | Concentration, size | ↑ 5 nm [AgNP] ↑ NETosis ↑ 100 nm [AgNP] --- NETosis |
| Gold [83] | Size | ↓ Size ↑ NETosis |
| Cationic liposomes [82] | Surface chemistry (cationic surfactants) | ↑ Surface charge ↑ NETosis |
| Iron oxide [78] | Surface chemistry (lauric acid, dextran, or albumin) | NET/NP aggregation ↓ for dextran or albumin coated NPs |
| Manganese oxide and iron oxide * | Metal oxide, 0–5% PLGA-PEG encapsulation | NETosis --- for bare NPs NETosis ↑ for NEMO particles NETosis ↑ for 2.5% PLGA-PEG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snoderly, H.T.; Freshwater, K.A.; Martinez de la Torre, C.; Panchal, D.M.; Vito, J.N.; Bennewitz, M.F. PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation. Biosensors 2022, 12, 123. https://doi.org/10.3390/bios12020123
Snoderly HT, Freshwater KA, Martinez de la Torre C, Panchal DM, Vito JN, Bennewitz MF. PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation. Biosensors. 2022; 12(2):123. https://doi.org/10.3390/bios12020123
Chicago/Turabian StyleSnoderly, Hunter T., Kasey A. Freshwater, Celia Martinez de la Torre, Dhruvi M. Panchal, Jenna N. Vito, and Margaret F. Bennewitz. 2022. "PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation" Biosensors 12, no. 2: 123. https://doi.org/10.3390/bios12020123
APA StyleSnoderly, H. T., Freshwater, K. A., Martinez de la Torre, C., Panchal, D. M., Vito, J. N., & Bennewitz, M. F. (2022). PEGylation of Metal Oxide Nanoparticles Modulates Neutrophil Extracellular Trap Formation. Biosensors, 12(2), 123. https://doi.org/10.3390/bios12020123





