How to Find the Right RNA-Sensing CRISPR-Cas System for an In Vitro Application
Abstract
:1. Introduction
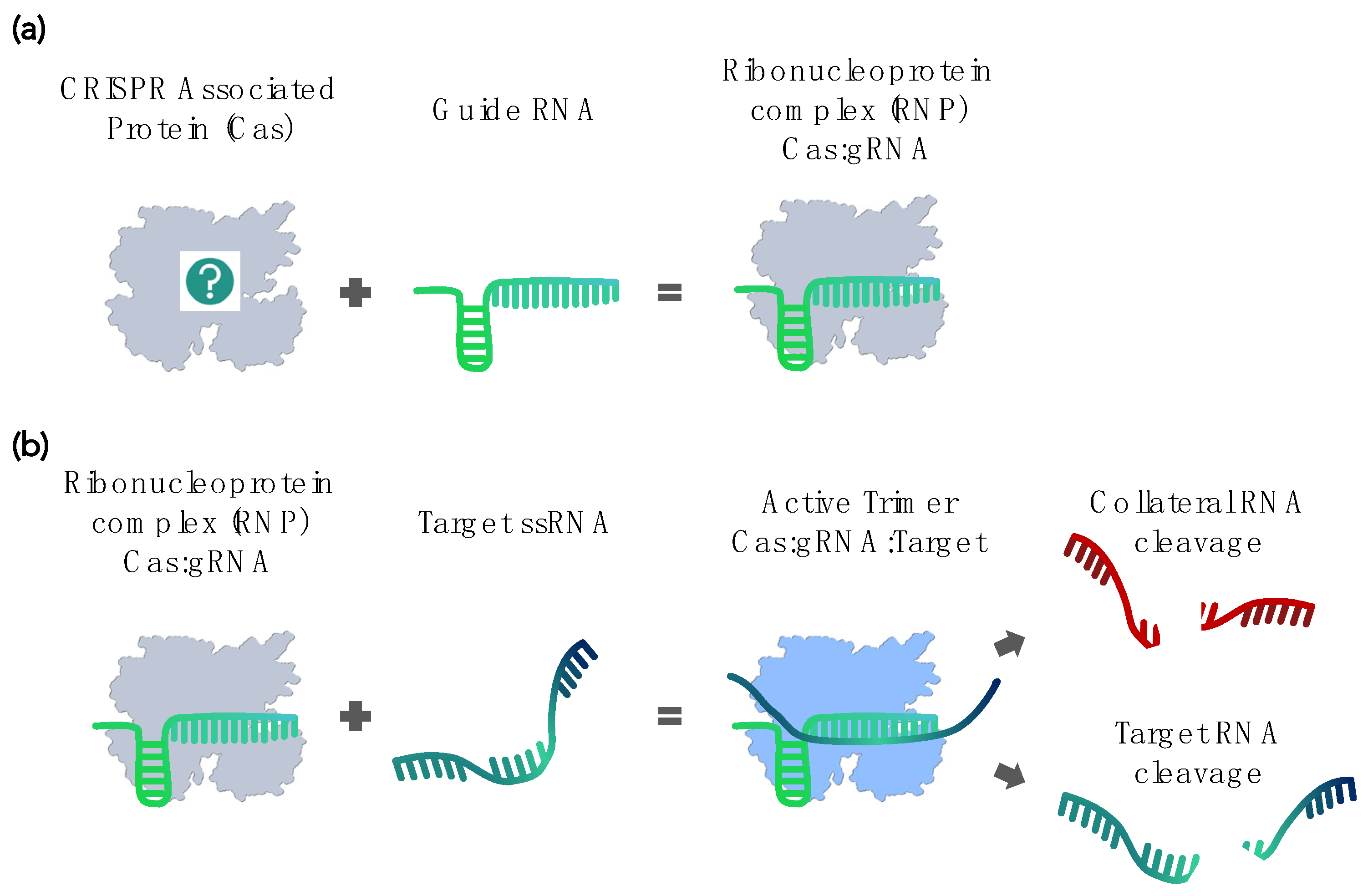
2. Current CRISPR-Cas–Based RNA Detection Methods
3. Computational Tools for CRISPR Identification
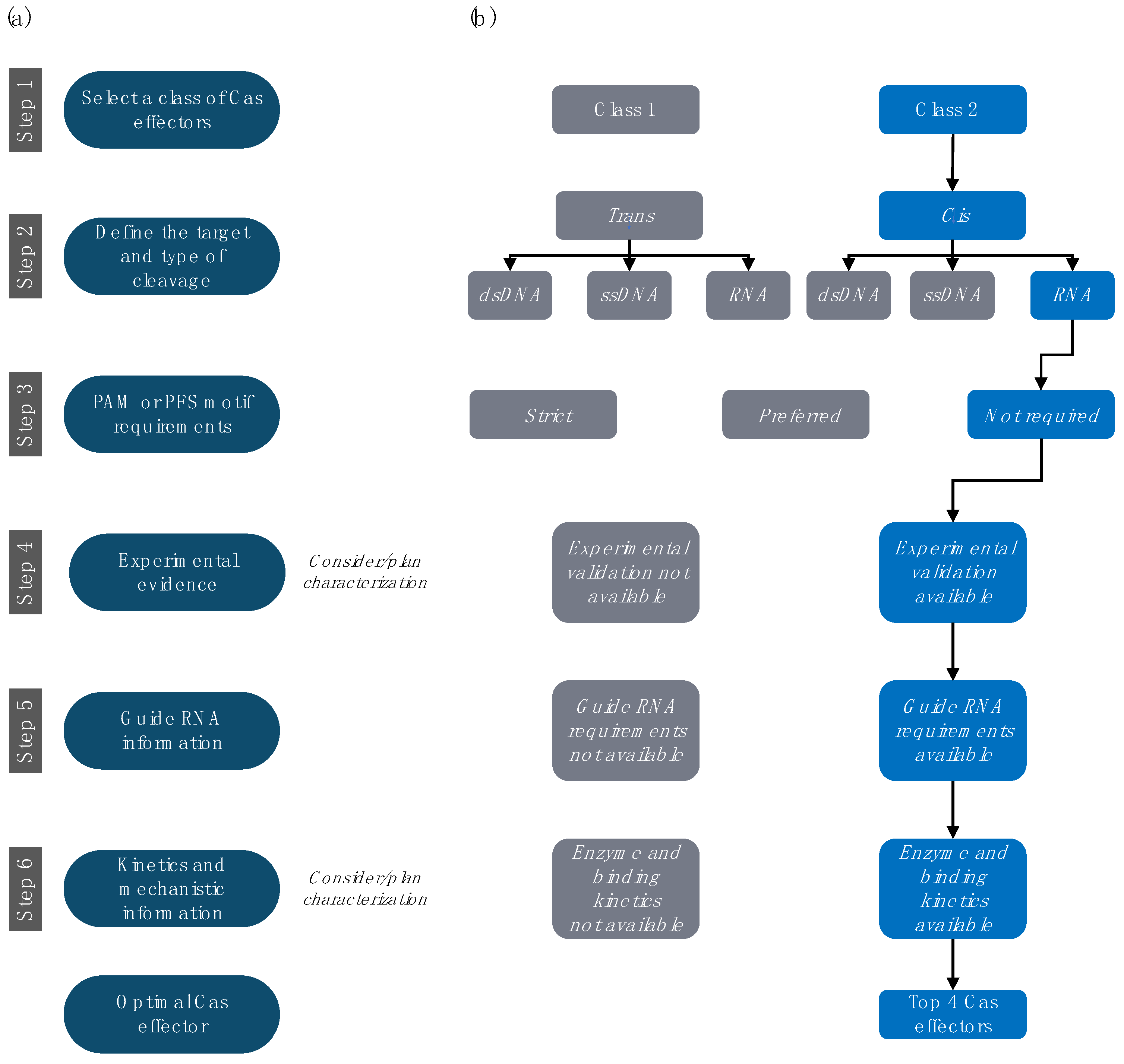
4. Step-by-Step Selection and Design of RNA-Targeting CRISPR-Cas Systems
4.1. Step 1. Select the Preferred Class of Cas Effectors
4.2. Step 2. Select the Preferred Target and Type of Cleavage
4.3. Step 3. Investigate PAM or PFS Motif Requirements
4.4. Step 4. Prioritize Effectors with Experimental Characterization
4.5. Step 5. Investigate gRNA Information
4.6. Step 6. Review Available Kinetics and Mechanistic Information
5. Detailed Profiles of Four Cas Effectors for In Vitro RNA Detection
5.1. Leptotrichia wadei (LwaCas13a)
5.2. Staphylococcus aureus (SauCas9)
5.3. Leptotrichia buccalis (LbuCas13a)
5.4. Eubacterium siraeum (EsCas13d)
5.5. Other Notable Cas Candidates
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [Green Version]
- Mojica, F.J.; Juez, G.; Rodríguez-Valera, F. Transcription at Different Salinities of Haloferax Mediterranei Sequences Adjacent to Partially Modified PstI Sites. Mol. Microbiol. 1993, 9, 613–621. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological Significance of a Family of Regularly Spaced Repeats in the Genomes of Archaea, Bacteria and Mitochondria. Mol. Microbiol. 2000, 36, 244–246. