Recent Advances in Single Fe-Based Nanoagents for Photothermal–Chemodynamic Cancer Therapy
Abstract
:1. Introduction
2. Nanoplatforms for PTT/CDT in the NIR-I Biowindow
3. Nanoplatforms for PTT/CDT in the NIR-II Biowindow
3.1. Iron Sulphide/Phosphide Nanoparticles
3.2. Copper Iron Sulfide Nanoparticles
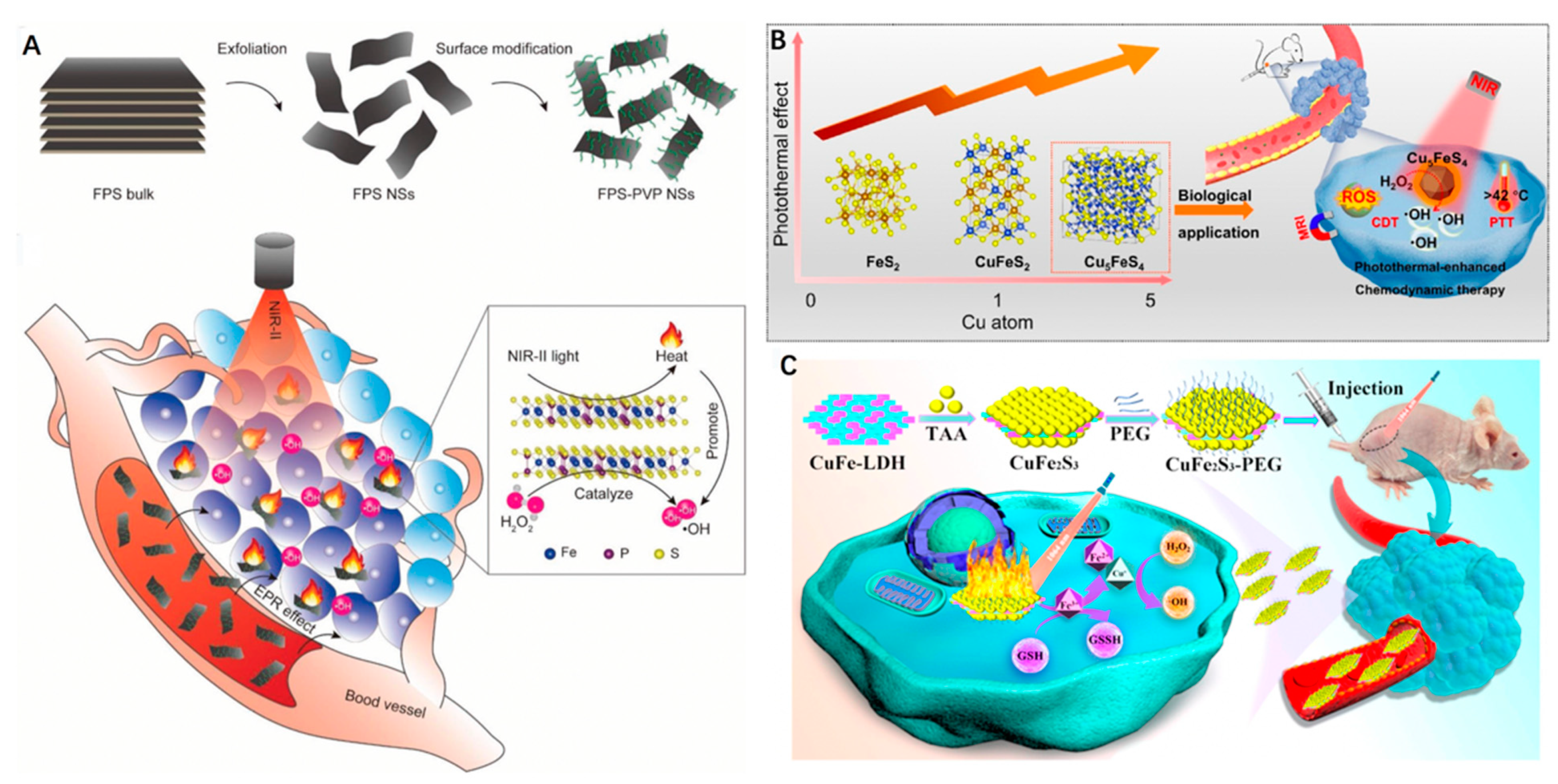
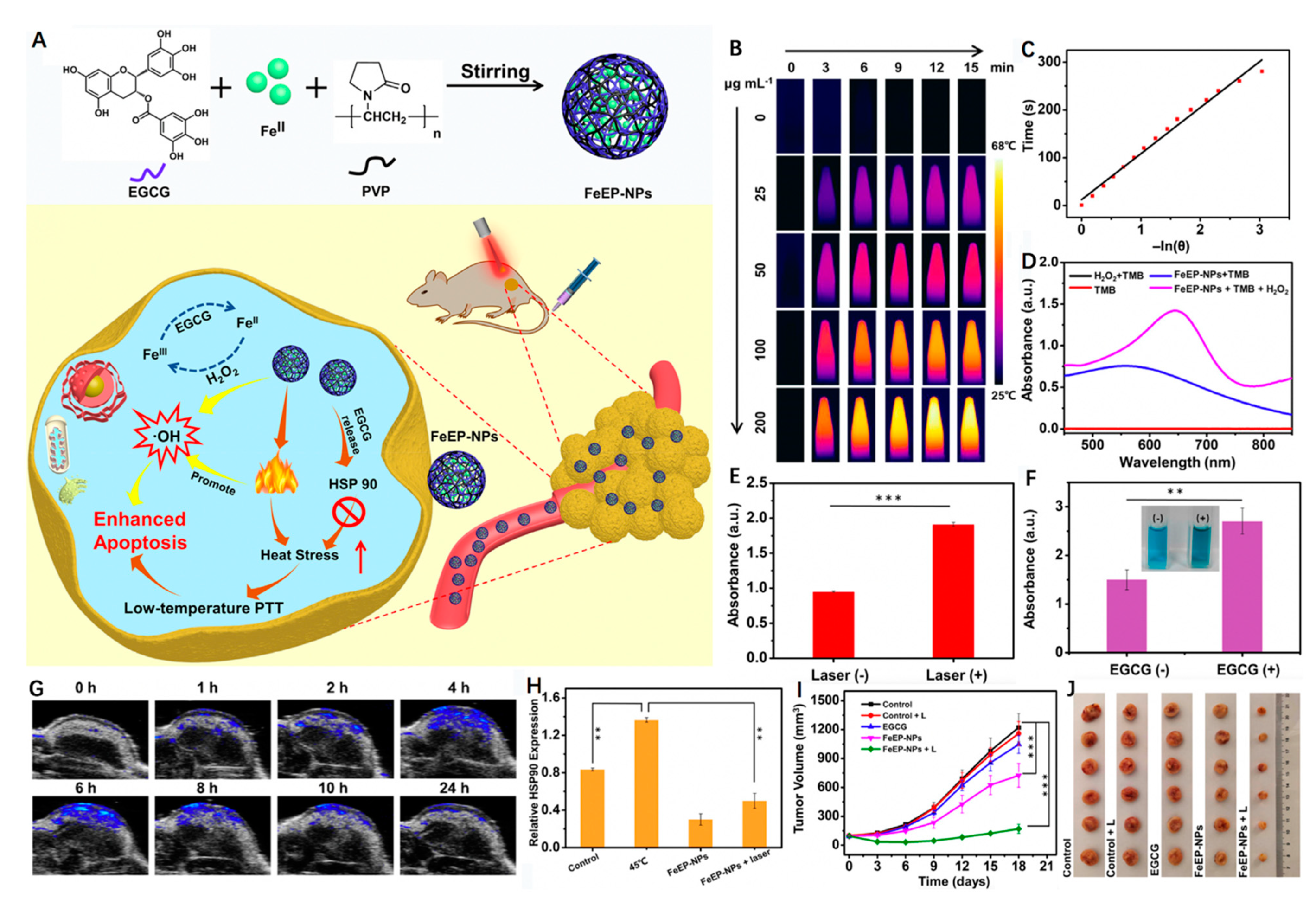
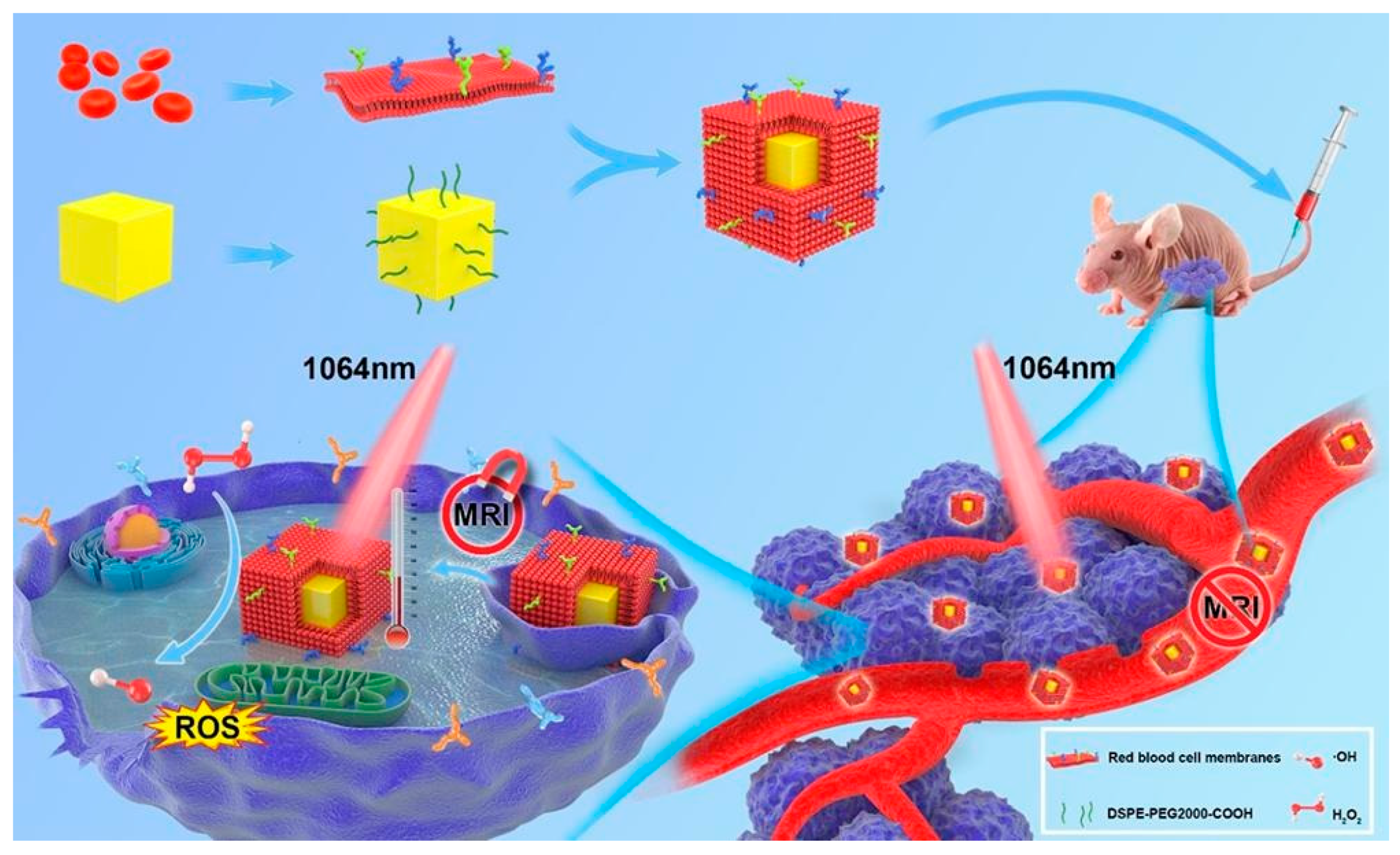
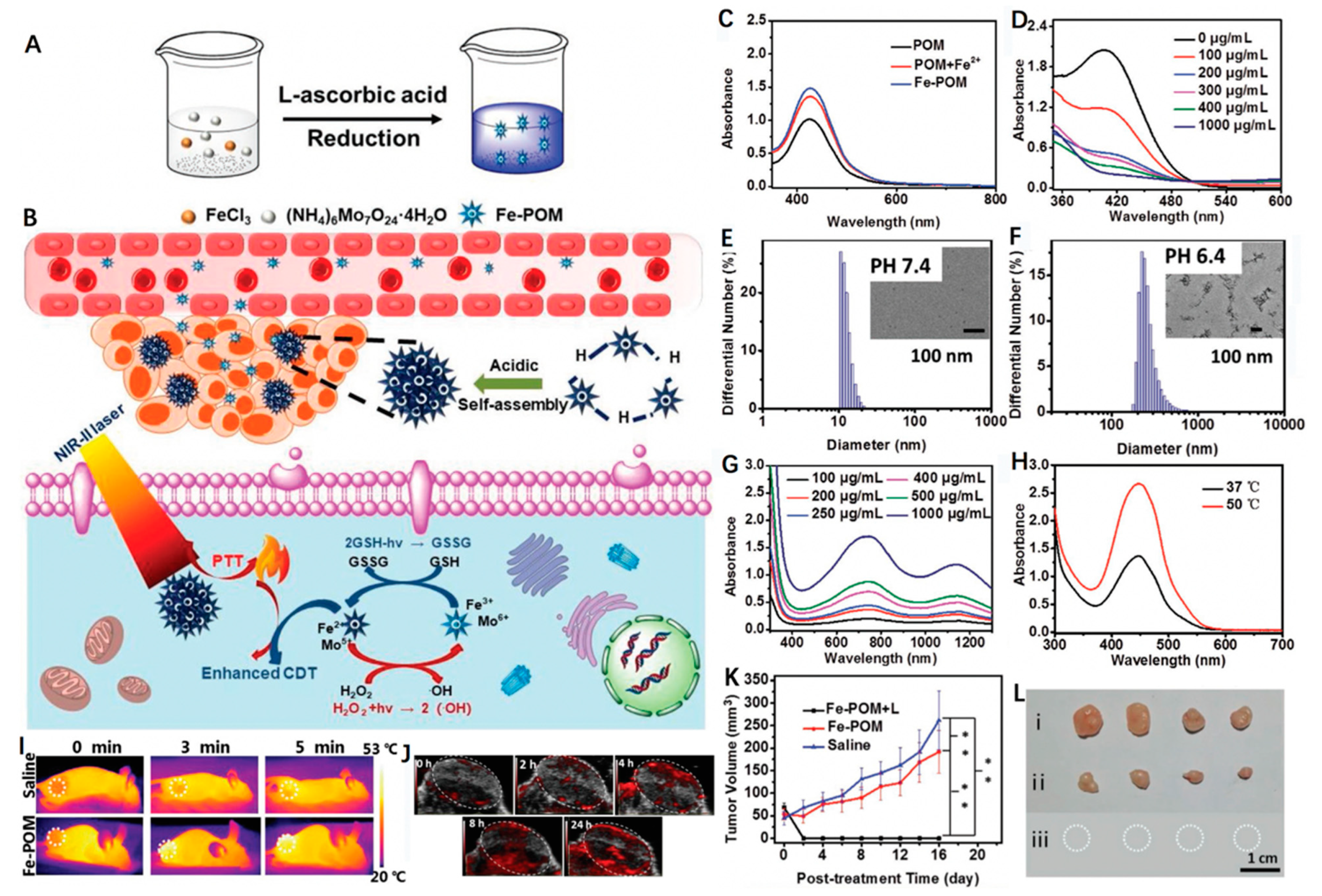
3.3. Iron-Doped Inorganic Nanoparticles
4. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Del. Rev. 2021, 178, 113907. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Tian, F.; Wang, S.; Shi, K.; Zhong, X.; Gu, Y.; Fan, Y.; Zhang, Y.; Yang, M. Dual-Depletion of Intratumoral Lactate and ATP with Radicals Generation for Cascade Metabolic-Chemodynamic Therapy. Adv. Sci. 2021, 2102595. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Qiu, M.; Zhao, K.; Hu, H.; Xu, Q.; Cao, J.; Luo, Y.; Liu, L.; Xu, Z.; Yi, C. Multifunctional phototheranostic nanoplatform based on polydopamine-manganese dioxide-IR780 iodide for effective magnetic resonance imaging-guided synergistic photodynamic/photothermal therapy. J. Colloid Interface Sci. 2022, 611, 193–204. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater. 2018, 30, 1802591. [Google Scholar] [CrossRef]
- Zhang, L.; Bei, H.P.; Piao, Y.; Wang, Y.; Yang, M.; Zhao, X. Polymer-Brush-Grafted Mesoporous Silica Nanoparticles for Triggered Drug Delivery. ChemPhysChem 2018, 19, 1956–1964. [Google Scholar] [CrossRef] [Green Version]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Lv, K.; Lin, H.; Qu, F. Biodegradable hollow Co3S4@ N-doped carbon as enhanced PTT/PDT agent for multimodal MR/thermal imaging and synergistic antitumor therapy. Chem. Eng. J. 2020, 392, 124555. [Google Scholar] [CrossRef]
- Urbanová, V.; Pumera, M. Biomedical and bioimaging applications of 2D pnictogens and transition metal dichalcogenides. Nanoscale 2019, 11, 15770–15782. [Google Scholar] [CrossRef]
