Lab-on-a-Chip Platforms for Airborne Particulate Matter Applications: A Review of Current Perspectives
Abstract
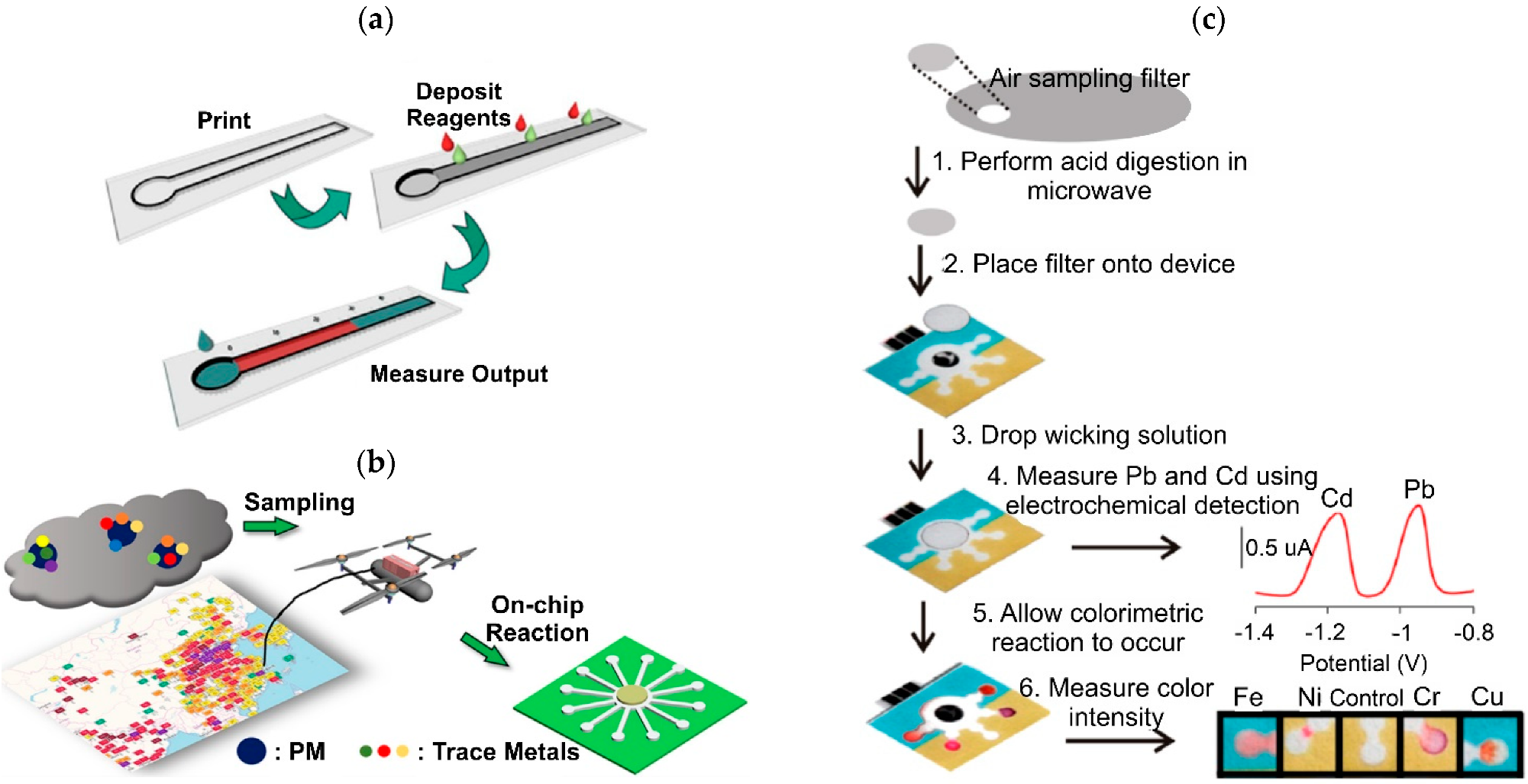
:1. Introduction
2. Classification Methods
2.1. Passive Classification Systems
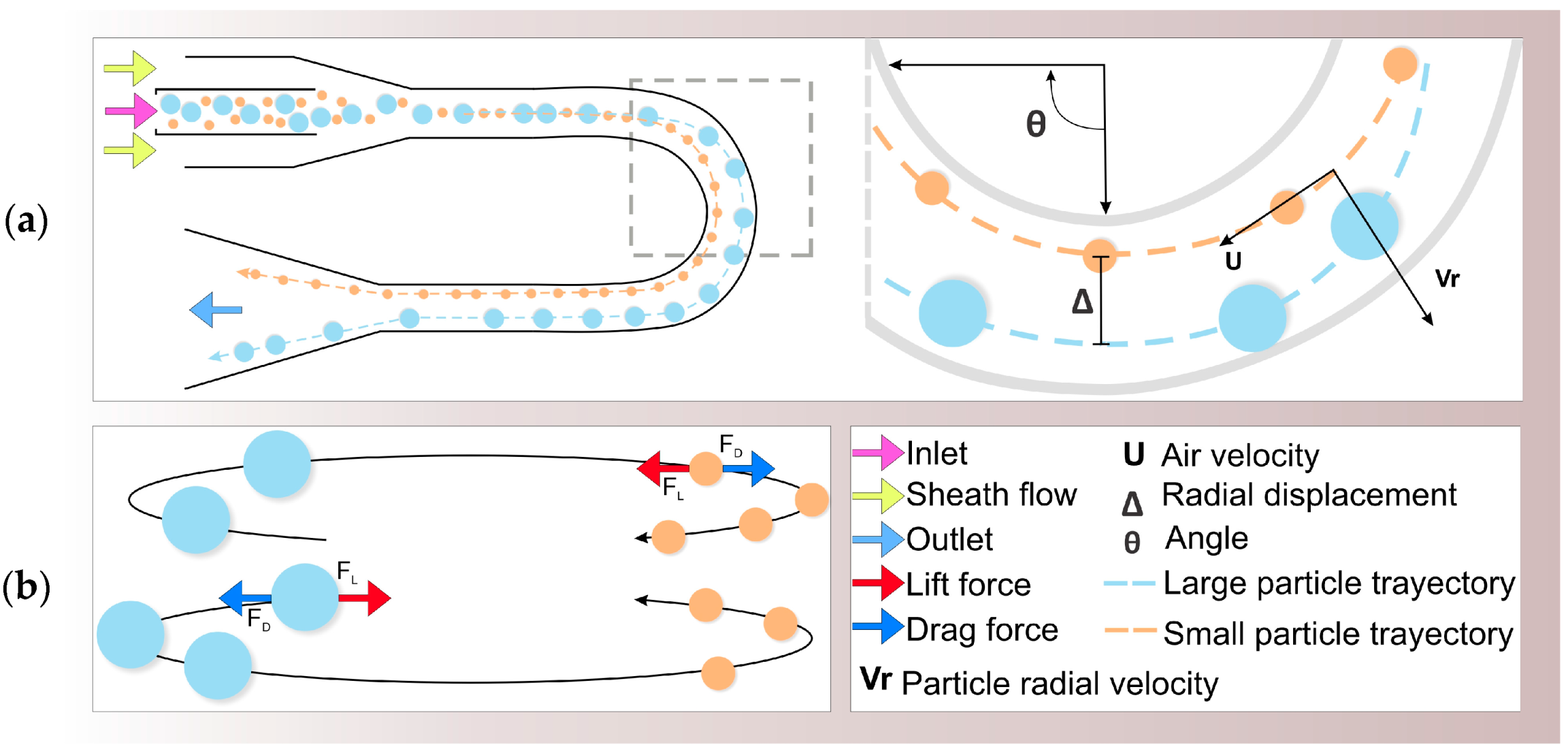
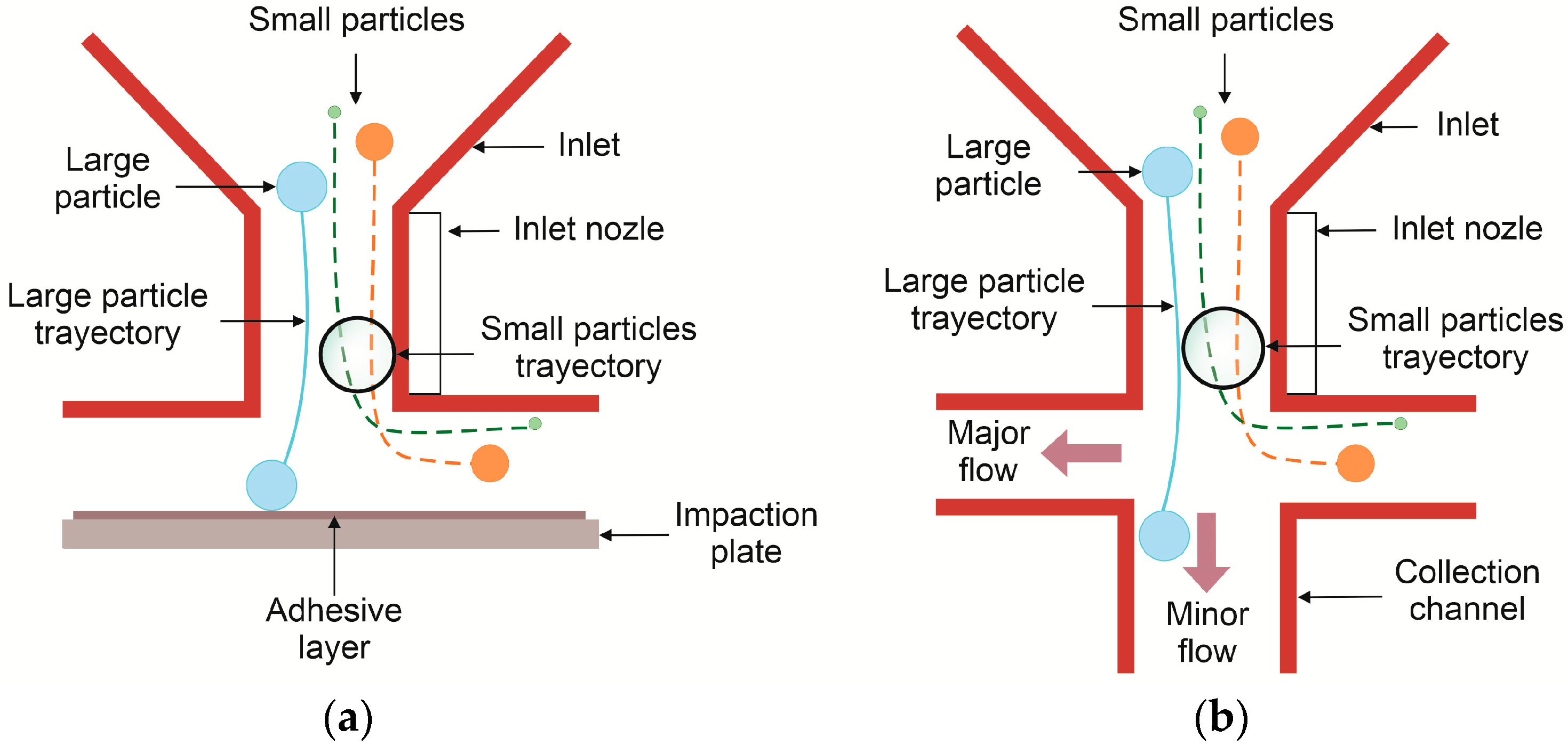
Inertial Impactors
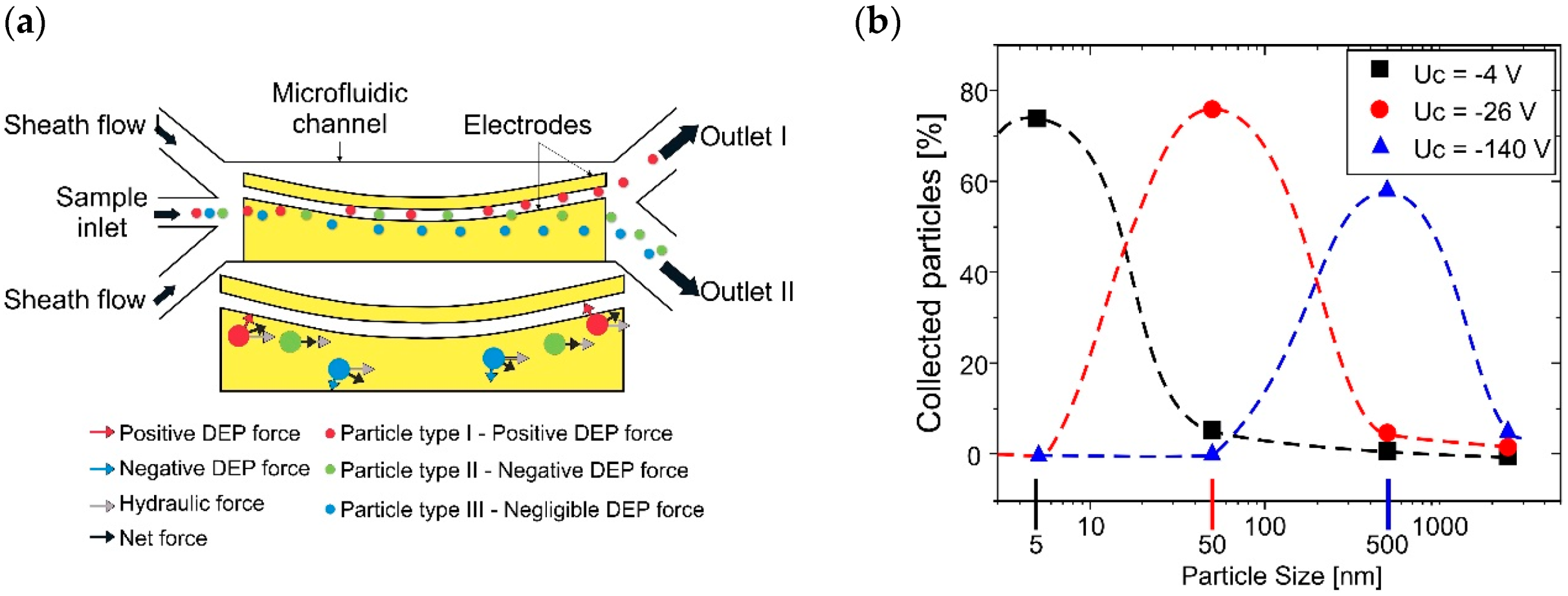
2.2. Active Classification Systems
3. Detection Methods
3.1. Electrical-Based Sensors
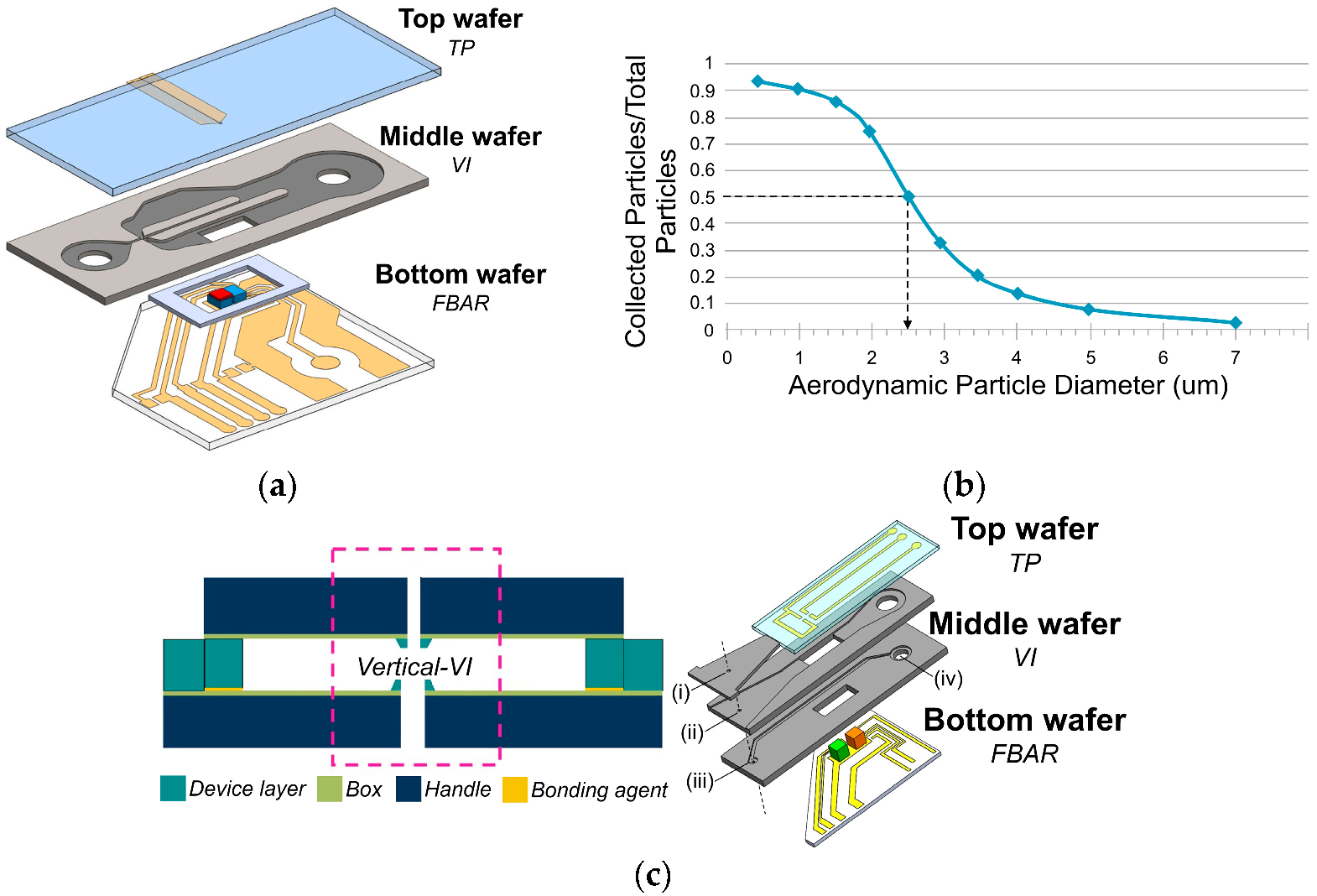
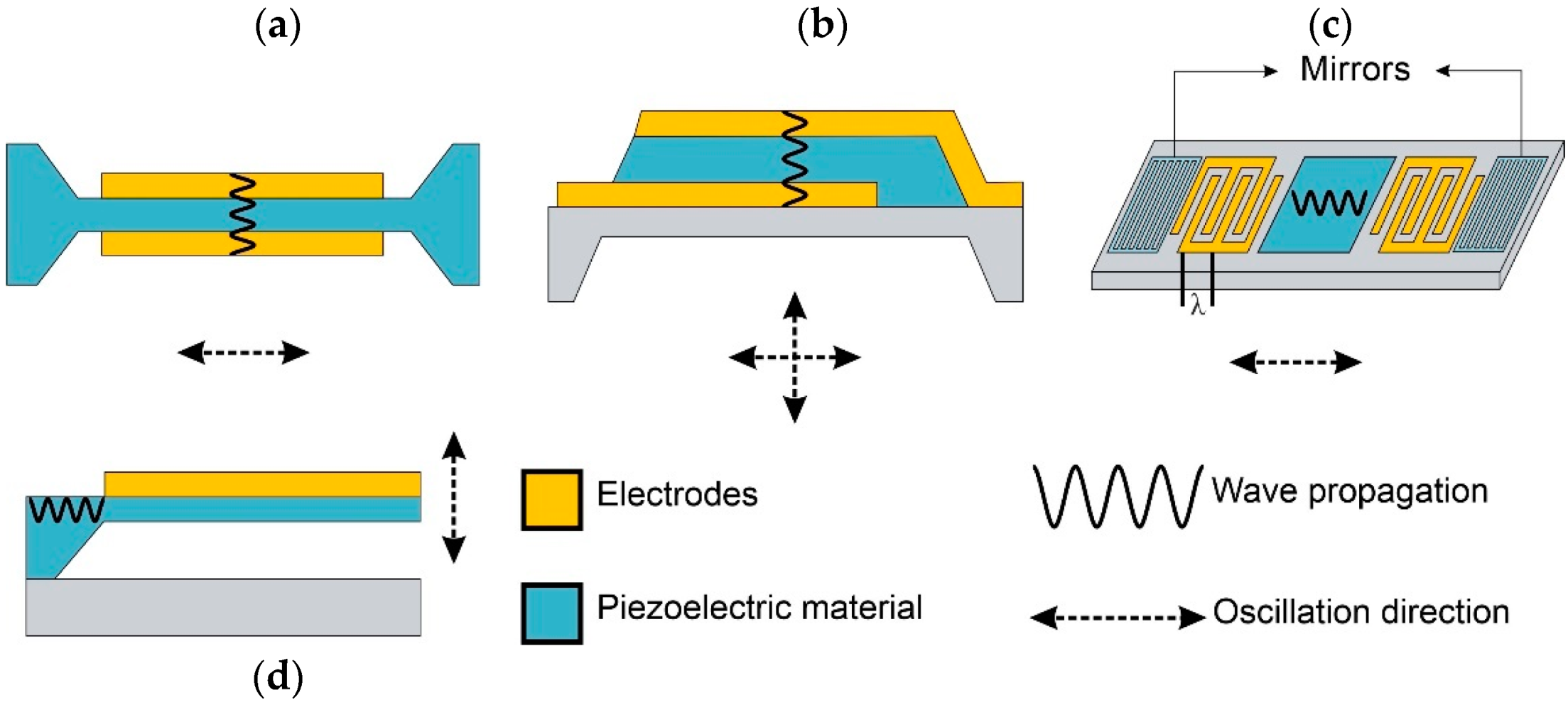
3.1.1. MEMS-Based Sensors
3.1.2. NEMS-Based Resonance Sensors
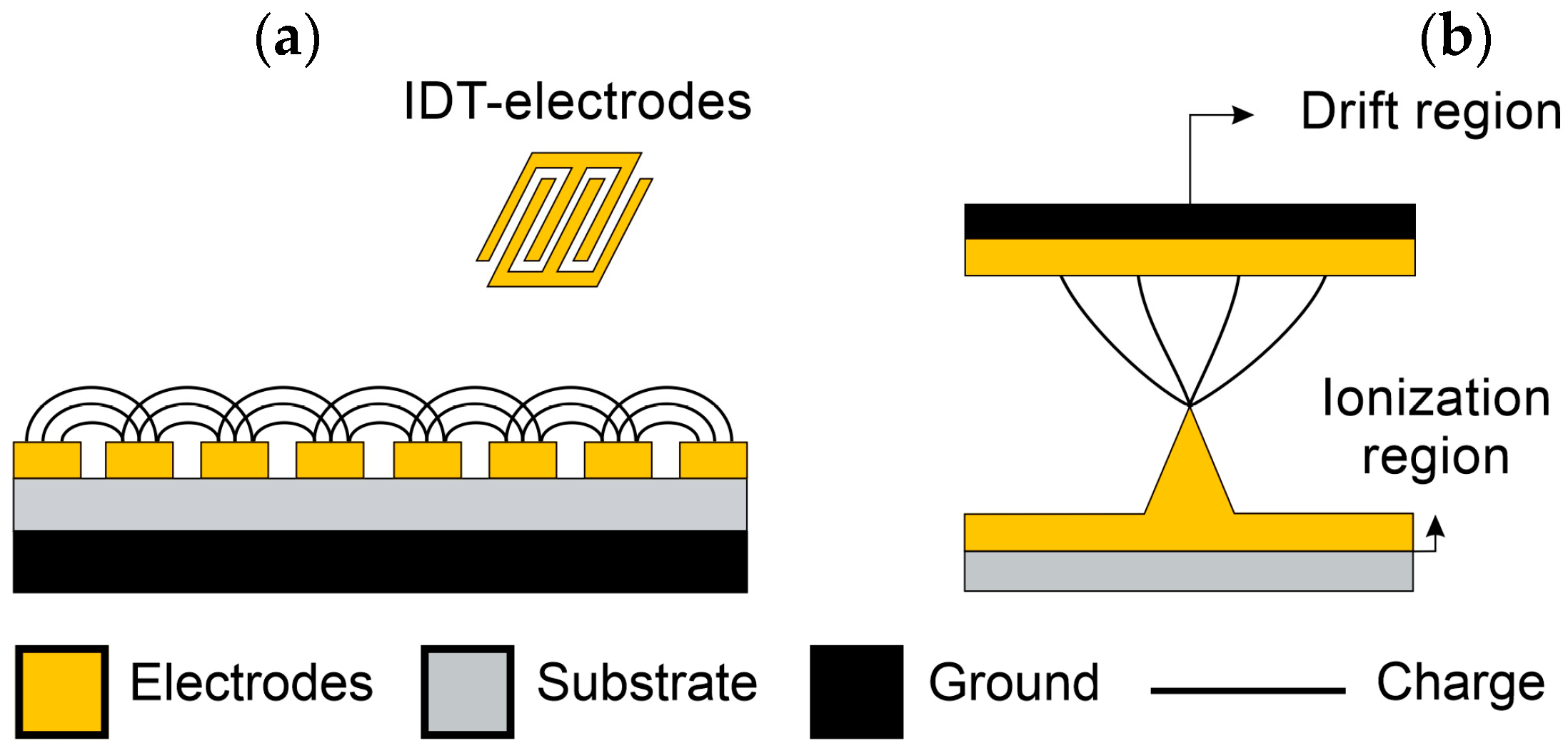
3.1.3. Capacitive-Based Sensors
3.1.4. Corona Discharge Sensors
3.2. Optical Sensors
4. Analytical Methods
4.1. Continuous Flow Microfluidics
4.1.1. Electrochemical-Based Detection
4.1.2. Optical-Based Detection
4.1.3. Spectroscopy-Based Detection
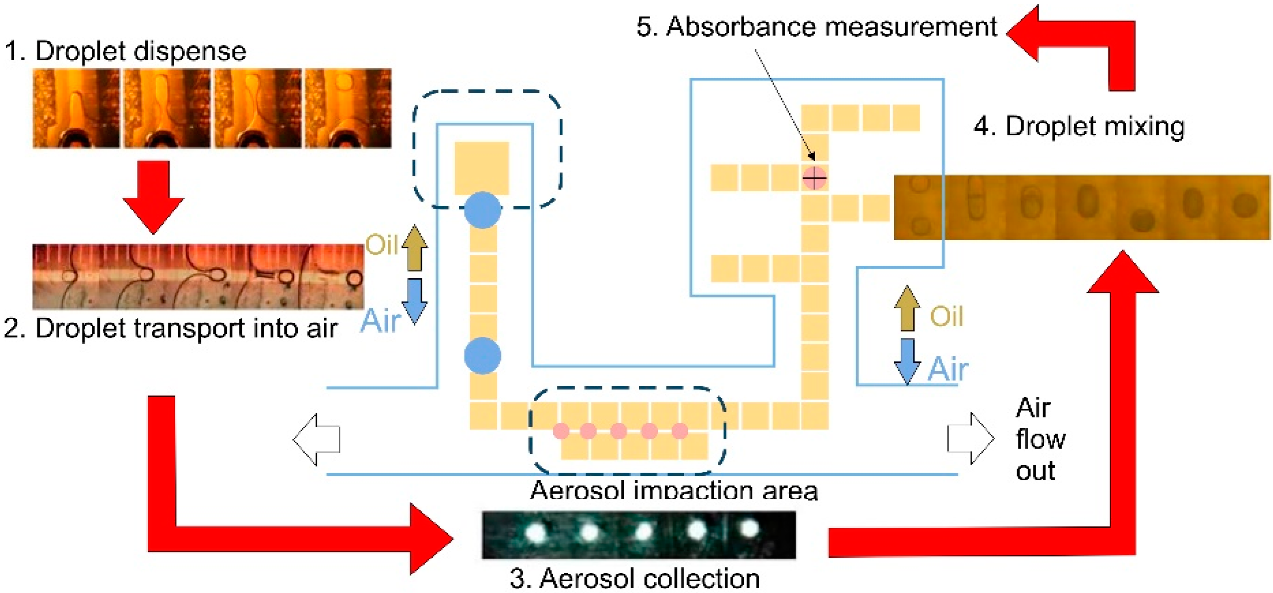
4.2. Droplet Microfluidics
Colorimetric-Based Detection
4.3. Paper Microfluidics
4.3.1. Colorimetric-Based Detection
4.3.2. Electrochemical-Based Detection
5. Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Type | Fabrication Method | Width W (μm) | Stk50 | Flow Rate Q (mL/min) | Cut-Off Point d50 (μm) | Experimental d50 at 50% (μm) | Sensing Device | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| µVI | 1 | a: DRIE on Si Wafer | – | 0.59 | 6 | 2.5 | – | FBAR | [84,88,89,97] |
| 1 | a: DRIE on Si Wafer | 190 | 0.24 | 9.5 | 2.5 | d50 at 45% | FBAR | [98] | |
| 1 | a: DRIE on Si Wafer | 200 | 0.59 | 6.5 | 2.5 | – | Optical | [94] | |
| 1 | a: Patterned DFP | 1000 | 0.479–0.59 | 300 | 2.5 | – | Optical | [92] | |
| 1 | a: Patterned DFP | 1000 | 0.479–0.59 | 300 | 2.5 | – | Capacitive | [93] | |
| 1 | a: Patterned DFP | 210 | 0.229 | 500 | 0.3 | 330 | Corona discharge | [95] | |
| 1 | a: Patterned SU8 | 200 | 0.229 | 300 | Type I—0.6 Type II—1.0 | Type I—550 Type II—1.1 | Corona discharge | [86] | |
| 1 | a: Patterned SU8 | 200 | 0.229 | 300 | 1.0 | 0.95 | – | [87] | |
| 1 | a: DRIE on Si Wafer | – | 0.59 | 5 | 2.5 | – | – | [99] | |
| 1 | a: Molded PDMS | 290 | 0.372 | 93.5 | 2.5 | 1.93 | – | [101] | |
| 1 | a: ICPE on Si Wafer | 290 | 0.55 | 12.5 | 2.5 | – | SAW | [96] | |
| 1 | c | 1000 | 0.229 | 440 | 1 | 1.05 | SAW | [109] | |
| 1 | c | 1200 | 0.59 | 480 | 2.5 | – | QCM | [108] | |
| 1 | c | 1000 | 0.58 | 270 | 2.5 | 2.65 | QCM | [106] | |
| 1 | b | 500 | 0.479–0.59 | 90 | 2.0 | d50 at 34% | – | [104] | |
| 1 | b | 1000 | 0.479–0.59 | 750 | 2.0 | – | Optical | [105] | |
| 2 | b | 1800 | 0.23 | 1000–900 | 3.15–2.5 | 3.2–2.28 | QCM | [102] | |
| 2 | d | 500–200 | 0.59 | 6.9 | 10–2.5 | – | – | [110] | |
| 3 | a: Patterned SU8 | 3700–1850–350 | 0.229 | 600–270–3 | 0.2–2.5–6 | 0.135–1.9–4.8 | – | [85] | |
| µCI | 1 | a: Molded PDMS | – | – | 500 | 2.5 | – | Optical | [91] |
| 2 | a: Molded PDMS | 500–140 | 0.4–0.8 | 12.5 | 5–1 | 4.83–0.98 | – | [100] | |
| 3 | a: Molded PDMS | 1287–472–263 | – | 120 | 2.02–0.88–0.54 | 2.24–0.91–0.49 | Optical | [90] | |
| 3 | b | 374–197–110 | 0.72 | 500 | 1.06–0.55–0.26 | 1.19–0.51–0.27 | – | [103] | |
| 4 | a: Molded PDMS | 890–660–460–300 | 0.59 | 300 | 1.7–1.2–0.8–0.5 | 1.63–1.11–0.82–0.48 | Corona discharge | [111] | |
| 5 | a: Molded PDMS | 570–496–403–314–184 | – | 550 | 1.2–1.0–0.8–0.6–0.3 | 1.17–0.94–0.71–0.54–0.23 | Corona discharge | [107] | |
| Type of Sensor | Particle Deposition | Sampling Method | Particle Size (μm) | Resonant Frequency (MHz) | Quality Factor (Qf) | Resolution (ng) | Sensitivity | LOD (μg m−3) | Integration Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| QCM | a: hydrogel film | μVI | PM2.5 | 11.98 | 830 | – | 8320 | 1 | [102] | |
| a: heated grease film | CI | PM2.5 | 10 | 570 | 0.095 hz (ng cm−2)−1 | 15 | 1 | [126] | ||
| a: thin photoresist film | μVI | PM2.5 | 4.98 | 33,000 | 3.47 (calculated) | 11 hz min−1 | 142 | 5 | [106] | |
| a | μVI | PM1 | 4.98 | 5.1 hz min−1 | 52.33 | 10 | [108] | |||
| SAW | a: glycerol film | μVI | PM1 | 147.24 | 190 | 7.46 hz min−1 per μg m−3 | [109] | |||
| e | – | PM1 | 894 | 7.5 khz per μg m−3 | [129] | |||||
| e | – | PM2.5 | 262 | 0.21 | 262 Hz ng−1 | [130] | ||||
| b | μVI | PM2.5 | 311 | 4500 | 0.17 | 93.96 hz min−1 per μg m−3 | 2 | 15 | [96] | |
| FBAR | b | – | PM2.5 | 1600 | 0.001 | – | 18 | 1 | [128] | |
| b | μVI | PM2.5 | 600 | – | 2 | 10 | [84,88,89] | |||
| b | μVI | PM2.5 | 600 | 5 | 1 | [97] | ||||
| b | μVI | PM2.5 | 600 | 7.05 hz min−1 per μg m−3 | 1 | 7 | [98] | |||
| TPR | c | Vacuum chamber | PM0.1 | 1: 0.2–1.7 | 20,000–4400 | 0.115 | 50–300 hz ng−1 | 121 max | [136] | |
| c | Vacuum chamber | PM0.1 | 1: 60–20 | 11,000–4000 | 45 × 10−4 to 25 × 10−4 | 1.2–1.6 kHz pg−1 | 25 | [134] | ||
| c | CI | PM0.1 | 2: 5.3 | – | 42 hz pg−1 | 60 | [133] | |||
| PCR | d | Air chamber | PM0.1 | 3: 16 × 10−4 | 155–300 | 12.1 | 8.31 × 10−3 Hz ng−1 | [139] | ||
| d | Air chamber | PM0.1 | 3: 2.6 | 480 | 8.9 | 11.15 × 10−3 Hz ng−1 | [138] | |||
| d | Air chamber | PM0.1 | 3: 44 | 1230 | 0.0048 | 8.33 Hz ng−1 | [137,141] | |||
| d | Air chamber | PM0.1 | 3: 44 | 1206 | 0.001 | 10 Hz ng−1 | [140] | |||
| d | Air chamber | PM0.1 | 4: 144.2 | 2100 | 32.75 Hz ng−1 | [142] | ||||
| d | Micro fan | PM0.1 | 5: 221.5 | 1950 in air | 5 × 10−6 | 36.51 Hz ng−1 | 15 | [143] | ||
| d | Micro fan | PM0.1 | 9.4 × 10−3 | 25 | 5 | [144] | ||||
| d | Micro fan | PM0.1 | 6: 200 | 4700 | 5 | 6 seg | [145] | |||
| d | Air chamber | PM0.1 | 7: 0.45 | 1200–1700 | 1.5 × 10−6 | 7220 kHz ng−1 | [146] | |||
| Capacitive | e | – | PM1 | 65 zF | [151] | |||||
| e | Air pump | PM2.5–10 | 1.2 aF | 10 ms | [150] | |||||
| b | μVI | PM2.5–10 | 4 | –56.8 pF μg−1 | [93] | |||||
| b | Air pump | PM2.5–10 | 0.48 zF | [152] | ||||||
| Corona discharge | f | μVI | PM0.1 | 8 × 10–7 pA (# cm−3)−1 | [86] | |||||
| f | μVI | PM0.1 | Comparable to * | [156] | ||||||
| f | μVI | PM0.1 | 320 to 106 # cm−3 | [95] | ||||||
| F | μCI | PM0.1 | Comparable to ** | [107] | ||||||
| Optical | f | NP condenser | PM0.1 | 0.21–105 # cm−3 | 0.3 s | [158] | ||||
| f | Air pump | PM2.5 | 32.8 | Real-time | [159] | |||||
| f | Air pump | PM2.5 | 10 | Real-time | [160] | |||||
| f | μVI | PM2.5 | 2.55 | Real-time | [94] |
| Detection Principle | Characteristics | Sampling Method | Reaction Principle | Target Analyte | LOD | Integration Time (s) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Continuous flow-based | Electrochemical | self-assembled monolayer (SAM)/multilevel air pillars | Hydrophilic-hydrophobic barrier/natural deposition | Nessler’s reaction | NH3 | – | 15 | [180] |
| modified Ca paste electrodes (CoPC-CPE) | PILS sampler | Oxidation of Dithiothreitol (DTT) | Urban oxidative activity | 7 ng to 214 ng | 180 | [182] | ||
| Capillary electrophoresis (CE) | – | Background electrolytes (BGE) dilution | Sox/NO3/Cl/C2O4 | 160 nM/260 nM/190 nM/180 nM | 25 | [178] | ||
| Glassy Ca electrodes | Modified 2,4-dinitrophenylhydrazine (DNPH)/Silica-gel cartridges | Aldehydes derivatization to form DNPH hydrazones | formaldehyde/acetaldehyde/2-propenal | 9.5 µM/7.2 µM/9.2 µM | 0.1 | [179] | ||
| Cu plate electrode | bioaerosol-in-hydrosol electrostatic sampler | Selective antibody-modified silicon nanowire transistors (SiNW-FET) | H3N2 airborne influenza virus | 104 viruses L−1 | 60–120 | [181] | ||
| Metallic coated microchannels | Microsampler | Commercial chemoresistive gas sensor | VOCs | – | 150 | [185,186] | ||
| Optical | EW: λ = 640 nm Spectrometer and cellphone camera detection | Button air sampler | Latex immunoagglutination assay | H1N1/2009 virus | 1 and 10 pg mL−1 | 300 | [188] | |
| CCD fluorescence microscope detection | capture and enrichment micro-chamber | Fluorimetric immune adsorption reaction Ag85B antigens | M. tuberculosis | 102 cells mL−1 | ~4 h | [190] | ||