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR Elements in Yersinia Pestis Acquire New Repeats by Preferential Uptake of Bacteriophage DNA, and Provide Additional Tools for Evolutionary Studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered Regularly Interspaced Short Palindrome Repeats (CRISPRs) Have Spacers of Extrachromosomal Origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; van der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Saikot, F.K.; Rahman, M.S.; Jamal, M.A.H.M.; Rahman, S.M.K.; Islam, S.M.R.; Kim, K.-H. Programmable Molecular Scissors: Applications of a New Tool for Genome Editing in Biotech. Mol. Ther.-Nucleic Acids 2019, 14, 212–238. [Google Scholar] [CrossRef] [Green Version]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Almendros, C. Short Motif Sequences Determine the Targets of the Prokaryotic CRISPR Defence System. Microbiology 2009, 155, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-CrRNA Ribonucleoprotein Complex Mediates Specific DNA Cleavage for Adaptive Immunity in Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 Is a Single-Component Programmable RNA-Guided RNA-Targeting CRISPR Effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- East-Seletsky, A.; O’Connell, M.R.; Knight, S.C.; Burstein, D.; Cate, J.H.D.; Tjian, R.; Doudna, J.A. Two Distinct RNase Activities of CRISPR-C2c2 Enable Guide-RNA Processing and RNA Detection. Nature 2016, 538, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA Editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Su, J.; Hu, X.; Zhou, C.; Li, H.; Chen, Z.; Xiao, Q.; Wang, B.; Wu, W.; Sun, Y.; et al. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 2020, 181, 590–603.e16. [Google Scholar] [CrossRef] [PubMed]
