Electrophoretic µPAD for Purification and Analysis of DNA Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Polymerase Chain Reactions
2.3. DNA/Protein-Mix Sample Preparation
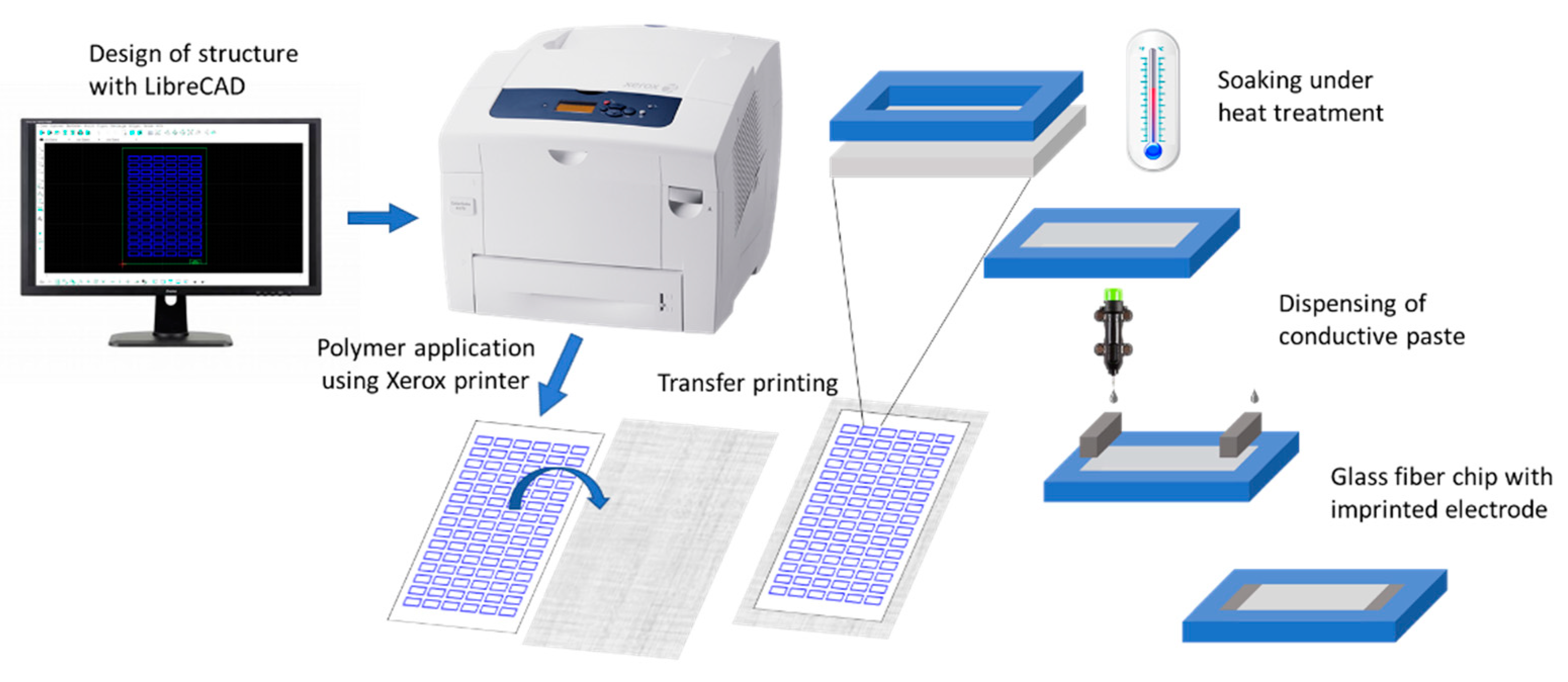
2.4. Preparation of the Electrophoresis Chip
2.5. Description of the Measurement Setup
2.6. DNA Electrophoresis on the Fiber Chip
3. Results
3.1. Manufacturing of the Fiber-Based Electrophoresis Chip
3.2. Characterization of the Fiber-Based Electrophoresis Chip
3.3. Conductive Fiber-Electrophoresis Chip for Movement of Nucleic Acid Mixtures
3.4. Movement of a Single DNA Fragment on the Electrophoresis Chip
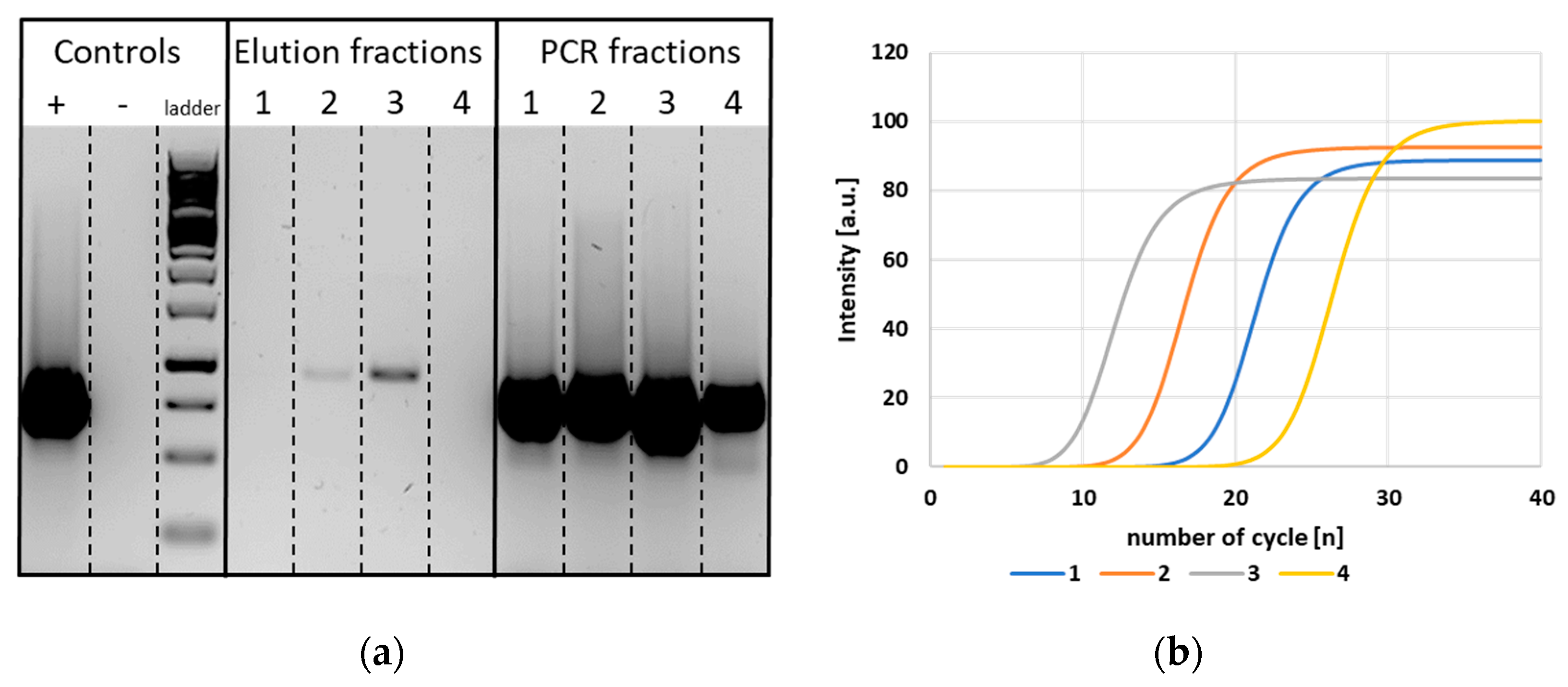
3.5. Separation of DNA from Other Potential Sample Components
3.6. Cross-Layer Chip Arrangement
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolník, V.; Liu, S.; Jovanovich, S. Capillary Electrophoresis on Microchip. Electrophoresis 2000, 21, 41–54. [Google Scholar] [CrossRef]
- Auroux, P.-A.; Iossifidis, D.; Reyes, D.R.; Manz, A. Micro Total Analysis Systems. 2. Analytical Standard Operations and Applications. Anal. Chem. 2002, 74, 2637–2652. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro Total Analysis Systems. 1. Introduction, Theory, and Technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Vilkner, T.; Janasek, D.; Manz, A. Micro Total Analysis Systems. Recent Developments. Anal. Chem. 2004, 76, 3373–3386. [Google Scholar] [CrossRef]
- Merrill, C.R.; Tarasowych, M.G.; Harrington, M.G. Chapter 7—Protein Staining and Detection Methods. In Gel Electrophoresis of Proteins; Dunn, M.J., Ed.; Butterworth-Heinemann: Waltham, MA, USA, 1986; pp. 323–362. [Google Scholar]
- Edmonds, C.G.; Loo, J.A.; Barinaga, C.J.; Udseth, H.R.; Smith, R.D. Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry. J. Chromatogr. A 1989, 474, 21–37. [Google Scholar] [CrossRef]
- König, P. Applicaçao Da Electrophorése Nos Trabalhos Chimicos Com Quantidades Pequenas. In Proceedings of the Actas e Trabalhos do Terceiro Congresso Sul-Americano, Rio de Janeiro, Sâo Paulo, Brazil; 1937; Volume 2, pp. 334–336. [Google Scholar]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-Printed Microfluidic Multianalyte Chemical Sensing Paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef]