| EW: λ = 365 nm | capture and enrichment micro-chamber | Loop-mediated isothermal amplification (LAMP) | S. aureus/E. coli/P. aeruginosa/C. koseri/K. pneumoniae | 24 cells | ~4 h | [191] | ||
| EW: λ = 470 nm Photodiode detection | capture and enrichment micro-chamber | LAMP | P. aeruginosa | – | 70 min | [193] | ||
| Bioluminescence photodiode detection | Bioaerosol sampler | Adenosine triphosphate (ATP)/D-luciferin reaction | B. subtilis/E. coli JM110 | concentration vs. intensity Linear growth | 120 | [198] | ||
| EW: λ = 470 nm CCD fluorescence microscope detection | μCI- stained agar plate | Direct bioaerosol staining with SYBR green I dye | S. epidermidis | concentration vs. intensity Linear growth | 10 | [90] | ||
| EW: λ = 510–550 nm CCD array camera | Biosampler | Direct bioaerosol staining with SYTO82 fluorescent dye medium | E. coli/B. subtilis/S. epidermidis | concentration vs. intensity Linear growth | 25–250 | [197] | ||
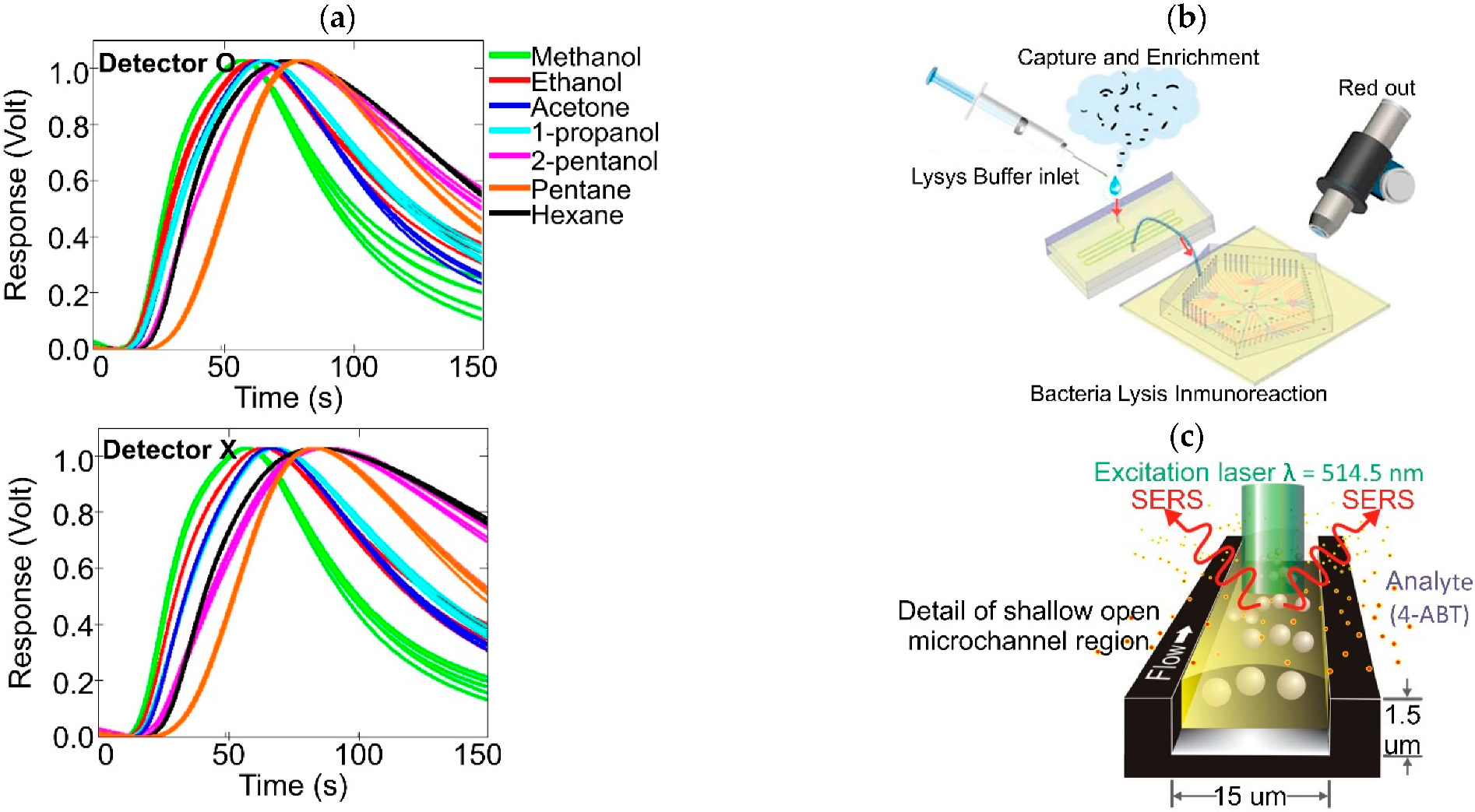
| Continuous flow-based | Spectroscopic | EW: λ = 514.5 nm | Sampling delivery gas system into an open microchannel | silver nanoparticles colloidal suspension to form SERS hot spots (AgNPs-SERS) | gaseous 4-aminobenzenethiol (4-ABT) | – | – | [199] |
| EW: λ = 658 nm | Sampling delivery gas system into an open microchannel | AgNPs-SERS | 2,4-dinitrotoluene (2,4-DNT) | 1 ppb | 120 | [200] | ||
| EW: λ = 648 nm | Sampling delivery gas system into a closed microchannel | AgNPs-SERS | 4-ABT vapor | <2.5 pg | 2.5 | [201] | ||
| Droplet-based | Colorimetric | CMOS inverted microscope | Air-into-liquid sampler | Nessler’s reaction | NH3 | – | – | [186] |
| Fluorimetric microscope | Aerodynamic lens | fluorescent profile of E. coli produced with propidium iodide (PI) | E. coli | – | 60 | [194] | ||
| High-speed camera | Filter-based and impinger sampling | Microdroplet freezing event from −5 °C to 35 °C | ice-nucleating particles (INPs) | – | 60–90 min | [195,196] | ||
| AW: λ = 540–365–608 nm Absorption spectrometer | μCI | MTB-barium complex | NO3/NH4/SO4 | NA/0.256/11 ppm | 60 min | [166] | ||
| Paper-based | Colorimetric | Scanned Images processed by image software | Filter-based personal sampler | Bathophenanthroline (Bphen) /Bathocuproine (BC)/ Dimethylglyoxime (DGM) | Fe/Cu/Ni | 1 to 1.5 ug Linear range: 1 to 17 ug | – | [167] |
| Scanned Images processed by image software | Filter-based sampling | 1,5-diphenylcarbazide (1,5-DPC) | Cr | 0.12 ug Linear range: 0.2–3.7 ug | – | [169] | ||
| Scanned Images processed by image software | Filter-based sampling | Bphen/BC/DGM/1,5-DPC | Fe/Cu/Ni/Cu | Linear range: 1.1–10/0.15–6/1–10/1.5–8 ug | – | [172] | ||
| Distance-based uPAD | Filter-based sampling | Bphen/dithiooxamide/DGM | Fe/Cu/Ni | <0.1 ug | – | [173] | ||
| Cellphone image software application | unmanned aerial vehicle (UAV) | chrysoidine-G/dithiooxamide/ Bphen | Co/Cu/Fe | 8.2/45.8/186.0 ng | – | [174] | ||
| Cellphone image software application | UAV | Bphen/DGM/4-(2-pyridylazo) resorcinol (PAR) | Fe/Ni/Mn | Linear range: 170–1440/81–684/9.2–85 ng | – | [175] | ||
| Paper-based | Colorimetric | Cellphone image software application Field reaction kit | UAV | Chrysoidine-G/dithiooxamide/Bphen/PAR/ 1,5-DPC/DGM | Co/Cu/Fe/Mn/Cr/Ni | Linear range 8–81/45–458/186–1860/10–100/152–3810/80–800 ng | – | [176] |
| Cellphone image software application | UAV | Graphene oxide nanosheetsBphen/dithiooxamide/DGM | Fe/Cu/Ni | 16/5/10 ng | – | [177] | ||
| Image software and distance-based uPAD | Filter-based sampling | DTT-oxidation | aerosol oxidative activity | Linear range: 0–75 ng and 5–25 ng | 20 min | [168] | ||
| Scanned Images processed by image software | Filter-based personal sampler | DTT-oxidation | aerosol oxidative activity | Linear range: 0–120 ng | 30 min | [170] | ||
| Electrochemical | Image software and modified Ca electrodes (Bi/ferricyanide) | Filter-based sampling | DGM/Bphen/BC/1,5-DPC | uPAD-Ni/Fe/Cu/Cr ePAD-Cd/Pb | uPAD-0.12 ug ePAD-0.25 ng | – | [183] | |
| modified Ca electrodes (Nafion/BiCSPE) | Ultrasonic personal sampler | 1,10- phenanthroline/DMG | Cu/Fe/Ni/Cd/Pb | 3.23/1.02/26.4/268.5/122.5 ng | – | [187] | ||
References
- Thompson, J.E. Airborne Particulate Matter Exposure and Health Effects. J. Occup. Environ. Med. 2018, 60, 392–423. [Google Scholar] [CrossRef]
- Monks, P.S.; Granier, C.; Fuzzi, S.; Stohl, A.; Williams, M.L.; Akimoto, H.; Amann, M.; Baklanov, A.; Baltensperger, U.; Bey, I.; et al. Atmospheric Composition Change-Global and Regional Air Quality. Atmos. Environ. 2009, 43, 5268–5350. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, D.L. Historical Highlights of Air Pollution Toxicology. Toxicol. Sci. 2018, 164, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Chen, L.C. Health Effects of Concentrated Ambient Air Particulate Matter (CAPs) and Its Components. Crit. Rev. Toxicol. 2009, 39, 865–913. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.R. Air Pollution and Mortality: A History. Atmos. Environ. 2009, 43, 142–152. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Mills, I.C.; Walton, H.A.; Anderson, H.R. Fine Particle Components and Health—A Systematic Review and Meta-Analysis of Epidemiological Time Series Studies of Daily Mortality and Hospital Admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Schraufnagel, D.E. The Health Effects of Ultrafine Particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, Source and Chemical Composition as Determinants of Toxicity Attributable to Ambient Particulate Matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Solimini, A.; Filipponi, F.; Fegatelli, D.A.; Caputo, B.; De Marco, C.M.; Spagnoli, A.; Vestri, A.R. A global association between COVID-19 cases and airborne particulate matter at regional level. Sci. Rep. 2021, 11, 6256. [Google Scholar] [CrossRef]
- Stanek, L.W.; Sacks, J.D.; Dutton, S.J.; Dubois, J.J.B. Attributing Health Effects to Apportioned Components and Sources of Particulate Matter: An Evaluation of Collective Results. Atmos. Environ. 2011, 45, 5655–5663. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air Pollution and Public Health: Emerging Hazards and Improved Understanding of Risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Richardson, B.; Takle, E.; Chai, L.; Schmitt, D.; Xin, H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019, 9, 11755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhangrazi, Z.S.; Sancini, G.; Hunter, A.C.; Moghimi, S.M. Airborne Particulate Matter and SARS-CoV-2 Partnership: Virus Hitchhiking, Stabilization and Immune Cell Targeting—A Hypothesis. Front. Immunol. 2020, 11, 579352. [Google Scholar] [CrossRef]
- Nor, N.S.M.; Yip, C.W.; Ibrahim, N.; Jaafar, M.H.; Rashid, Z.Z.; Mustafa, N.; Hamid, H.H.A.; Chandru, K.; Latif, M.T.; Saw, P.E.; et al. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2021, 11, 2508. [Google Scholar] [CrossRef]
- Reyna, M.A.; Schwander, S.; Avitia, R.L.; Bravo-Zanoguera, M.E.; Reyna, M.E.; Nava, M.L.; Siqueiros, M.; Osornio-Vargas, A.R. Particulate Matter Air Pollution Effects on Pulmonary Tuberculosis Activation in a Semi-Desert City on the US-Mexican Border. Atmósfera 2021, 35, 545–556. [Google Scholar] [CrossRef]
- Sullivan, R.C.; Gorkowski, K.; Jahn, L. Characterization of Individual Aerosol Particles. In Physical Chemistry of Gas-Liquid Interfaces; Faust, J.A., House, J.E., Eds.; Elsevier: Alpharetta, GA, USA, 2018; pp. 353–402. ISBN 9780128136416. [Google Scholar]