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.; et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell 2020, 181, 865–876.e12. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Conferring Resistance to Plant RNA Viruses with the CRISPR/CasRx System. Virol. Sin. 2021, 36, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, W.; Shi, T.; Lu, P.; Zhuang, M.; Liu, J.-L. Capturing RNA–Protein Interaction via CRUIS. Nucleic Acids Res. 2020, 48, e52. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tokuyasu, T.A. CRISPR-Cas13-Based RNA-Interacting Protein Detection in Living Cells. Biochemistry 2020, 59, 1791–1792. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic Acid Detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR–Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Strutt, S.C.; Torrez, R.M.; Kaya, E.; Negrete, O.A.; Doudna, J.A. RNA-Dependent RNA Targeting by CRISPR-Cas9. eLife 2018, 7, e32724. [Google Scholar] [CrossRef] [PubMed]
- Fozouni, P.; Son, S.; de Derby, M.D.L.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-Free Detection of SARS-CoV-2 with CRISPR-Cas13a and Mobile Phone Microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef]
- Ali, Z.; Aman, R.; Mahas, A.; Rao, G.S.; Tehseen, M.; Marsic, T.; Salunke, R.; Subudhi, A.K.; Hala, S.M.; Hamdan, S.M.; et al. ISCAN: An RT-LAMP-Coupled CRISPR-Cas12 Module for Rapid, Sensitive Detection of SARS-CoV-2. Virus Res. 2020, 288, 198129. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and Portable Nucleic Acid Detection Platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-Y.; Cheng, Q.-X.; Wang, J.-M.; Li, X.-Y.; Zhang, Z.