- Pelton, R. Bioactive Paper Provides a Low-Cost Platform for Diagnostics. TrAC Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Niedl, R.R.; Beta, C. Hydrogel-Driven Paper-Based Microfluidics. Lab Chip 2015, 15, 2452–2459. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-Dimensional Microfluidic Devices Fabricated in Layered Paper and Tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [Green Version]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Use of Multiple Colorimetric Indicators for Paper-Based Microfluidic Devices. Anal. Chim. Acta 2010, 674, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ge, L.; Huang, J.; Wang, S.; Ge, S. Microfluidic Paper-Based Chemiluminescence Biosensor for Simultaneous Determination of Glucose and Uric Acid. Lab Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical Detection for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-Based Fluorescent and Visual Biosensor for Multiplexed Monitoring of Cancer Cells in Microfluidic Paper-Based Analytical Devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Choopara, I.; Suea-Ngam, A.; Teethaisong, Y.; Howes, P.D.; Schmelcher, M.; Leelahavanichkul, A.; Thunyaharn, S.; Wongsawaeng, D.; deMello, A.J.; Dean, D.; et al. Fluorometric Paper-Based, Loop-Mediated Isothermal Amplification Devices for Quantitative Point-of-Care Detection of Methicillin-Resistant Staphylococcus Aureus (MRSA). ACS Sens. 2021, 6, 742–751. [Google Scholar] [CrossRef]
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards Smart Personalized Perspiration Analysis: An IoT-Integrated Cellulose-Based Microfluidic Wearable Patch for Smartphone Fluorimetric Multi-Sensing of Sweat Biomarkers. Biosens. Bioelectron. 2020, 168, 112450. [Google Scholar] [CrossRef]
- Gao, B.; Elbaz, A.; He, Z.; Xie, Z.; Xu, H.; Liu, S.; Su, E.; Liu, H.; Gu, Z. Bioinspired Kirigami Fish-Based Highly Stretched Wearable Biosensor for Human Biochemical–Physiological Hybrid Monitoring. Adv. Mater. Technol. 2018, 3, 1700308. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.Q.; Soenksen, L.R.; Donghia, N.M.; Angenent-Mari, N.M.; de Puig, H.; Huang, A.; Lee, R.; Slomovic, S.; Galbersanini, T.; Lansberry, G.; et al. Wearable Materials with Embedded Synthetic Biology Sensors for Biomolecule Detection. Nat. Biotechnol. 2021, 39, 1366–1374. [Google Scholar] [CrossRef]