- Li, W.; Shao, L.; Zhang, D.; Ro, C.U.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A Review of Single Aerosol Particle Studies in the Atmosphere of East Asia: Morphology, Mixing State, Source, and Heterogeneous Reactions. J. Clean. Prod. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- Elmes, M.; Gasparon, M. Sampling and Single Particle Analysis for the Chemical Characterisation of Fine Atmospheric Particulates: A Review. J. Environ. Manag. 2017, 202, 137–150. [Google Scholar] [CrossRef]
- Galvão, E.S.; Santos, J.M.; Lima, A.T.; Reis, N.C.; Orlando, M.T.D.A.; Stuetz, R.M. Trends in Analytical Techniques Applied to Particulate Matter Characterization: A Critical Review of Fundaments and Applications. Chemosphere 2018, 199, 546–568. [Google Scholar] [CrossRef]
- Huffman, J.A.; Perring, A.E.; Savage, N.J.; Clot, B.; Crouzy, B.; Tummon, F.; Shoshanim, O.; Damit, B.; Schneider, J.; Sivaprakasam, V.; et al. Real-Time Sensing of Bioaerosols: Review and Current Perspectives. Aerosol Sci. Technol. 2020, 54, 465–495. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Cordero, J.L.; Ricco, A.J. Lab-on-a-Chip (General Philosophy). In Encyclopedia of Microfluidics and Nanofluidics, 2nd ed.; Li, D., Ed.; Springer: New York, NY, USA, 2015; pp. 1501–1510. [Google Scholar] [CrossRef]
- Dekker, S.; Isgor, P.K.; Feijten, T.; Segerink, L.I.; Odijk, M. From Chip-in-a-Lab to Lab-on-a-Chip: A Portable Coulter Counter Using a Modular Platform. Microsyst. Nanoeng. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-J.; Hwang, E.-S.; Kim, Y.-H.; Yoon, S.-I.; Park, S. MEMS-Based Biosensor. In Encyclopedia of Microfluidics and Nanofluidics, 1st ed.; Li, D., Ed.; Springer: New York, NY, USA, 2014; pp. 1747–1758. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-Chip or Chip-in-a-Lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A Review of Digital Microfluidics as Portable Platforms for Lab-on a-Chip Applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, A.R.; Narayan, S.; Dutcher, C.S. A Review of Microfluidic Concepts and Applications for Atmospheric Aerosol Science. Aerosol Sci. Technol. 2018, 52, 310–329. [Google Scholar] [CrossRef]
- Jiang, M.; Qian, S.; Liu, Z. Inertial Migration of Aerosol Particles in Confined Microfluidic Channels. arXiv 2018, arXiv:1812.02323, 1–21. [Google Scholar]
- Carminati, M.; Ferrari, G.; Sampietro, M. Emerging Miniaturized Technologies for Airborne Particulate Matter Pervasive Monitoring. Measurement 2017, 101, 250–256. [Google Scholar] [CrossRef]
- Poenar, D.P. Microfluidic and micromachined/MEMS devices for separation, discrimination and detection of airborne particles for pollution monitoring. Micromachines 2019, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.P.; Zugates, C.T.; Treado, P.J.; Casuccio, G.S.; Exline, D.L.; Schlaegle, S.F. Combining Raman Chemical Imaging and Scanning Electron Microscopy to Characterize Ambient Fine Particulate Matter. Aerosol Sci. Technol. 2001, 34, 108–117. [Google Scholar] [CrossRef]
- Godoi, R.H.M.; Potgeieter-Vermaak, S.; De Hong, J.; Kaegi, R.; Van Grieken, R. Substrate Selection for Optimum Qualitative and Quantitative Single Atmospheric Particles Analysis Using Nano-Manipulation, Sequential Thin-Window Electron Probe X-Ray Microanalysis and Micro-Raman Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 375–388. [Google Scholar] [CrossRef]
- Amaral, S.S.; De Carvalho, J.A.; Costa, M.A.M.; Pinheiro, C. An Overview of Particulate Matter Measurement Instruments. Atmosphere 2015, 6, 1327–1345. [Google Scholar] [CrossRef] [Green Version]
- Worobiec, A.; Potgieter-Vermaak, S.; Brooker, A.; Darchuk, L.; Stefaniak, E.; Van Grieken, R. Interfaced SEM/EDX and Micro-Raman Spectrometry for Characterisation of Heterogeneous Environmental Particles—Fundamental and Practical Challenges. Microchem. J. 2010, 94, 65–72. [Google Scholar] [CrossRef]
- Goienaga, N.; Sarmiento, A.; Olivares, M.; Carrero, J.A.; Fernández, L.A.; Madariaga, J.M. Emerging Application of a Structural and Chemical Analyzer for the Complete Characterization of Metal-Rich Particulate Matter. Anal. Chem. 2013, 85, 7173–7181. [Google Scholar] [CrossRef]
- Vineyard, M.F.; Labrake, S.M.; Ali, S.F.; Nadareski, B.J.; Safiq, A.D.; Smith, J.W.; Yoskowitz, J.T. Characterization of Atmospheric Aerosols in the Adirondack Mountains Using PIXE, SEM/EDX, and Micro-Raman Spectroscopies. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 350, 77–80. [Google Scholar] [CrossRef]
- Cardell, C.; Guerra, I. An Overview of Emerging Hyphenated SEM-EDX and Raman Spectroscopy Systems: Applications in Life, Environmental and Materials Sciences. TrAC Trends Anal. Chem. 2016, 77, 156–166. [Google Scholar] [CrossRef]
- Ahmed, M.; Guo, X.; Zhao, X.M. Spectroscopic and Microscopic Characterization of Atmospheric Particulate Matter. Instrum. Sci. Technol. 2017, 45, 659–682. [Google Scholar] [CrossRef]
- Saffaripour, M.; Tay, L.L.; Thomson, K.A.; Smallwood, G.J.; Brem, B.T.; Durdina, L.; Johnson, M. Raman Spectroscopy and TEM Characterization of Solid Particulate Matter Emitted from Soot Generators and Aircraft Turbine Engines. Aerosol Sci. Technol. 2017, 51, 518–531. [Google Scholar] [CrossRef]
- Chen, Q.X.; Huang, C.L.; Xiao, T.; Yuan, Y.; Mao, Q.J.; Tan, H.P. Characterization of Atmospheric Aerosols and Source Apportionment Analyses in Urban Harbin, Northeast China. Infrared Phys. Technol. 2019, 103, 103109. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Bilo, F.; Borgese, L.; Wambui, A.; Assi, A.; Zacco, A.; Federici, S.; Eichert, D.M.; Tsuji, K.; Lucchini, R.G.; Placidi, D.; et al. Comparison of Multiple X-ray Fluorescence Techniques for Elemental Analysis of Particulate Matter Collected on Air Filters. J. Aerosol Sci. 2018, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Mascolo, D.; Coclite, A.; Gentile, F.; Francardi, M. Quantitative Micro-Raman Analysis of Micro-Particles in Drug Delivery. Nanoscale Adv. 2019, 1, 1541–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, A.; Yazdi, S.; Ardekani, A.M. Hydrodynamic Mechanisms of Cell and Particle Trapping in Microfluidics. Biomicrofluidics 2013, 7, 021501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields IV, W.; Reyes, C.D.; Lopez, G.P. Microfluidic Cell Sorting: A Review of the Advances in the Separation of Cells from Debulking to Rare Cell Isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Yalikun, Y.; Tanaka, Y. Recent Advances in Microfluidic Cell Sorting Systems. Sens. Actuators B Chem. 2019, 282, 268–281. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Onck, P.; Den Toonder, J. A Concise Review of Microfluidic Particle Manipulation Methods. Microfluid. Nanofluidics 2020, 24, 24. [Google Scholar] [CrossRef] [Green Version]
- Sajeesh, P.; Sen, A.K. Particle Separation and Sorting in Microfluidic Devices: A Review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in Microfluidics for Nanoparticle Separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Yousuff, C.M.; Ho, E.T.W.; Ismail Hussain, K.; Hamid, N.H.B. Microfluidic Platform for Cell Isolation and Manipulation Based on Cell Properties. Micromachines 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Zhao, Q.; Yan, S.; Tang, S.Y.; Alici, G.; Zhang, J.; Li, W. Recent Progress of Particle Migration in Viscoelastic Fluids. Lab Chip 2018, 18, 551–567. [Google Scholar] [CrossRef] [Green Version]
- Choi, S. Hydrophoresis—A Microfluidic Principle for Directed Particle Migration in Flow. Biochip J. 2020, 14, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Paiè, P.; Zandrini, T.; Vázquez, R.M.; Osellame, R.; Bragheri, F. Particle Manipulation by Optical Forces in Microfluidic Devices. Micromachines 2018, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, X.; Chen, P.; Huang, X.; Li, S.; Liu, B.F. Microfluidic Chip Electrophoresis for Biochemical Analysis. J. Sep. Sci. 2020, 43, 258–270. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Wang, J.C.; Tu, Q.; Ren, L.; Wang, Y.; Wang, J. Dynamic trapping and high-throughput patterning of cells using pneumatic microstructures in an integrated microfluidic device. Lab Chip 2012, 12, 1702–1709. [Google Scholar] [CrossRef]