-L.; Gao, S.; Cao, R.-B.; Zhao, G.-P.; Wang, J. CRISPR-Cas12a-Assisted Nucleic Acid Detection. Cell Discov. 2018, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, S.; Wu, N.; Wu, J.; Wang, G.; Zhao, G.; Wang, J. HOLMESv2: A CRISPR-Cas12b-Assisted Platform for Nucleic Acid Detection and DNA Methylation Quantitation. ACS Synth. Biol. 2019, 8, 2228–2237. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, R.; Huang, M.; Shen, J.; Shan, Y.; Xing, D. CRISPR/Cas13a Powered Portable Electrochemiluminescence Chip for Ultrasensitive and Specific MiRNA Detection. Adv. Sci. 2020, 7, 1903661. [Google Scholar] [CrossRef]
- Shinoda, H.; Taguchi, Y.; Nakagawa, R.; Makino, A.; Okazaki, S.; Nakano, M.; Muramoto, Y.; Takahashi, C.; Takahashi, I.; Ando, J.; et al. Amplification-Free RNA Detection with CRISPR–Cas13. Commun. Biol. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, S.; Chen, A.; He, B.; Zhou, Y.; Chai, G.; Guo, F.; Huang, J. CasPDB: An Integrated and Annotated Database for Cas Proteins from Bacteria and Archaea. Database 2019, 2019, baz093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhao, S.; Ren, C.; Zhu, Y.; Zhou, H.; Lai, Y.; Zhou, F.; Jia, Y.; Zheng, K.; Huang, Z. CRISPRminer Is a Knowledge Base for Exploring CRISPR-Cas Systems in Microbe and Phage Interactions. Commun. Biol. 2018, 1, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A Web Tool to Identify Clustered Regularly Interspaced Short Palindromic Repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CRISPR-CAS++. Available online: https://crisprcas.i2bc.paris-saclay.fr/ (accessed on 5 July 2020).