- Su, Z.; Dong, S.; Zhao, S.-C.; Liu, K.; Tan, Y.; Jiang, X.; Assaraf, Y.G.; Qin, B.; Chen, Z.-S.; Zou, C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updates 2021, 58, 100777. [Google Scholar] [CrossRef]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew. Chem. Int. Ed. 2016, 128, 2141–2146. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Liu, F.; Zhang, S.; Duan, J.; Li, Z.; Kong, Y.; Sang, Y.; Liu, H.; Bu, W. Self-assembled copper–amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2018, 141, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, X.; Chen, J.; Wang, X.; Wei, L.; Fang, L.; Kumar, A.; Zhuang, S.; Liu, J. Recent advances in MOF-based nanoplatforms generating reactive species for chemodynamic therapy. Dalton Trans. 2020, 49, 11045–11058. [Google Scholar] [CrossRef]
- Tian, Q.; Xue, F.; Wang, Y.; Cheng, Y.; An, L.; Yang, S.; Chen, X.; Huang, G. Recent advances in enhanced chemodynamic therapy strategies. Nano Today 2021, 39, 101162. [Google Scholar] [CrossRef]
- Xin, J.; Deng, C.; Aras, O.; Zhou, M.; Wu, C.; An, F. Chemodynamic nanomaterials for cancer theranostics. J. Nanobiotechnol. 2021, 19, 192. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.; Liu, P.; Wang, J.; Yi, C.; Xu, Z.; Ren, J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1–T2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, Z.; Liu, P.; Wang, J.; Cao, J.; Shen, A.; Xu, Z. An efficient tumor-inducible nanotheranostics for magnetic resonance imaging and enhanced photodynamic therapy. Chem. Eng. J. 2019, 358, 969–979. [Google Scholar] [CrossRef]
- Du, J.; Shi, T.; Long, S.; Chen, P.; Sun, W.; Fan, J.; Peng, X. Enhanced photodynamic therapy for overcoming tumor hypoxia: From microenvironment regulation to photosensitizer innovation. Coord. Chem. Rev. 2021, 427, 213604. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Z.; Liu, B.; He, F.; Gai, S.; Yang, P.; Yang, D.; Li, C.; Lin, J. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coord. Chem. Rev. 2022, 451, 214267. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, L.; Wei, J.; Li, R.; Xu, Q.; Hu, H.; Xu, Z.; Ren, J.; Wong, C.-Y. Tumor acidity-activatable photothermal/Fenton nanoagent for synergistic therapy. J. Colloid Interface Sci. 2022, 612, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-S.; Huang, T.; Song, J.; Ou, X.-Y.; Wang, Z.; Deng, H.; Tian, R.; Liu, Y.; Wang, J.-F.; Liu, Y. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 2018, 130, 4996–5000. [Google Scholar] [CrossRef]