- Leikauf, G.D. Toxic Responses of the Respiratory System. In Casarett & Doull’s: Essentials of Toxicology, 3rd ed.; Klaassen, C.D., Watkins, J.B., Eds.; Mc Graw Hill Education: New York, NY, USA, 2015; pp. 223–236. ISBN 9780071847094. [Google Scholar]
- Zhang, J.; Li, W.; Alici, G. Inertial Microfluidics: Mechanism and Applications. In Advanced Mechatronics and MEMS Devices II, 1st ed.; Zhang, D., Wei, B., Eds.; Springer: New York, NY, USA, 2017; pp. 563–593. [Google Scholar] [CrossRef] [Green Version]
- Stoecklein, D.; Di Carlo, D. Nonlinear Microfluidics. Anal. Chem. 2019, 91, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Inertial Focusing in Microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, A.J. A Minireview on Inertial Microfluidics Fundamentals: Inertial Particle Focusing and Secondary Flow. Biochip J. 2019, 13, 53–63. [Google Scholar] [CrossRef]
- Kim, G.Y.; Han, J.I.; Park, J.K. Inertial Microfluidics-Based Cell Sorting. Biochip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Masaeli, M.; Sollier, E.; Amini, H.; Mao, W.; Camacho, K.; Doshi, N.; Mitragotri, S.; Alexeev, A.; Di Carlo, D. Continuous Inertial Focusing and Separation of Particles by Shape. Phys. Rev. X 2012, 2, 031017. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Lin, Y. Inertial Focusing and Separation of Particles in Similar Curved Channels. Sci. Rep. 2019, 9, 16575. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, N.; Neubauer, P.; Birkholz, M. Spiral Microfluidic Devices for Cell Separation and Sorting in Bioprocesses. Biomicrofluidics 2019, 13, 061501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid Microfluidics Combined with Active and Passive Approaches for Continuous Cell Separation. Electrophoresis 2017, 38, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, G.; Jiang, X.; Sun, J. Inertial Focusing of Spherical Particles in Rectangular Microchannels over a Wide Range of Reynolds Numbers. Lab Chip 2015, 15, 1168–1177. [Google Scholar] [CrossRef]
- Crane, R.I.; Evans, R.L. Inertial Deposition of Particles in a Bent Pipe. J. Aerosol Sci. 1977, 8, 161–170. [Google Scholar] [CrossRef]
- Hinds, W.C. Acceleration and Curvilinear Particle Motion. In Aerosol Technology: Properties, Behavior, and Measurement and Airborne Particles; Wiley: Hoboken, NJ, USA, 1982; pp. 104–126. ISBN 0471087262. [Google Scholar]
- Marple, V.A.; Olson, B.A. Sampling and Measurement Using Inertial, Gravitational, Centrifugal, and Thermal Techniques. In Aerosol Measurement. Principles, Techniques and Applications, 3rd ed.; Kulkarni, P., Baron, P.A., Willeke, K., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 129–151. [Google Scholar]
- Schaap, A.M.; Chu, W.C.; Stoeber, B. Continuous Size-Separation of Airborne Particles in a Microchannel for Aerosol Monitoring. IEEE Sens. J. 2011, 11, 2790–2797. [Google Scholar] [CrossRef]
- Schaap, A.; Chu, W.C.; Stoeber, B. Transport of Airborne Particles in Straight and Curved Microchannels. Phys. Fluids 2012, 24, 083301. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.C.; Kang, J.S.; Lee, J.E.; Kim, S.S.; Jung, J.H. Continuous Aerosol Size Separator Using Inertial Microfluidics and Its Application to Airborne Bacteria and Viruses. Lab Chip 2015, 15, 1889–1897. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, R.; Yang, N.; Kwabena Oppong, P.; Sun, J.; Wang, P. High-Precision Extraction and Concentration Detection of Airborne Disease Microorganisms Based on Microfluidic Chip. Biomicrofluidics 2019, 13, 024110. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous Inertial Focusing, Ordering, and Separation of Particles in Microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, S.J. Measurement of Dean Flow in a Curved Micro-Tube Using Micro Digital Holographic Particle Tracking Velocimetry. Exp. Fluids 2009, 46, 255–264. [Google Scholar] [CrossRef]
- Gou, Y.; Jia, Y.; Wang, P.; Sun, C. Progress of Inertial Microfluidics in Principle and Application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marple, V.A. History of Impactors—The First 110 Years. Aerosol Sci. Technol. 2004, 38, 247–292. [Google Scholar] [CrossRef]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol Sampling: Sampling Mechanisms, Bioefficiency and Field Studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Marple, V.A.; Willeke, K. Impactor Design. Atmos. Environ. 1976, 10, 891–896. [Google Scholar] [CrossRef]
- Chen, B.T.; Yeh, H.C. An improved virtual impactor: Design and performance. J. Aerosol Sci. 1987, 18, 203–214. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology. Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Paprotny, I.; Doering, F.; Solomon, P.A.; White, R.M.; Gundel, L.A. Microfabricated Air-Microfluidic Sensor for Personal Monitoring of Airborne Particulate Matter: Design, Fabrication, and Experimental Results. Sens. Actuators A Phys. 2013, 201, 506–516. [Google Scholar] [CrossRef]
- Kim, Y.H.; Maeng, J.Y.; Park, D.; Jung, I.H.; Hwang, J.; Kim, Y.J. Micromachined Cascade Virtual Impactor with a Flow Rate Distributor for Wide Range Airborne Particle Classification. Appl. Phys. Lett. 2007, 91, 043512. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, D.; Hwang, J.; Kim, Y.J. Integrated Particle Detection Chip for Environmental Monitoring. Lab Chip 2008, 8, 1950–1956. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, D.; Hwang, J.; Kim, Y.J. A Hybrid Chip Based on Aerodynamics and Electrostatics for the Size-Dependent Classification of Ultrafine and Nano Particles. Lab Chip 2009, 9, 2722–2728. [Google Scholar] [CrossRef]
- Paprotny, I.; Doering, F.; White, R.M. MEMS Particulate Matter (PM) Monitor for Cellular Deployment. In Proceedings of the IEEE Sensors, Waikoloa, HI, USA, 1–4 November 2010; pp. 2435–2440. [Google Scholar] [CrossRef]
- Doering, F.L.; Paprotny, I.; White, R.M. Mems Air-Microfluidic Sensor for Portable Monitoring of Airborne Particulates. In Proceedings of the 2012 Solid-State Sensors, Actuators and Microsystems Workshop, San Diego, CA, USA, 3–7 June 2012; pp. 315–319. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, K.S.; Kim, S.S.; Bae, G.N.; Jung, J.H. Real-Time Detection of an Airborne Microorganism Using Inertial Impaction and Mini-Fluorescent Microscopy. Lab Chip 2014, 14, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Yuen, L.; Chu, W.C.; Stoeber, B. Microfluidic-Based Real-Time Detector for Fine Particulate Matter. In Proceedings of the 2014 IEEE Sensors, Valencia, Spain, 2–5 November 2014. [Google Scholar] [CrossRef]
- Jianwen, S.; Kun, Y.; Zewen, L.; Yanwu, L. A System of Continuous Particles Monitoring Using Virtual Impactor. In Proceedings of the 2015 IEEE 12th International Conference on Electronic Measurement and Instruments (ICEMI), Qingdao, China, 16–18 July 2015; Volume 3, pp. 1183–1187. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Yang, K.; Lu, Y. A Miniature System for Particulate Matter (PM) Measurement. In Proceedings of the 2015 IEEE Sensors, Busan, Korea, 1–4 November 2015; pp. 5–8. [Google Scholar] [CrossRef]
- Dong, M.; Iervolino, E.; Santagata, F.; Zhang, G.; Zhang, G. Integrated Virtual Impactor Enabled PM2.5 Sensor. IEEE Sens. J. 2017, 17, 2814–2821. [Google Scholar] [CrossRef]