- Özcan, A.; Krajeski, R.; Ioannidi, E.; Lee, B.; Gardner, A.; Makarova, K.S.; Koonin, E.V.; Abudayyeh, O.O.; Gootenberg, J.S. Programmable RNA Targeting with the Single-Protein CRISPR Effector Cas7-11. Nature 2021, 597, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F. Development of CRISPR-Cas Systems for Genome Editing and Beyond. Q. Rev. Biophys. 2019, 52, E6. [Google Scholar] [CrossRef] [Green Version]
- Hale, C.R.; Zhao, P.; Olson, S.; Duff, M.O.; Graveley, B.R.; Wells, L.; Terns, R.M.; Terns, M.P. RNA-Guided RNA Cleavage by a CRISPR RNA-Cas Protein Complex. Cell 2009, 139, 945–956. [Google Scholar] [CrossRef] [Green Version]
- East-Seletsky, A.; O’Connell, M.R.; Burstein, D.; Knott, G.J.; Doudna, J.A. RNA Targeting by Functionally Orthogonal Type VI-A CRISPR-Cas Enzymes. Mol. Cell 2017, 66, 373–383.e3. [Google Scholar] [CrossRef] [Green Version]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic Acid Detection with CRISPR Nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Chatterjee, P.; Jakimo, N.; Lee, J.; Amrani, N.; Rodríguez, T.; Koseki, S.R.T.; Tysinger, E.; Qing, R.; Hao, S.; Sontheimer, E.J.; et al. An Engineered ScCas9 with Broad PAM Range and High Specificity and Activity. Nat. Biotechnol. 2020, 38, 1154–1158. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA Targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Tang, L. PAM-Less Is More. Nature Methods 2020, 17, 559. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.R.; Oakes, B.L.; Sternberg, S.H.; East-Seletsky, A.; Kaplan, M.; Doudna, J.A. Programmable RNA Recognition and Cleavage by CRISPR/Cas9. Nature 2014, 516, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Nelles, D.A.; Fang, M.Y.; O’Connell, M.R.; Xu, J.L.; Markmiller, S.J.; Doudna, J.A.; Yeo, G.W. Programmable RNA Tracking in Live Cells with CRISPR/Cas9. Cell 2016, 165, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, B.A.; Hou, Z.; Gramelspacher, M.J.; Zhang, Y. Programmable RNA Cleavage and Recognition by a Natural CRISPR-Cas9 System from Neisseria Meningitidis. Molecular Cell 2018, 69, 906–914.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickar-Oliver, A.; Black, J.B.; Lewis, M.M.; Mutchnick, K.J.; Klann, T.S.; Gilcrest, K.A.; Sitton, M.J.; Nelson, C.E.; Barrera, A.; Bartelt, L.C.; et al. Targeted Transcriptional Modulation with Type I CRISPR-Cas Systems in Human Cells. Nat. Biotechnol. 2019, 37, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Classification and Nomenclature of CRISPR-Cas Systems: Where from Here? CRISPR J. 2018, 1, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-H.; An, Y.; Woo, E.-J. In vitro Assembly of Thermostable Csm Complex in CRISPR-Cas Type III/A System. Methods Enzym. 2019, 616, 173–189. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An Updated Evolutionary Classification of CRISPR–Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koonin, E.V.; Makarova, K.S. Origins and Evolution of CRISPR-Cas Systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180087. [Google Scholar] [CrossRef] [PubMed]