- Fu, L.-H.; Hu, Y.-R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable manganese-doped calcium phosphate nanotheranostics for traceable cascade reaction-enhanced anti-tumor therapy. ACS Nano 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Ge, K.; Li, Z.; Liu, H.; Dai, X.; Zhang, Y.; Chen, S.; Liang, X.; Zhang, J. Self-supply of O2 and H2O2 by a Nanocatalytic medicine to enhance combined chemo/Chemodynamic therapy. Adv. Sci. 2019, 6, 1902137. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, Y.; Wang, C.; Jiang, Y.; Chu, X.; Wu, F.; Wu, Y.; Cai, X.; Cao, Y.; Liu, Y. NIR-Triggered Intracellular H+ Transients for Lamellipodia-Collapsed Antimetastasis and Enhanced Chemodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 21905–21910. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef]
- Jin, Q.; Yan, S.; Hu, H.; Jin, L.; Pan, Y.; Zhang, J.; Huang, J.; Xiao, H.; Cao, P. Enhanced Chemodynamic Therapy and Chemotherapy via Delivery of a Dual Threat ArtePt and Iodo-Click Reaction Mediated Glutathione Consumption. Small Methods 2021, 5, 2101047. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, K.; Fu, C.; Liu, H.; Lei, M.; Bao, J.; Fu, A.; Yu, Y.; Zhang, W. Interfacial engineered gadolinium oxide nanoparticles for magnetic resonance imaging guided microenvironment-mediated synergetic chemodynamic/photothermal therapy. Biomaterials 2019, 219, 119379. [Google Scholar] [CrossRef]
- Yan, Y.; Hou, Y.; Zhang, H.; Gao, W.; Han, R.; Yu, J.; Xu, L.; Tang, K. CeO2 QDs anchored on MnO2 nanoflowers with multiple synergistic effects for amplified tumour therapy. Colloids Surf. B. Biointerfaces 2021, 208, 112103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Meng, X.; Yang, Z.; Dong, H.; Zhang, X. Enhanced cancer therapy by hypoxia-responsive copper metal-organic frameworks nanosystem. Biomaterials 2020, 258, 120278. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Song, L.; Xu, J.; Wang, R.; Shi, J.; Chen, M.; Zhang, Y. Smart Manganese Dioxide-Based Lanthanide Nanoprobes for Triple-Negative Breast Cancer Precise Gene Synergistic Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 35444–35455. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Li, X.; Lu, N.; Ge, Z. Polymersome Nanoreactor-Mediated Combination Chemodynamic-Immunotherapy via ROS Production and Enhanced STING Activation. Adv. Ther. 2021, 4, 2100130. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, J.; Guo, H.; Sun, A.; Chen, P.; Xi, L.; Huang, W.; Song, X.; Dong, X. Mo2C-derived polyoxometalate for NIR-II photoacoustic imaging-guided chemodynamic/photothermal synergistic therapy. Angew. Chem. Int. Ed. 2019, 58, 18641–18646. [Google Scholar] [CrossRef]