- Kim, H.L.; Han, J.; Lee, S.M.; Kwon, H.B.; Hwang, J.; Kim, Y.J. MEMS-Based Particle Detection System for Measuring Airborne Ultrafine Particles. Sens. Actuators A Phys. 2018, 283, 235–244. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; Liu, M.; Liang, Y.; He, S. A Novel Particulate Matter 2.5 Sensor Based on Surface Acoustic Wave Technology. Appl. Sci. 2018, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Fahimi, D.; Mahdavipour, O.; Cados, T.; Kirchstetter, T.; Solomon, P.; Gundel, L.; White, R.M.; Fukushima, N.; Nagai, H.; Saitoh, M.; et al. MEMS Air-Microfluidic Lab-on-a-Chip Sensor for Personal Monitoring of Airborne Particulate Matter. In Proceedings of the 2016 Solid-State Sensors, Actuators and Microsystems Workshop, Hilton Head, SC, USA, 5–9 June 2016; pp. 336–339. [Google Scholar] [CrossRef]
- Fahimi, D.; Mahdavipour, O.; Sabino, J.; White, R.M.; Paprotny, I. Vertically-Stacked MEMS PM2.5 Sensor for Wearable Applications. Sens. Actuators A Phys. 2019, 299, 111569. [Google Scholar] [CrossRef]
- Mahdavipour, O.; Fahimi, D.; Paprotny, I. Microfabricated Air-Microfluidics Virtual Impactor with Groove- Based Envelope-Flow Particle Focusing System. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, S.; Yang, N.; Wang, A.; Fordjour, A.; Chen, S. The Collection Method for Crop Fungal Spores Based on an Efficient Microfluidic Device. Aerosol Air Qual. Res. 2020, 20, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yuan, S.; Yang, N.; Wang, A. Performance Evaluation of a Virtual Impactor with an Additional Pretreatment Structure for Particle Separation. Aerosol Air Qual. Res. Aerosol Air Qual. Res. 2021, 21, 200269. [Google Scholar] [CrossRef]
- Liang, D.; Shih, W.P.; Chen, C.S.; Dai, C.A. A Miniature System for Separating Aerosol Particles and Measuring Mass Concentrations. Sensors 2010, 10, 3641–3654. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.S.; Lee, K.S.; Lee, K.H.; Sung, H.J.; Kim, S.S. Characterization of a Microscale Cascade Impactor. Aerosol Sci. Technol. 2012, 46, 966–972. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Qu, H.; Zhang, M.; Pang, W.; Duan, X. A Machined Virtual Impactor for PM 2 Detection. In Proceedings of the IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Pang, W.; Sun, C.; Zhou, Q.; Lin, Z.; Chang, Y.; Li, Q.; Zhang, M.; Duan, X. Smartphone-Enabled Aerosol Particle Analysis Device. IEEE Access 2019, 7, 101117–101124. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, M.; Liang, L.; Wang, W.; Xie, J. Airborne Particulate Matter Classification and Concentration Detection Based on 3D Printed Virtual Impactor and Quartz Crystal Microbalance Sensor. Sens. Actuators A Phys. 2016, 238, 379–388. [Google Scholar] [CrossRef]
- Kwon, H.B.; Kim, H.L.; Hong, U.S.; Yoo, S.J.; Kim, K.; Han, J.; Kim, M.K.; Hwang, J.; Kim, Y.J. Particle Size Spectrometer Using Inertial Classification and Electrical Measurement Techniques for Real-Time Monitoring of Particle Size Distribution. Lab Chip 2018, 18, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, W.; Chen, D.; Wu, C.; Xie, J. A Miniature System for Classification and Concentration Detection of PM Based on 3D Printed Virtual Impactor and QCM Sensor. In Proceedings of the 13th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Singapore, 22–26 April 2018; 288, pp. 67–74. [Google Scholar]
- Wang, Y.; Wang, Y.; Liu, W.; Chen, D.; Wu, C.; Xie, J. An Aerosol Sensor for PM 1 Concentration Detection Based on 3D Printed Virtual Impactor and SAW Sensor. Sens. Actuators A Phys. 2019, 288, 67–74. [Google Scholar] [CrossRef]
- Chen, T.; Sun, J.; Ma, T.; Li, T.; Liu, C.; Zhu, X.; Xue, N. Design and Analysis of Particulate Matter Air-Microfluidic Grading Chip Based on MEMS. Micromachines 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.L.; Han, J.S.; Lee, S.M.; Gown, H.B.; Hwang, J.; Kim, Y.J. Aerosol Particle Size Spectrometer Using a Micromachined Cascade Impactor. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 84–86. [Google Scholar] [CrossRef]
- Moon, H.S.; Nam, Y.W.; Jae, C.P.; Jung, H.I. Dielectrophoretic Separation of Airborne Microbes and Dust Particles Using a Microfluidic Channel for Real-Time Bioaerosol Monitoring. Environ. Sci. Technol. 2009, 43, 5857–5863. [Google Scholar] [CrossRef]
- Mohamadi Nasrabadi, A.; Han, J.S.; Massoudi Farid, M.; Lee, S.G.; Hwang, J. Real-Time Separation of Aerosolized Staphylococcus Epidermidis and Polystyrene Latex Particles with Similar Size Distributions. Aerosol Sci. Technol. 2017, 51, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Hong, J.; Lee, S.-B. Real-time detection of airborne dust particles using paddle-type silicon cantilevers. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2009, 27, 3120. [Google Scholar] [CrossRef]
- Bertke, M.; Xu, J.; Fahrbach, M.; Setiono, A.; Wasisto, H.S.; Peiner, E. Strategy toward Miniaturized, Self-out-Readable Resonant Cantilever and Integrated Electrostatic Microchannel Separator for Highly Sensitive Airborne Nanoparticle Detection. Sensors 2019, 19, 901. [Google Scholar] [CrossRef] [Green Version]
- Bertke, M.; Xu, J.; Setiono, A.; Kirsch, I.; Uhde, E.; Peiner, E. Fabrication of a Microcantilever-Based Aerosol Detector with Integrated Electrostatic on-Chip Ultrafine Particle Separation and Collection. J. Micromech. Microeng. 2020, 30, 014001. [Google Scholar] [CrossRef]
- Steinle, S.; Reis, S.; Sabel, C.E.; Semple, S.; Twigg, M.M.; Braban, C.F.; Leeson, S.R.; Heal, M.R.; Harrison, D.; Lin, C.; et al. Personal exposure monitoring of PM2.5 in indoor and outdoor microenvironments. Sci. Total Environ. 2015, 508, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Bedekar, V.N.; Tantawi, K.H. MEMS Sensors and Actuators. In Advanced Mechatronics and MEMS Devices II-Microsystems and Nanosystems, 1st ed.; Howe, R.T., Ricco, A.J., Eds.; Springer: Cham, Switzerland, 2015; pp. 195–216. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An Overview of High Frequency Acoustic. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [Green Version]
- Baracu, A.M.; Gugoasa, A.D. Recent Advances in Microfabrication, Desing and Applications of Amperometric Sensors and Biosensors. J. Electrochem. Soc. 2021, 168, 037503. [Google Scholar] [CrossRef]
- Cali, K.; Tuccori, E.; Persaud, K.C. Gravimetric biosensors. In Methods in Enzymology; Pelosi, P., Knoll, W., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 435–468. [Google Scholar] [CrossRef]
- Montagut, Y.; Vicente Garcia, J.; Jimenez, Y.; March, C.; Montoya, A.; Arnau, A. QCM Technology in Biosensors. In Biosensors–Emerging Materials and Applications; Serra, P.A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 153–178. [Google Scholar]
- Owen, T.W.; Al-Kaysi, R.O.; Bardeen, C.J.; Cheng, Q. Microgravimetric Immunosensor for Direct Detection of Aerosolized Influenza A Virus Particles. Sens. Actuators B Chem. 2007, 126, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Akin, D.; Savran, C.A.; Bashir, R. Real-time detection of airborne viruses on a mass-sensitive device. Appl. Phys. Let. 2008, 93, 13901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, D.R.P.; Fatisson, J.; Olsson, A.L.J.; Tufenkji, N.; Ferro, A.R. Real-time monitoring of airborne cat allergen using a QCM-based immunosensor. Sens. Actuators B Chem. 2014, 190, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Zampetti, E.; Macagnano, A.; Papa, P.; Bearzotti, A.; Petracchini, F.; Paciucci, L.; Pirrone, N. Exploitation of an Integrated Microheater on QCM Sensor in Particulate Matter Measurements. Sens. Actuators A Phys. 2017, 264, 205–211. [Google Scholar] [CrossRef]
- Nirschl, M.; Schreiter, M.; Vörös, J. Comparison of FBAR and QCM-D sensitivity dependence on adlayer thickness and viscosity. Sens. Actuators A Phys. 2011, 165, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Black, J.P.; Elium, A.; White, R.M.; Apte, M.G.; Gundel, L.A.; Cambie, R. MEMS-Enabled Miniaturized Particulate Matter Monitor Employing 1.6 GHz Aluminum Nitride Thin-Film Bulk Acoustic Wave Resonator (FBAR) and Thermophoretic Precipitator. In Proceedings of the IEEE Ultrasonics Symposium, New York, NY, USA, 28–31 October 2007; pp. 476–479. [Google Scholar] [CrossRef]