- Chylinski, K.; Makarova, K.S.; Charpentier, E.; Koonin, E.V. Classification and Evolution of Type II CRISPR-Cas Systems. Nucleic Acids Res. 2014, 42, 6091–6105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Gao, A.; Zhan, Q.; Wang, Y.; Feng, H.; Liu, S.; Gao, G.; Serganov, A.; Gao, P. Diverse Mechanisms of CRISPR-Cas9 Inhibition by Type IIC Anti-CRISPR Proteins. Mol. Cell 2019, 74, 296–309.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.X.; Hunnewell, P.; Alfonse, L.E.; Carte, J.M.; Keston-Smith, E.; Sothiselvam, S.; Garrity, A.J.; Chong, S.; Makarova, K.S.; Koonin, E.V.; et al. Functionally Diverse Type V CRISPR-Cas Systems. Science 2019, 363, 88–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellman, L.M.; Fried, M.G. Electrophoretic Mobility Shift Assay (EMSA) for Detecting Protein–Nucleic Acid Interactions. Nat. Protoc. 2007, 2, 1849–1861. [Google Scholar] [CrossRef]
- Tambe, A.; East-Seletsky, A.; Knott, G.J.; Doudna, J.A.; O’Connell, M.R. RNA-Binding and HEPN-Nuclease Activation Are Decoupled in CRISPR-Cas13a. Cell Rep. 2018, 24, 1025–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandera, K.G.; Beisel, C.L. Rapidly Characterizing CRISPR-Cas13 Nucleases Using Cell-Free Transcription-Translation Systems. In Post-Transcriptional Gene Regulation; Dassi, E., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; pp. 135–153. ISBN 978-1-07-161851-6. [Google Scholar]
- Cléry, A.; Gillioz, L.; Nguyen, C.K.X.; Allain, F.H.-T. A Step-by-Step Guide to Study Protein–RNA Interactions. CHIMIA Int. J. Chem. 2019, 73, 406–414. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and Evolution of Class 2 CRISPR–Cas Systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Wessels, H.-H.; Méndez-Mancilla, A.; Guo, X.; Legut, M.; Daniloski, Z.; Sanjana, N.E. Massively Parallel Cas13 Screens Reveal Principles for Guide RNA Design. Nat. Biotechnol. 2020, 38, 722–727. [Google Scholar] [CrossRef]
- Gorski, S.A.; Vogel, J.; Doudna, J.A. RNA-Based Recognition and Targeting: Sowing the Seeds of Specificity. Nat. Rev. Mol. Cell Biol. 2017, 18, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ye, Y.; Ye, W.; Perčulija, V.; Jiang, H.; Chen, Y.; Li, Y.; Chen, J.; Lin, J.; Wang, S.; et al. Two HEPN Domains Dictate CRISPR RNA Maturation and Target Cleavage in Cas13d. Nat. Commun. 2019, 10, 2544. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Corsi, G.I.; Anthon, C.; Qu, K.; Pan, X.; Liang, X.; Han, P.; Dong, Z.; Liu, L.; Zhong, J.; et al. Enhancing CRISPR-Cas9 GRNA Efficiency Prediction by Data Integration and Deep Learning. Nat. Commun. 2021, 12, 3238. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.; Tsuji, M.H.; Shimizu, Y.; Usami, K.; Lee, S.; Takei, N.K.; Yoshitome, K.; Nishimura, Y.; Otsuki, T.; Ito, T. Structure-Based Design of GRNA for Cas13. Sci. Rep. 2020, 10, 11610. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Rahman, J.A.; Wessels, H.-H.; Méndez-Mancilla, A.; Haro, D.; Chen, X.; Sanjana, N.E. Transcriptome-Wide Cas13 Guide RNA Design for Model Organisms and Viral RNA Pathogens. Cell Genom. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Wang, Q.; Wang, Y.; Kang, C. Rapid Design and Development of CRISPR-Cas13a Targeting SARS-CoV-2 Spike Protein. Theranostics 2021, 11, 649–664. [Google Scholar] [CrossRef]
- RNAfold Web Server. Available online: http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on 21 December 2021).
- RNAxs Web Server. Available online: http://rna.tbi.univie.ac.at/cgi-bin/RNAxs/RNAxs.cgi (accessed on 21 December 2021).