- Zhou, B.; Yin, C.; Feng, Q.; Wu, Y.; Pan, X.; Liu, C.; Tian, J.; Geng, S.; Wang, K.; Xing, J. Polypyrrole-based nanotheranostic agent for MRI guided photothermal-chemodynamic synergistic cancer therapy. Nanoscale 2021, 13, 19085–19097. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Li, A.; Xu, S.; Manghnani, P.N.; Liu, C.; Guo, L.; Zheng, H.; Liu, B. Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Ren, J.; Ba, L.; He, W.; Wong, C.-Y. Multifunctional oxygen-enriching nano-theranostics for cancer-specific magnetic resonance imaging and enhanced photodynamic/photothermal therapy. Nano Res. 2020, 13, 1389–1398. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Fu, J.; Zhang, B.; Xie, N.; Nie, G.; Ågren, H.; Qiu, M.; Zhang, H. NIR-II Responsive Inorganic 2D Nanomaterials for Cancer Photothermal Therapy: Recent Advances and Future Challenges. Adv. Funct. Mater. 2021, 2101625. [Google Scholar] [CrossRef]
- Yin, C.; Li, X.; Wang, Y.; Liang, Y.; Zhou, S.; Zhao, P.; Lee, C.S.; Fan, Q.; Huang, W. Organic Semiconducting Macromolecular Dyes for NIR-II Photoacoustic Imaging and Photothermal Therapy. Adv. Funct. Mater. 2021, 31, 2104650. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, J.; Pu, K. Second Near-Infrared Absorbing Agents for Photoacoustic Imaging and Photothermal Therapy. Small Methods 2019, 3, 1900553. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Feng, T.; Wan, Y.; Blum, N.T.; Liu, C.; Zheng, C.; Zhao, Z.; Jiang, T.; Wang, J. Plasmonic modulation of gold nanotheranostics for targeted NIR-II photothermal-augmented immunotherapy. Nano Today 2020, 35, 100987. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, B.; Jiang, X.; Yuan, Z.; Chen, S.; Sun, P.; Fan, Q.; Huang, W. Double-acceptor conjugated polymers for NIR-II fluorescence imaging and NIR-II photothermal therapy applications. J. Mater. Chem. B 2021, 9, 1002–1008. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, X.; Huang, J.; Li, J.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.; Duan, H. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat. Commun. 2020, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Liu, T.; Yang, S.; Zhou, M.; Wang, W.; Yu, H. Iron-based theranostic nanoplatform for improving chemodynamic therapy of cancer. ACS Biomater. Sci. Eng. 2020, 6, 4834–4845. [Google Scholar] [CrossRef]