- Thomas, S.; Cole, M.; Villa-Lopez, F.H.; Gardner, J.W.; Peters, J.; Theunis, J. A Low-Cost Acoustic Microsensor Based System in Package for Air Quality Monitoring. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 5–7. [Google Scholar] [CrossRef]
- Thomas, S.; Cole, M.; Villa-López, F.H.; Gardner, J.W. High Frequency Surface Acoustic Wave Resonator-Based Sensor for Particulate Matter Detection. Sens. Actuators A Phys. 2016, 244, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Z.; Wang, Y.; Xie, J. A study on AIN film-based SAW attenuation in liquids and their potential as liquid ethanol sensors. Sensors 2017, 17, 1813. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, H.; Gan, X.; Xia, X.; Xu, P.; Li, J.; Liu, M.; Li, Y. Integrated MEMS/NEMS resonant cantilevers for ultrasensitive biological detection. J. Sens. 2009, 2009, 637874. [Google Scholar] [CrossRef]
- Maldonado-Garcia, M.; Kumar, V.; Wilson, J.C.; Pourkamali, S. Chip-Scale Implementation and Cascade Assembly of Particulate Matter Collectors with Embedded Resonant Mass Balances. IEEE Sens. J. 2017, 17, 1617–1625. [Google Scholar] [CrossRef]
- Hajjam, A.; Wilson, J.C.; Pourkamali, S. Individual Air-Borne Particle Mass Measurement Using High-Frequency Micromechanical Resonators. IEEE Sens. J. 2011, 11, 2883–2890. [Google Scholar] [CrossRef]
- Schmid, S.; Kurek, M.; Adolphsen, J.Q.; Boisen, A. Real-time single airborne nanoparticle detection with nanomechanical resonant filter-fiber. Sci. Rep. 2013, 3, 3–7. [Google Scholar] [CrossRef]
- Hajjam, A.; Wilson, J.C.; Rahafrooz, A.; Pourkamali, S. Fabrication and Characterization of Thermally Actuated Micromechanical Resonators for Airborne Particle Mass Sensing: II. Device Fabrication and Characterization. J. Micromech. Microeng. 2010, 20, 125019. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Waag, A.; Kirsch, I.; Uhde, E.; Salthammer, T.; Peiner, E. Determination of Exposure to Engineered Carbon Nanoparticles Using a Self-Sensing Piezoresistive Silicon Cantilever Sensor. Microsyst. Technol. 2012, 18, 905–915. [Google Scholar] [CrossRef]
- Merzsch, S.; Wasisto, H.S.; Sökmen, Ü.; Waag, A.; Uhde, E.; Salthammer, T.; Peiner, E. Mass measurement of nanoscale aerosol particles using a piezoelectrically actuated resonant sensor. IEEE Sens. Conf. Open Poster 2010, 1, 1. [Google Scholar]
- Sökmen, Ü.; Stranz, A.; Waag, A.; Ababneh, A.; Seidel, H.; Schmid, U.; Peiner, E. Evaluation of resonating Si cantilevers sputter-deposited with AlN piezoelectric thin films for mass sensing applications. J. Micromech. Microeng. 2010, 20, 064007. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Waag, A.; Uhde, E.; Salthammer, T.; Peiner, E. Airborne engineered nanoparticle mass sensor based on a silicon resonant cantilever. Sens. Actuators B Chem. 2013, 180, 77–89. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Stranz, A.; Waag, A.; Kirsch, I.; Uhde, E.; Salthammer, T.; Peiner, E. A Resonant Cantilever Sensor for Monitoring Airborne Nanoparticles. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 1116–1119. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Waag, A.; Kirsch, I.; Uhde, E.; Salthammer, T.; Peiner, E. Enhanced airborne nanoparticles mass sensing using a high-mode resonant silicon cantilever sensor. In Proceedings of the Sensors, 2011 IEEE, Limerick, Ireland, 28–31 October 2011. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Waag, A.; Uhde, E.; Salthammer, T.; Peiner, E. Portable Cantilever-Based Airborne Nanoparticle Detector. Sens. Actuators B Chem. 2013, 187, 118–127. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Uhde, E.; Waag, A.; Peiner, E. Partially Integrated Cantilever-Based Airborne Nanoparticle Detector for Continuous Carbon Aerosol Mass Concentration Monitoring. J. Sens. Sens. Syst. 2015, 4, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Wasisto, H.S.; Merzsch, S.; Uhde, E.; Waag, A.; Peiner, E. Handheld Personal Airborne Nanoparticle Detector Based on Microelectromechanical Silicon Resonant Cantilever. Microelectron. Eng. 2015, 145, 96–103. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Stranz, A.; Waag, A.; Uhde, E.; Salthammer, T.; Peiner, E. Silicon Resonant Nanopillar Sensors for Airborne Titanium Dioxide Engineered Nanoparticle Mass Detection. Sens. Actuators B Chem. 2013, 189, 146–156. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Steib, F.; Waag, A.; Peiner, E. Vertical Silicon Nanowire Array-Patterned Microcantilever Resonators for Enhanced Detection of Cigarette Smoke Aerosols. Micro Nano Lett. 2014, 9, 676–679. [Google Scholar] [CrossRef]
- Merzsch, S.; Wasisto, H.; Kirsch, I.; Uhde, E.; Salthammer, T.; Peiner, E. Recycling of Cantilevers for Nanoparticle Detection by Lift-off Technique. In Proceedings of the 14th IMCS, Nuremberg, Germany, 20–23 May 2012; pp. 916–919. [Google Scholar] [CrossRef]
- Wasisto, H.S.; Merzsch, S.; Waag, A.; Uhde, E.; Salthammer, T.; Peiner, E. Evaluation of Photoresist-Based Nanoparticle Removal Method for Recycling Silicon Cantilever Mass Sensors. Sens. Actuators A Phys. 2013, 202, 90–99. [Google Scholar] [CrossRef]
- Carminati, M.; Pedalà, L.; Bianchi, E.; Nason, F.; Dubini, G.; Cortelezzi, L.; Ferrari, G.; Sampietro, M. Capacitive Detection of Micrometric Airborne Particulate Matter for Solid-State Personal Air Quality Monitors. Sens. Actuators A Phys. 2014, 219, 80–87. [Google Scholar] [CrossRef]
- Ciccarella, P.; Carminati, M.; Sampietro, M.; Ferrari, G. Multichannel 65 ZF Rms Resolution CMOS Monolithic Capacitive Sensor for Counting Single Micrometer-Sized Airborne Particles on Chip. IEEE J. Solid-State Circuits 2016, 51, 2545–2553. [Google Scholar] [CrossRef]
- Oluwasanya, P.W.; Rughoobur, G.; Occhipinti, L.G. Design, Modeling and Simulation of a Capacitive Size-Discriminating Particulate Matter Sensor for Personal Air Quality Monitoring. IEEE Sens. J. 2020, 20, 1971–1979. [Google Scholar] [CrossRef]
- Woo, K.; Chen, D.; Pui, D.Y.H.; Wilson, W.E. Use of Continuous Measurements of Integral Aerosol Parameters to Estimate Particle Surface Area. Aerosol Sci. Technol. 2001, 34, 57–65. [Google Scholar] [CrossRef]
- Park, D.; An, M.; Hwang, J. Development and performance test of a unipolar diffusion charger for real-time measurements of submicron aerosol particles having a log-normal size distribution. J. Aerosol Sci. 2007, 38, 420–430. [Google Scholar] [CrossRef]
- Park, D.; Kim, Y.H.; Lee, S.G.; Kim, C.; Hwang, J.; Kim, Y.J. Development and Performance Test of a Micromachined Unipolar Charger for Measurements of Submicron Aerosol Particles Having a Log-Normal Size Distribution. J. Aerosol Sci. 2010, 41, 490–500. [Google Scholar] [CrossRef]
- Kim, H.L.; Han, J.S.; Lee, S.M.; Kwon, H.B.; Hwang, J.; Kim, Y.J. Ultrafine Particle Counter Using a MEMS-Based Particle Processing Chip. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 559–562. [Google Scholar] [CrossRef]
- Schaap, A.; Chu, W.C.; Antonio, M.I.; Stoeber, B. Microchannel-Based Size Detector for Airborne Particles. In Proceedings of the Sensors, IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 2441–2446. [Google Scholar] [CrossRef]
- Kwon, H.B.; Yoo, S.J.; Kim, Y.J. Microfluidic Condensation Nanoparticle Counter Using Water as the Condensing Liquid for Assessing Individual Exposure to Airborne Nanoparticles. Lab Chip 2020, 20, 1092–1102. [Google Scholar] [CrossRef]
- Li, X.; Iervolino, E.; Santagata, F.; Wei, J.; Yuan, C.A.; Sarro, P.M.; Zhang, G.Q. Miniaturized Particulate Matter Sensor for Portable Air Quality Monitoring Devices. In Proceedings of the IEEE Sensors, Valencia, Spain, 2–5 November 2014; pp. 2151–2154. [Google Scholar] [CrossRef]