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef] [Green Version]
- Kannan, S.; Altae-Tran, H.; Jin, X.; Madigan, V.J.; Oshiro, R.; Makarova, K.S.; Koonin, E.V.; Zhang, F. Compact RNA Editors with Small Cas13 Proteins. Nat. Biotechnol. 2021, 1–4. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, Y.; Xiao, Q.; He, B.; Geng, G.; Wang, Z.; Cao, B.; Wang, X.; Zhou, D.; Yuan, T.; et al. Novel Miniature CRISPR–Cas13 Systems from Uncultivated Microbes Effective in Degrading SARS-CoV-2 Sequences and Influenza Viruses. Res. Square 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Konermann, S.; Brideau, N.J.; Lotfy, P.; Wu, X.; Novick, S.J.; Strutzenberg, T.; Griffin, P.R.; Hsu, P.D.; Lyumkis, D. Structural Basis for the RNA-Guided Ribonuclease Activity of CRISPR-Cas13d. Cell 2018, 175, 212–223.e17. [Google Scholar] [CrossRef] [Green Version]
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Mol. Cell 2019, 76, 826–837.e11. [Google Scholar] [CrossRef] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Kellner, M.J.; Zhang, F. Nucleic Acid Detection of Plant Genes Using CRISPR-Cas13. CRISPR J. 2019, 2, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Mahas, A.; Aman, R.; Mahfouz, M. CRISPR-Cas13d Mediates Robust RNA Virus Interference in Plants. Genome Biol. 2019, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Nishimasu, H.; Cong, L.; Yan, W.X.; Ran, F.A.; Zetsche, B.; Li, Y.; Kurabayashi, A.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Staphylococcus Aureus Cas9. Cell 2015, 162, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In Vivo Genome Editing Using Staphylococcus Aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Friedland, A.E.; Baral, R.; Singhal, P.; Loveluck, K.; Shen, S.; Sanchez, M.; Marco, E.; Gotta, G.M.; Maeder, M.L.; Kennedy, E.M.; et al. Characterization of Staphylococcus Aureus Cas9: A Smaller Cas9 for All-in-One Adeno-Associated Virus Delivery and Paired Nickase Applications. Genome Biol. 2015, 16, 257. [Google Scholar] [CrossRef] [Green Version]
- Batra, R.; Nelles, D.A.; Pirie, E.; Blue, S.M.; Marina, R.J.; Wang, H.; Chaim, I.A.; Thomas, J.D.; Zhang, N.; Nguyen, V.; et al. Elimination of Toxic Microsatellite Repeat Expansion RNA by RNA-Targeting Cas9. Cell 2017, 170, 899–912.e10. [Google Scholar] [CrossRef]
- Yourik, P.; Fuchs, R.T.; Mabuchi, M.; Curcuru, J.L.; Robb, G.B. Staphylococcus Aureus Cas9 Is a Multiple-Turnover Enzyme. RNA 2019, 25, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Mekler, V.; Kuznedelov, K.; Severinov, K. Quantification of the Affinities of CRISPR–Cas9 Nucleases for Cognate Protospacer Adjacent Motif (PAM) Sequences. J. Biol. Chem. 2020, 295, 6509–6517. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, X.; Wang, J.; Wang, M.; Chen, P.; Yin, M.; Li, J.; Sheng, G.; Wang, Y. Two Distant Catalytic Sites Are Responsible for C2c2 RNase Activities. Cell 2017, 168, 121–134.e12. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, X.; Ma, J.; Li, Z.; You, L.; Wang, J.; Wang, M.; Zhang, X.; Wang, Y. The Molecular Architecture for RNA-Guided RNA Cleavage by Cas13a. Cell 2017, 170, 714–726.e10. [Google Scholar] [CrossRef]
- Yan, W.X.; Chong, S.; Zhang, H.; Makarova, K.S.; Koonin, E.V.; Cheng, D.R.; Scott, D.A. Cas13d Is a Compact RNA-Targeting Type VI CRISPR Effector Positively Modulated by a WYL-Domain-Containing Accessory Protein. Mol. Cell 2018, 70, 327–339.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, A.; Huyke, D.A.; Sharma, E.; Sahoo, M.K.; Huang, C.; Banaei, N.; Pinsky, B.A.; Santiago, J.G. Electric Field-Driven Microfluidics for Rapid CRISPR-Based Diagnostics and Its Application to Detection of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 29518–29525. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Xiao, R.; Han, R.; Chang, L. Cryo-EM Structure of the RNA-Guided Ribonuclease Cas12g. Nat. Chem. Biol. 2021, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]