- Colozza, N.; Kehe, K.; Dionisi, G.; Popp, T.; Tsoutsoulopoulos, A.; Steinritz, D.; Moscone, D.; Arduini, F. A Wearable Origami-like Paper-Based Electrochemical Biosensor for Sulfur Mustard Detection. Biosens. Bioelectron. 2019, 129, 15–23. [Google Scholar] [CrossRef]
- Mehl, J.W. Introduction to Paper Electrophoresis and Related Methods: Michael Lederer. Elsevier Publishing Co., Amsterdam, The Netherlands; 1955, 206 Pages, 70 Figures, 378, 6d. Anal. Chim. Acta 1956, 1, 300. [Google Scholar] [CrossRef]
- Fujita, K.; Nagatsu, T.; Shinpo, K.; Maruta, K.; Teradaira, R.; Nakamura, M. Improved Analysis for Urinary Polyamines by Use of High-Voltage Electrophoresis on Paper. Clin. Chem. 1980, 26, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.J.; Urbin, M.C.; Williamson, M.B. Ionography: Some Aspects of Ion Migration on Paper in an Electric Field. J. Colloid Sci. 1951, 6, 236–244. [Google Scholar] [CrossRef]
- McLoughlin, J.V. Splitting of Serum Albumin during Electrophoresis on Paper. Nature 1961, 191, 1305–1306. [Google Scholar] [CrossRef]
- Smith, J.D. Paper Electrophoresis of Nucleic Acid Components. In Methods in Enzymology; Nucleic Acids, Part A; Academic Press: Cambridge, MA, USA, 1967; Volume 12, pp. 350–361. [Google Scholar]
- Ge, L.; Wang, S.; Ge, S.; Yu, J.; Yan, M.; Li, N.; Huang, J. Electrophoretic Separation in a Microfluidic Paper-Based Analytical Device with an on-Column Wireless Electrogenerated Chemiluminescence Detector. Chem. Commun. 2014, 50, 5699–5702. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, X.; Crooks, R.M. Low-Voltage Origami-Paper-Based Electrophoretic Device for Rapid Protein Separation. Anal. Chem. 2014, 86, 12390–12397. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Kang, B.-H.; Ku, J.; Kim, Y.; Jeong, K.-H. On-Chip Paper Electrophoresis for Ultrafast Screening of Infectious Diseases. BioChip J. 2021, 15, 305–311. [Google Scholar] [CrossRef]