- Dong, M.; Iervolino, E.; Santagata, F.; Zhang, G.; Zhang, G. Silicon Microfabrication Based Particulate Matter Sensor. Sens. Actuators A Phys. 2016, 247, 115–124. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in Microfluidics for Environmental Analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef]
- Yew, M.; Ren, Y.; Koh, K.S.; Sun, C.; Snape, C. A Review of State-of-the-Art Microfluidic Technologies for Environmental Applications: Detection and Remediation. Glob. Chall. 2019, 3, 1800060. [Google Scholar] [CrossRef] [Green Version]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-Based Analytical Devices for Environmental Analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.T.; Hou, C.Y.; Wang, Y.N.; Fu, L.M. Microfluidic Paper-Based Analytical Devices for Environmental Analysis of Soil, Air, Ecology and River Water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Fair, R.B.; Khlystov, A.; Srinivasan, V.; Pamula, V.K.; Weaver, K.N. Integrated Chemical/Biochemical Sample Collection, Pre-Concentration, and Analysis on a Digital Microfluidic Lab-on-a-Chip Platform. Lab Chip Platf. Devices Appl. 2004, 5591, 113. [Google Scholar] [CrossRef]
- Huang, S.; Connolly, J.; Khlystov, A.; Fair, R.B. Digital Microfluidics for the Detection of Selected Inorganic Ions in Aerosols. Sensors 2020, 20, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentele, M.M.; Cunningham, J.; Koehler, K.; Volckens, J.; Henry, C.S. Microfluidic Paper-Based Analytical Device for Particulate Metals. Anal. Chem. 2012, 84, 4474–4480. [Google Scholar] [CrossRef]
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of Aerosol Oxidative Activity Using Silver Nanoparticle Aggregation on Paper-Based Analytical Devices. Analyst 2013, 138, 6766–6773. [Google Scholar] [CrossRef] [Green Version]
- Rattanarat, P.; Dungchai, W.; Cate, D.M.; Siangproh, W.; Volckens, J.; Chailapakul, O.; Henry, C.S. A Microfluidic Paper-Based Analytical Device for Rapid Quantification of Particulate Chromium. Anal. Chim. Acta 2013, 800, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Sameenoi, Y.; Panymeesamer, P.; Supalakorn, N.; Koehler, K.; Chailapakul, O.; Henry, C.S.; Volckens, J. Microfluidic Paper-Based Analytical Device for Aerosol Oxidative Activity. Environ. Sci. Technol. 2013, 47, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cate, D.M.; Dungchai, W.; Cunningham, J.C.; Volckens, J.; Henry, C.S. Simple, Distance-Based Measurement for Paper Analytical Devices. Lab Chip 2013, 13, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Nanthasurasak, P.; Riwkulkajorn, P.; L’Orange, C.; Henry, C.S.; Volckens, J. Rapid Detection of Transition Metals in Welding Fumes Using Paper-Based Analytical Devices. Ann. Occup. Hyg. 2014, 58, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cate, D.M.; Noblitt, S.D.; Volckens, J.; Henry, C.S. Multiplexed Paper Analytical Device for Quantification of Metals Using Distance-Based Detection. Lab Chip 2015, 15, 2808–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Dong, H.; Zheng, J.; Sun, H. Portable Detection of Trace Metals in Airborne Particulates and Sediments via ΜPADs and Smartphone. Biomicrofluidics 2017, 11, 064101. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, W.; Zheng, J.; Ni, Z.; Sun, H. Spatial Varying Profiling of Air PM Constituents Using Paper-Based Microfluidics. Biomicrofluidics 2019, 13, 054103. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Dong, H.; Fan, L.; Zheng, J. Multiplex Quantification of Metals in Airborne Particulate Matter via Smartphone and Paper-Based Microfluidics. Anal. Chim. Acta 2018, 1044, 110–118. [Google Scholar] [CrossRef]
- Sun, H.; Jia, Y.; Dong, H.; Fan, L. Graphene Oxide Nanosheets Coupled with Paper Microfluidics for Enhanced On-Site Airborne Trace Metal Detection. Microsyst. Nanoeng. 2019, 5, 4. [Google Scholar] [CrossRef]
- Noblitt, S.D.; Schwandner, F.M.; Hering, S.V.; Collett, J.L.; Henry, C.S. High-Sensitivity Microchip Electrophoresis Determination of Inorganic Anions and Oxalate in Atmospheric Aerosols with Adjustable Selectivity and Conductivity Detection. J. Chromatogr. A 2009, 1216, 1503–1510. [Google Scholar] [CrossRef]
- Dossi, N.; Susmel, S.; Toniolo, R.; Pizzariello, A.; Bontempelli, G. Application of Microchip Electrophoresis with Electrochemical Detection to Environmental Aldehyde Monitoring. Electrophoresis 2009, 30, 3465–3471. [Google Scholar] [CrossRef]
- Greenwood, J.D.; Liu, Y.; Busacker, D.E.; Cheng, D.; Jiang, H. Collection of Gaseous and Aerosolized Samples Using Microfluidic Devices with Gas-Liquid Interfaces. IEEE Sens. J. 2010, 10, 952–959. [Google Scholar] [CrossRef]
- Shen, F.; Tan, M.; Wang, Z.; Yao, M.; Xu, Z.; Wu, Y.; Wang, J.; Guo, X.; Zhu, T. Integrating Silicon Nanowire Field Effect Transistor, Microfluidics and Air Sampling Techniques for Real-Time Monitoring Biological Aerosols. Environ. Sci. Technol. 2011, 45, 7473–7480. [Google Scholar] [CrossRef] [PubMed]
- Sameenoi, Y.; Koehler, K.; Shapiro, J.; Boonsong, K.; Sun, Y.; Collett, J.; Volckens, J.; Henry, C.S. Microfluidic Electrochemical Sensor for On-Line Monitoring of Aerosol Oxidative Activity. J. Am. Chem. Soc. 2012, 134, 10562–10568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanarat, P.; Dungchai, W.; Cate, D.; Volckens, J.; Chailapakul, O.; Henry, C.S. Multilayer Paper-Based Device for Colorimetric and Electrochemical Quantification of Metals. Anal. Chem. 2014, 86, 3555–3562. [Google Scholar] [CrossRef] [PubMed]
- Tirandazi, P.; Hidrovo, C.H. An Integrated Gas-Liquid Droplet Microfluidic Platform for Digital Sampling and Detection of Airborne Targets. Sens. Actuators B Chem. 2018, 267, 279–293. [Google Scholar] [CrossRef]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of Channel Coating and Dimensions of Microfluidic-Based Gas Detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective Detection of Volatile Organic Compounds in Microfluidic Gas Detectors Based on “like Dissolves Like”. Sci. Rep. 2019, 9, 161. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Volckens, J.; Henry, C.S. Janus Electrochemical Paper-Based Analytical Devices for Metals Detection in Aerosol Samples. Anal. Chem. 2020, 92, 1439–1446. [Google Scholar] [CrossRef]
- Kwon, H.J.; Fronczek, C.F.; Angus, S.V.; Nicolini, A.M.; Yoon, J.Y. Rapid and Sensitive Detection of H1N1/2009 Virus from Aerosol Samples with a Microfluidic Immunosensor. J. Lab. Autom. 2013, 19, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; Zhao, W.; Liu, S.; Li, L.; Tsai, C.T.; Fan, X.; Wu, W.; Li, J.; Yang, X.; Sui, G. Microfluidic Device for Efficient Airborne Bacteria Capture and Enrichment. Anal. Chem. 2013, 85, 5255–5262. [Google Scholar] [CrossRef]
- Jing, W.; Jiang, X.; Zhao, W.; Liu, S.; Cheng, X.; Sui, G. Microfluidic Platform for Direct Capture and Analysis of Airborne Mycobacterium Tuberculosis. Anal. Chem. 2014, 86, 5815–5821. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Liu, Q.; Jing, W.; Qin, K.; Sui, G. Rapid Capture and Analysis of Airborne Staphylococcus Aureus in the Hospital Using a Microfluidic Chip. Micromachines 2016, 7, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Jing, W.; Sun, X.; Liu, Q.; Yang, C.; Liu, S.; Qin, K.; Sui, G. High-Throughput Microfluidic Device for LAMP Analysis of Airborne Bacteria. ACS Sens. 2016, 1, 958–962. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Jing, W.; Liu, S.; Zhang, D.; Sui, G. First Airborne Pathogen Direct Analysis System. Analyst 2016, 141, 1637–1640. [Google Scholar] [CrossRef]