- Liu, X.; Liang, T.; Zhang, R.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Iron-Based Metal–Organic Frameworks in Drug Delivery and Biomedicine. ACS Appl. Mater. Interfaces 2021, 13, 9643–9655. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huo, M.; Shi, J. Nanocatalytic Medicine of Iron-Based Nanocatalysts. CCS Chem. 2021, 3, 2445–2463. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021, 50, 8669–8742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Q.; Guo, W.; Zhang, F.; Yu, K.; Yang, C.; Qu, F. Non-stoichiometric cobalt sulfide nanodots enhance photothermal and chemodynamic therapies against solid tumor. J. Colloid Interface Sci. 2021, 600, 390–402. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, C.; Li, B.; Liu, Z.; Gu, B.; He, S.; Li, P.; Sun, Y.; Song, S. A core-shell Au@Cu2-xSe heterogeneous metal nanocomposite for photoacoustic and computed tomography dual-imaging-guided photothermal boosted chemodynamic therapy. J. Nanobiotechnol. 2021, 19, 410. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS nanodots with ultrahigh efficient renal clearance for positron emission tomography imaging and image-guided photothermal therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef] [Green Version]
- Longmire, M.R.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Biologically optimized nanosized molecules and particles: More than just size. Bioconjug. Chem. 2011, 22, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Wang, X.; Li, B.; Zhang, W.; Cui, Z.; Lu, X.; Zou, R.; Hu, J. “Transformed” Fe3S4 tetragonal nanosheets: A high-efficiency and body-clearable agent for magnetic resonance imaging guided photothermal and chemodynamic synergistic therapy. Nanoscale 2018, 10, 17902–17911. [Google Scholar] [CrossRef]
- Qin, Z.; Qiu, M.; Zhang, Q.; Yang, S.; Liao, G.; Xiong, Z.; Xu, Z. Development of copper vacancy defects in a silver-doped CuS nanoplatform for high-efficiency photothermal–chemodynamic synergistic antitumor therapy. J. Mater. Chem. B 2021, 9, 8882–8896. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Y.; Du, W.; Liu, Z.; Zhang, S.; Yang, J.; Yao, H.; Liu, T.; Ma, M.; Chen, H. Clearable theranostic platform with a pH-independent chemodynamic therapy enhancement strategy for synergetic photothermal tumor therapy. ACS Appl. Mater. Interfaces 2019, 11, 18133–18144. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, M.; Zhang, L.; Ha, E.; Hu, X.; Zou, R.; Yan, L.; Hu, J. Tumor Microenvironment Responsive Biodegradable Fe-Doped MoOx Nanowires for Magnetic Resonance Imaging Guided Photothermal-Enhanced Chemodynamic Synergistic Antitumor Therapy. Adv. Healthc. Mater. 2021, 10, 2001665. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Zhang, Y.; Ge, W.; Zhong, L.; Huang, Y.; Si, W.; Wang, W.; Zhao, Y.; Dong, X. A three-dimensional BODIPY–iron (iii) compound with improved H2O2-response for NIR-II photoacoustic imaging guided chemodynamic/photothermal therapy. Chem. Commun. 2020, 56, 6281–6284. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ma, M.; Liang, K.; Shen, J.; Lan, Z.; Chen, H. A self-assembled metal-polyphenolic nanomedicine for mild photothermal-potentiated chemodynamic therapy of tumors. Appl. Mater. Today 2021, 25, 101235. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, C.; Liu, S.; Cai, L.; Zhou, Y.; Pang, M. Facile synthesis of Fe–baicalein nanoparticles for photothermal/chemodynamic therapy with accelerated Fe III/Fe II conversion. J. Mater. Chem. B 2021, 9, 3295–3299. [Google Scholar] [CrossRef]
- Kong, X.; Wan, G.; Li, B.; Wu, L. Recent advances of polyoxometalates in multi-functional imaging and photothermal therapy. J. Mater. Chem. B 2020, 8, 8189–8206. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Chen, S.; Wang, R.; Chen, Q.; Li, J.; Luo, Y.; Wang, X.; Chen, H. Photothermal fenton nanocatalysts for synergetic cancer therapy in the second near-infrared window. ACS Appl. Mater. Interfaces 2020, 12, 30145–30154. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Lei, H.; Geng, Y.; Zhao, Q.; Gong, F.; Yang, Z.; Dong, Z.; Liu, Z.; Cheng, L. Hollow Cu2Se nanozymes for tumor photothermal-catalytic therapy. Chem. Mater. 2019, 31, 6174–6186. [Google Scholar] [CrossRef]
- She, D.; Peng, S.; Liu, L.; Huang, H.; Zheng, Y.; Lu, Y.; Geng, D.; Yin, B. Biomimic FeS2 nanodrug with hypothermal photothermal effect by clinical approved NIR-Ⅱ light for augmented chemodynamic therapy. Chem. Eng. J. 2020, 400, 125933. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, T.; Zhang, S.; Zhao, Y.; Song, S.; Li, C. One-dimensional Fe2P acts as a Fenton agent in response to NIR II light and ultrasound for deep tumor synergetic theranostics. Angew. Chem. Int. Ed. 2019, 131, 2429–2434. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Q.; Chen, Q.; Zhao, X.; Pennycook, S.J.; Chen, H. Highly Efficient 2D NIR-II Photothermal Agent with Fenton Catalytic Activity for Cancer Synergistic Photothermal-Chemodynamic Therapy. Adv. Sci. 2020, 7, 1902576. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, H.; Cheng, X.; Wu, L.; Wang, Y.; Zhou, Z.; He, J.; Chen, H.-Y.; Ye, D. Degradable hybrid CuS nanoparticles for imaging-guided synergistic cancer therapy via low-power NIR-II light excitation. CCS Chem. 2021, 3, 1336–1349. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y.; Yang, Z.; Yang, M.; Wong, C.-Y. NIR-II-driven and glutathione depletion-enhanced hypoxia-irrelevant free radical nanogenerator for combined cancer therapy. J. Nanobiotechnol. 2021, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Guo, H.; Yu, N.; Ren, Q.; Jiang, Q.; Xia, J.; Peng, C.; Zhang, H.; Chen, Z. Synthesis of one-for-all type Cu5FeS4 nanocrystals with improved near infrared photothermal and Fenton effects for simultaneous imaging and therapy of tumor. J. Colloid Interface Sci. 2021, 592, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, T.; Wang, G.; Wang, Z.; Yan, D.; Liang, R.; Tan, C. Ultrathin CuFe2S3 nanosheets derived from CuFe-layered double hydroxide as an efficient nanoagent for synergistic chemodynamic and NIR-II photothermal therapy. Chem. Eng. J. 2021, 419, 129458. [Google Scholar] [CrossRef]
- Yu, Z.; Chan, W.K.; Zhang, Y.; Tan, T.T.Y. Near-infrared-II activated inorganic photothermal nanomedicines. Biomaterials 2021, 269, 120459. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Huang, H.; Cao, C.; Yin, J.; Xu, W.; Wang, W.; Song, X.; Zhang, Y.; Dong, X. Fe-doped Polyoxometalate as acid-aggregated Nanoplatform for NIR-II Photothermal-enhanced Chemodynamic therapy. Adv. Healthc. Mater. 2020, 9, 2000005. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhang, H.; Huang, H.; Sun, L.; Ma, L.; Du, Y.; Pei, C.; Zhang, Q.; Li, H. Activating layered metal oxide nanomaterials via structural engineering as biodegradable nanoagents for photothermal cancer therapy. Small 2021, 17, 2007486. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, Q.; Liu, J.; Zhang, S.; Zhang, T.; Wang, D.; Wang, Y.; Jin, L.; Zhang, H. Carambola-like Bi2Te3 superstructures with enhanced photoabsorption for highly efficient photothermal therapy in the second near-infrared biowindow. J. Mater. Chem. B 2021, 9, 7271–7277. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, 2006484. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, B.; Liang, S.; Zhao, Y.; Cheng, Z.; Xing, B.; Lin, J. Intelligent MoS2–CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/immunotherapy. Biomaterials 2021, 268, 120545. [Google Scholar] [CrossRef]
- Jiao, X.; Sun, L.; Zhang, W.; Ren, J.; Zhang, L.; Cao, Y.; Xu, Z.; Kang, Y.; Xue, P. Engineering oxygen-deficient ZrO2-x nanoplatform as therapy-activated “immunogenic cell death (ICD)” inducer to synergize photothermal-augmented sonodynamic tumor elimination in NIR-II biological window. Biomaterials 2021, 272, 120787. [Google Scholar] [CrossRef] [PubMed]



| Material | PCE | Laser Wavelength | Tumor Model | Reference |
|---|---|---|---|---|
| PVP-Fe3S4 | 63.4% | 915 nm | HeLa tumor | [58] |
| BSA-CuFeS2 | 38.8% | 808 nm | – | [60] |
| FMO | 48.5% | 808 nm | HeLa tumor | [61] |
| FeEP | 33.6% | 808 nm | 4T1 tumor | [63] |
| Fe–BaP | 45.6% | 808 nm | 4T1 tumor | [64] |
| FeS2@RBCs | 30.2% | 1064 nm | 4T1 tumor | [68] |
| FP NRs | 56.6% | 1064 nm | U14 tumor | [69] |
| FPS-PVP | 43.3% | 1064 nm | HeLa tumor | [70] |
| Cu5FeS4 | 45.9% | 1064 nm | 4T1 tumor | [73] |
| CuFe2S3-PEG | ~55.86% | 1064 nm | HepG2 tumor | [74] |
| Fe-POM | 51.4% | 1060 nm | HeLa tumor | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Forgham, H.; Shen, A.; Qiao, R.; Guo, B. Recent Advances in Single Fe-Based Nanoagents for Photothermal–Chemodynamic Cancer Therapy. Biosensors 2022, 12, 86. https://doi.org/10.3390/bios12020086
Zhang L, Forgham H, Shen A, Qiao R, Guo B. Recent Advances in Single Fe-Based Nanoagents for Photothermal–Chemodynamic Cancer Therapy. Biosensors. 2022; 12(2):86. https://doi.org/10.3390/bios12020086
Chicago/Turabian StyleZhang, Li, Helen Forgham, Ao Shen, Ruirui Qiao, and Bing Guo. 2022. "Recent Advances in Single Fe-Based Nanoagents for Photothermal–Chemodynamic Cancer Therapy" Biosensors 12, no. 2: 86. https://doi.org/10.3390/bios12020086
APA StyleZhang, L., Forgham, H., Shen, A., Qiao, R., & Guo, B. (2022). Recent Advances in Single Fe-Based Nanoagents for Photothermal–Chemodynamic Cancer Therapy. Biosensors, 12(2), 86. https://doi.org/10.3390/bios12020086