- Rosenfeld, T.; Bercovici, M. 1000-Fold Sample Focusing on Paper-Based Microfluidic Devices. Lab Chip 2014, 14, 4465–4474. [Google Scholar] [CrossRef]
- Li, X.; Luo, L.; Crooks, R.M. Low-Voltage Paper Isotachophoresis Device for DNA Focusing. Lab Chip 2015, 15, 4090–4098. [Google Scholar] [CrossRef] [Green Version]
- Briggs, D.R.; Garner, E.F.; Smith, F. Separation of Carbohydrates by Electrophoresis on Glass Filter Paper. Nature 1956, 178, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Smith, F. The heterogeneity of polysaccharides as revealed by electrophoresis on glass-fiber paper. J. Am. Chem. Soc. 1957, 79, 3929–3931. [Google Scholar] [CrossRef]
- Bourne, E.J.; Foster, A.B.; Grant, P.M. 831. Ionophoresis of Carbohydrates. Part IV. Separations of Carbohydrates on Fibreglass Sheets. J. Chem. Soc. 1956, 4, 4311–4314. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Qin, J.; Lin, B. Fabrication and Characterization of Paper-Based Microfluidics Prepared in Nitrocellulose Membrane By Wax Printing. Anal. Chem. 2010, 82, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Xu, C.; Lin, S.; Luo, J.; Wu, M.; Yang, F. A Simple Paper-Based Sensor Fabricated by Selective Wet Etching of Silanized Filter Paper Using a Paper Mask. Biomicrofluidics 2014, 8, 056504. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liang, Y.; Zhang, Y.; Le, S.; Li, D.; Zhang, S. One-Step Patterning of Hollow Microstructures in Paper by Laser Cutting to Create Microfluidic Analytical Devices. Analyst 2013, 138, 671–676. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-Based Microfluidic Devices by Plasma Treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef]
- Brewster, J.D.; Paoli, G.C. DNA Extraction Protocol for Rapid PCR Detection of Pathogenic Bacteria. Anal. Biochem. 2013, 442, 107–109. [Google Scholar] [CrossRef]
- Gawron, A.J.; Martin, R.S.; Lunte, S.M. Fabrication and Evaluation of a Carbon-Based Dual-Electrode Detector for Poly(Dimethylsiloxane) Electrophoresis Chips. Electrophoresis 2001, 22, 242–248. [Google Scholar] [CrossRef]
- McCreery, R.L.; Cline, K.K. Laboratory Techniques in Electroanalytical Chemistry; Kissinger, P.T., Heineman, W.R., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 293–332. [Google Scholar]