- Damit, B. Droplet-based microfluidics detector for bioaerosol detection. Aerosol Sci. Technol. 2017, 51, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Tarn, M.D.; Sikora, S.N.F.; Porter, G.C.E.; O’Sullivan, D.; Adams, M.; Whale, T.F.; Harrison, A.D.; Vergara-Temprado, J.; Wilson, T.W.; Shim, J.-U.; et al. The Study of Atmospheric Ice-Nucleating Particles via Microfluidically Generated Droplets. Microfluid. Nanofluidics 2018, 22, 52. [Google Scholar] [CrossRef] [Green Version]
- Tarn, M.D.; Sikora, S.N.F.; Porter, G.C.E.; Wyld, B.V.; Alayof, M.; Reicher, N.; Harrison, A.D.; Rudich, Y.; Shim, J.U.; Murray, B.J. On-Chip Analysis of Atmospheric Ice-Nucleating Particles in Continuous Flow. Lab Chip 2020, 20, 2889–2910. [Google Scholar] [CrossRef]
- Choi, J.; Kang, M.; Jung, J.H. Integrated Micro-Optofluidic Platform for Real-Time Detection of Airborne Microorganisms. Sci. Rep. 2015, 5, 15983. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.S.; Im, H.T.; Jung, H.I. A Microfluidic ATP-Bioluminescence Sensor for the Detection of Airborne Microbes. Sens. Actuators B Chem. 2008, 132, 443–448. [Google Scholar] [CrossRef]
- Piorek, B.D.; Seung, J.L.; Santiago, J.G.; Moskovits, M.; Banerjee, S.; Meinhart, C.D. Free-Surface Microfluidic Control of Surface-Enhanced Raman Spectroscopy for the Optimized Detection of Airborne Molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 18898–18901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piorek, B.D.; Lee, S.J.; Moskovits, M.; Meinhart, C.D. Free-Surface Microfluidics/Surface-Enhanced Raman Spectroscopy for Real-Time Trace Vapor Detection of Explosives. Anal. Chem. 2012, 84, 9700–9705. [Google Scholar] [CrossRef] [PubMed]
- Piorek, B.D.; Andreou, C.; Moskovits, M.; Meinhart, C.D. Discrete Free-Surface Millifluidics for Rapid Capture and Analysis of Airborne Molecules Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2014, 86, 1061–1066. [Google Scholar] [CrossRef]
- Chrimes, A.F.; Khoshmanesh, K.; Stoddart, P.R.; Mitchell, A.; Kalantar-Zadeh, K. Microfluidics and Raman Microscopy: Current Applications and Future Challenges. Chem. Soc. Rev. 2013, 42, 5880–5906. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, L.; Mukhitov, N.; Schrell, A.M.; Dhumpa, R.; Roper, M.G. Microfluidics-to-Mass Spectrometry: A Review of Coupling Methods and Applications. J. Chromatogr. A 2015, 1382, 98–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perro, A.; Lebourdon, G.; Henry, S.; Lecomte, S.; Servant, L.; Marre, S. Combining Microfluidics and FT-IR Spectroscopy: Towards Spatially Resolved Information on Chemical Processes. React. Chem. Eng. 2016, 1, 577–594. [Google Scholar] [CrossRef]
- Gao, D.; Jin, F.; Zhou, M.; Jiang, Y. Recent Advances in Single Cell Manipulation and Biochemical Analysis on Microfluidics. Analyst 2019, 144, 766–781. [Google Scholar] [CrossRef]
- Cho, S.K.; Zhao, Y.; Kim, C.J. Concentration and Binary Separation of Micro Particles for Droplet-Based Digital Microfluidics. Lab Chip 2007, 7, 490–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Chung, S.K.; Yi, U.C.; Cho, S.K. Droplet Manipulation and Microparticle Sampling on Perforated Microfilter Membranes. J. Micromech. Microeng. 2008, 18, 025030. [Google Scholar] [CrossRef]
- He, J.L.; Chen, A.T.; Lee, J.H.; Fan, S.K. Digital Microfluidics for Manipulation and Analysis of a Single Cell. Int. J. Mol. Sci. 2015, 16, 22319–22332. [Google Scholar] [CrossRef]
- Martínez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Koehler, K.A.; Shapiro, J.; Sameenoi, Y.; Henry, C.; Volckens, J. Laboratory Evaluation of a Microfluidic Electrochemical Sensor for Aerosol Oxidative Load. Aerosol Sci. Technol. 2014, 48, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Miller-Lionberg, D.; Reilly, T.; Volckens, J.; Henry, C.S. Low-Cost Reusable Sensor for Cobalt and Nickel Detection in Aerosols Using Adsorptive Cathodic Square-Wave Stripping Voltammetry. J. Electroanal. Chem. 2017, 805, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Luo, X.; Fu, P.; Li, X. Airborne particulate matter pollution in urban China: A chemical mixture perspective from sources to impacts. Nat. Sci. Rev. 2016, 4, 593–610. [Google Scholar] [CrossRef] [Green Version]
- Krieger, U.K.; Marcolli, C.; Reid, J.P. Exploring the complexity of aerosol particle properties and processes using single particle techniques. Chem. Soc. Rev. 2012, 41, 6631–6662. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Wang, C. Optical configurations for photophoretic trap of single particles in air. Rev. Sci. Instrum. 2016, 87, 103104. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.L.; Videen, G.; Wang, C. The temporal evolution process from fluorescence bleaching to clean Raman spectra of single solid particles optically trapped in air. Chem. Phys. Lett. 2017, 689, 100–104. [Google Scholar] [CrossRef]
- Kalume, A.; Beresnev, L.A.; Santarpia, J.; Pan, Y.L. Detection and Characterization of Chemical and Biological Aerosols Using Laser-Trapping Single-Particle Raman Spectroscopy. Appl. Opt. 2017, 56, 6577–6582. [Google Scholar] [CrossRef]
- Kalume, A.; Wang, C.; Santarpia, J.; Pan, Y.L. Study of Single Airborne Particle Using Laser-Trapped Submicron Position-Resolved Temporal Raman Spectroscopy. Chem. Phys. Lett. 2018, 706, 255–260. [Google Scholar] [CrossRef]
- Gómez-Castaño, J.; Boussekey, L.; Verwaerde, J.; Moreau, M.; Tobon, Y.A. Enhancing Double-Beam Laser Tweezers Raman Spectroscopy (LTRS) for the Photochemical Study of Individual Airborne Microdroplets. Molecules 2019, 24, 3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dochow, S.; Krafft, C.; Neugebauer, U.; Bocklitz, T.; Henkel, T.; Mayer, G.; Albert, J.; Popp, J. Tumour cell identification by means of Raman spectroscopy in combination with optical traps and microfluidic environments. Lab Chip 2011, 11, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Liberale, C.; Cojoc, G.; Bragheri, F.; Minzioni, P.; Perozziello, G.; La Rocca, R.; Ferrara, L.; Rajamanickam, V.; Di Fabrizio, E.; Cristiani, I. Integrated Microfluidic Device for Single-Cell Trapping and Spectroscopy. Sci. Rep. 2013, 3, 1258. [Google Scholar] [CrossRef] [Green Version]
- Perozziello, G.; Candeloro, P.; De Grazia, A.; Esposito, F.; Allione, M.; Coluccio, M.L.; Tallerico, R.; Valpapuram, I.; Tirinato, L.; Das, G.; et al. Microfluidic Device for Continuous Single Cells Analysis via Raman Spectroscopy Enhanced by Integrated Plasmonic Nanodimers. Opt. Express 2016, 24, A180–A190. [Google Scholar] [CrossRef] [Green Version]
- Pilát, Z.; Bernatová, S.; Ježek, J.; Kirchhoff, J.; Tannert, A.; Neugebauer, U.; Samek, O.; Zemánek, P. Microfluidic Cultivation and Laser Tweezers Raman Spectroscopy of E. coli under Antibiotic Stress. Sensors 2018, 18, 1623. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Chiou, P.Y. Light-Driven Droplet Manipulation Technologies for Lab-on-a-Chip Applications. Adv. OptoElectron. 2011, 2011, 909174. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezrre, S.; Reyna, M.A.; Anguiano, C.; Avitia, R.L.; Márquez, H. Lab-on-a-Chip Platforms for Airborne Particulate Matter Applications: A Review of Current Perspectives. Biosensors 2022, 12, 191. https://doi.org/10.3390/bios12040191
Ezrre S, Reyna MA, Anguiano C, Avitia RL, Márquez H. Lab-on-a-Chip Platforms for Airborne Particulate Matter Applications: A Review of Current Perspectives. Biosensors. 2022; 12(4):191. https://doi.org/10.3390/bios12040191
Chicago/Turabian StyleEzrre, Sharon, Marco A. Reyna, Citlalli Anguiano, Roberto L. Avitia, and Heriberto Márquez. 2022. "Lab-on-a-Chip Platforms for Airborne Particulate Matter Applications: A Review of Current Perspectives" Biosensors 12, no. 4: 191. https://doi.org/10.3390/bios12040191
APA StyleEzrre, S., Reyna, M. A., Anguiano, C., Avitia, R. L., & Márquez, H. (2022). Lab-on-a-Chip Platforms for Airborne Particulate Matter Applications: A Review of Current Perspectives. Biosensors, 12(4), 191. https://doi.org/10.3390/bios12040191