| Class | Features | Type | Features | Key Effectors | Target | Ref. |
|---|---|---|---|---|---|---|
| 1 | Effector, adaptation, and accessory functions distributed over multiple proteins | I |
| Cas1, Cas2, Cas4, Cas5, Cas6, Cas3, Cas8 | dsDNA | [25,49,50] |
| III |
| Cas1, Cas2, Cas5, Cas6, Cas7, Cas10 | dsDNA, RNA | [51,52] | ||
| IV |
| Cas5, Cas7, Csf1 | dsDNA | [52,53] | ||
| 2 | Single protein with multiple domains combines crRNA-binding, catalytic activity, and pre-crRNA processing | II |
| Cas9 | dsDNA, RNA | [12,25,54,55] |
| V |
| Cas12, Cas14 | dsDNA, ssDNA, RNA | [25,56] | ||
| VI |
| Cas13 | RNA | [15,25,41,54] |
| Identifier | Source Organism | Features | gRNA |
|---|---|---|---|
| LwaCas13a | Leptotrichia wadeii | Size: 1389 aa Structure: not available Nuclease domain: HEPN dCas mutations: D403G, R474A, and R1046A [44] PFS: not required Optimal spacer length: 20–28 nt Specificity: collateral cleavage in vitro but not in mammalian cells Turnover kinetics: not available Others: used for SHERLOCK diagnostics | 5′-GATTTAGACTACCCCAAAAACGAAGGGGACTAAAAC-SPACER |
| SauCas9 | Staphylococcus aureus | Size: 1053 aa Structure: 5AXW Nuclease domain: RuvC and HNH dCas mutations: D10A (RuvC), N580A (HNH) [26] PAM/PFS: PFS not required for ssRNA targeting; PAM required for dsDNA targeting (5’NNGRRT) Optimal spacer length: 23 nt Specificity: High DNA target specificity due to long PAM; mismatch tolerance characterization available for its RNA-targeting role; no collateral activity Turnover kinetics: DNA (multiple), RNA (single) Others: target secondary structure affects affinity | 5′-SPACER- GTTTTAGTACTCTGGAAACAGAATCTACTAAAACAAGGCAAAATGCCGTGTTTATCTCGTCAACTTGTTGGCGAGATTT |
| LbuCas13a | Leptotrichia buccalis | Size: 1159 aa Structure: 5XWP Nuclease domain: HEPN dCas mutations: R472A, H477A, R1048A and H1053A [84] PFS: not required Optimal spacer length: 20–24 nt Activity: collateral activity with high turnover (104 turnovers per target RNA recognized) [41] Turnover kinetics: not available for target cleavage but multiple turnover for collateral cleavage | 5′- GGCCACCCCAAAAATGAAGGGGACTAAAACA-SPACER |
| EsCas13d | Eubacterium siraeum | Size: 954 aa Structure: 6E9F Nuclease domain: HEPN dCas mutations: R295A, H300A, R849A and H854A [71] PFS: not required Optimal spacer length: 20–30 nt Specificity: collateral activity Turnover kinetics: not available Others: robust expression in E. coli; limited activity in mammalian cells [71] | 5′ AACTACACCCGTGCAAAAATGCAGGGGTCTAAAAC-SPACER |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Galicia, E.; Grünberg, R.; Arold, S.T. How to Find the Right RNA-Sensing CRISPR-Cas System for an In Vitro Application. Biosensors 2022, 12, 53. https://doi.org/10.3390/bios12020053
Díaz-Galicia E, Grünberg R, Arold ST. How to Find the Right RNA-Sensing CRISPR-Cas System for an In Vitro Application. Biosensors. 2022; 12(2):53. https://doi.org/10.3390/bios12020053
Chicago/Turabian StyleDíaz-Galicia, Escarlet, Raik Grünberg, and Stefan T. Arold. 2022. "How to Find the Right RNA-Sensing CRISPR-Cas System for an In Vitro Application" Biosensors 12, no. 2: 53. https://doi.org/10.3390/bios12020053
APA StyleDíaz-Galicia, E., Grünberg, R., & Arold, S. T. (2022). How to Find the Right RNA-Sensing CRISPR-Cas System for an In Vitro Application. Biosensors, 12(2), 53. https://doi.org/10.3390/bios12020053