- Vandaveer, W.R.; Pasas, S.A.; Martin, R.S.; Lunte, S.M. Recent Developments in Amperometric Detection for Microchip Capillary Electrophoresis. Electrophoresis 2002, 23, 3667–3677. [Google Scholar] [CrossRef]
- Noor, M.O.; Krull, U.J. Camera-Based Ratiometric Fluorescence Transduction of Nucleic Acid Hybridization with Reagentless Signal Amplification on a Paper-Based Platform Using Immobilized Quantum Dots as Donors. Anal. Chem. 2014, 86, 10331–10339. [Google Scholar] [CrossRef] [PubMed]
- Pänke, O.; Kirbs, A.; Lisdat, F. Voltammetric Detection of Single Base-Pair Mismatches and Quantification of Label-Free Target SsDNA Using a Competitive Binding Assay. Biosens. Bioelectron. 2007, 22, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical Paper-Based Peptide Nucleic Acid Biosensor for Detecting Human Papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Ozkan, D.; Kara, P.; Meric, B.; Gooding, J.J.; Ozsoz, M. Voltammetric Determination of DNA Hybridization Using Methylene Blue and Self-Assembled Alkanethiol Monolayer on Gold Electrodes. Anal. Chim. Acta 2002, 462, 39–47. [Google Scholar] [CrossRef]
- Riedel, M.; Kartchemnik, J.; Schöning, M.J.; Lisdat, F. Impedimetric DNA Detection—Steps Forward to Sensorial Application. Anal. Chem. 2014, 86, 7867–7874. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinsohn, N.K.; Niedl, R.R.; Anielski, A.; Lisdat, F.; Beta, C. Electrophoretic µPAD for Purification and Analysis of DNA Samples. Biosensors 2022, 12, 62. https://doi.org/10.3390/bios12020062
Heinsohn NK, Niedl RR, Anielski A, Lisdat F, Beta C. Electrophoretic µPAD for Purification and Analysis of DNA Samples. Biosensors. 2022; 12(2):62. https://doi.org/10.3390/bios12020062
Chicago/Turabian StyleHeinsohn, Natascha Katharina, Robert Raimund Niedl, Alexander Anielski, Fred Lisdat, and Carsten Beta. 2022. "Electrophoretic µPAD for Purification and Analysis of DNA Samples" Biosensors 12, no. 2: 62. https://doi.org/10.3390/bios12020062
APA StyleHeinsohn, N. K., Niedl, R. R., Anielski, A., Lisdat, F., & Beta, C. (2022). Electrophoretic µPAD for Purification and Analysis of DNA Samples. Biosensors, 12(2), 62. https://doi.org/10.3390/bios12020062





