Real-Time Monitoring the Cytotoxic Effect of Andrographolide on Human Oral Epidermoid Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagent Preparation
2.2. Impedance Measurement Using ECIS
2.3. AlamarBlue Viability Test
2.4. Annexin V/7-AAD Binding Assay
2.5. Statistical Analysis
3. Results
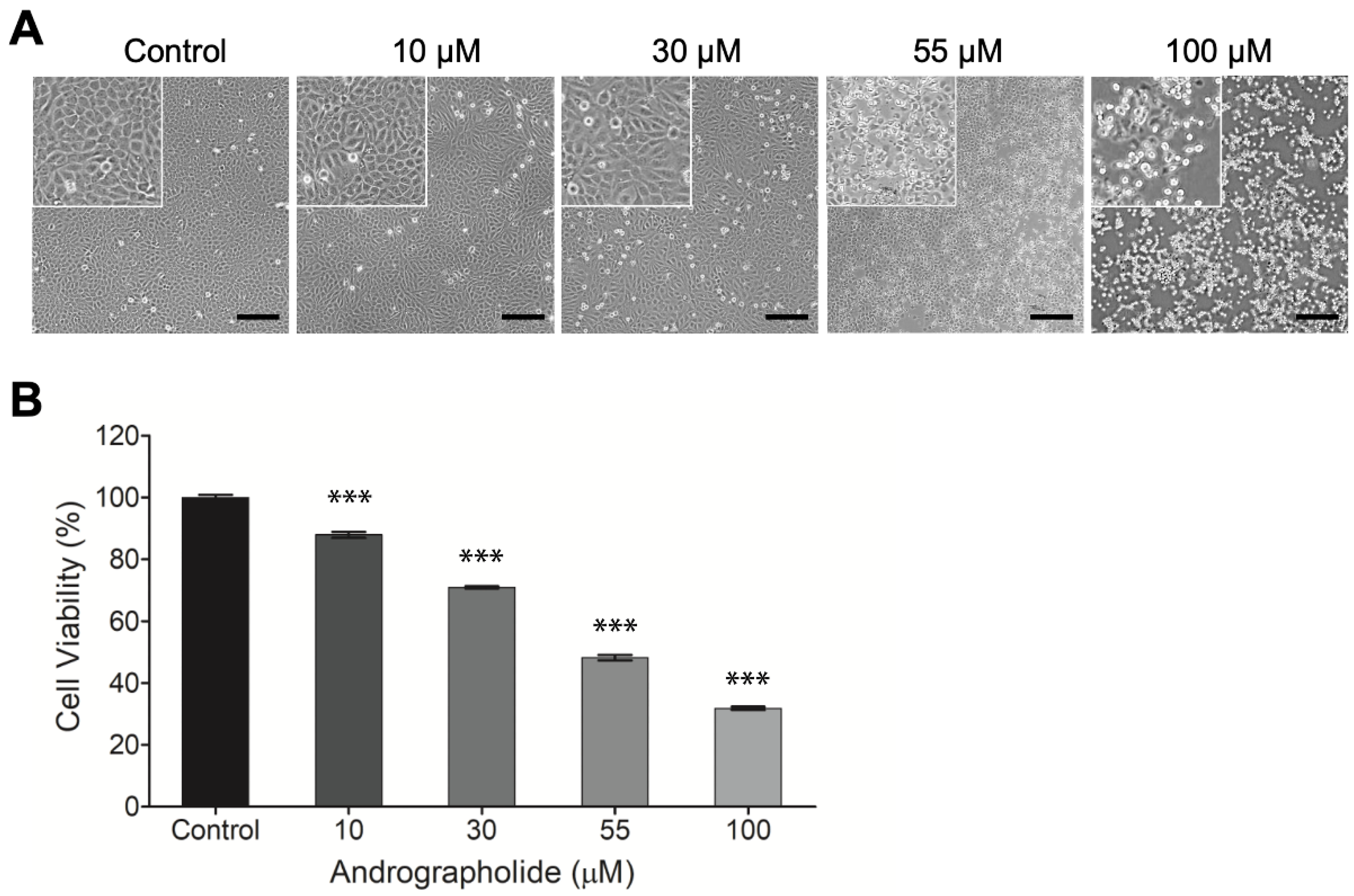
3.1. The Effects of Andrographolide on Cell Viability and Morphology in OEC-M1 Cells
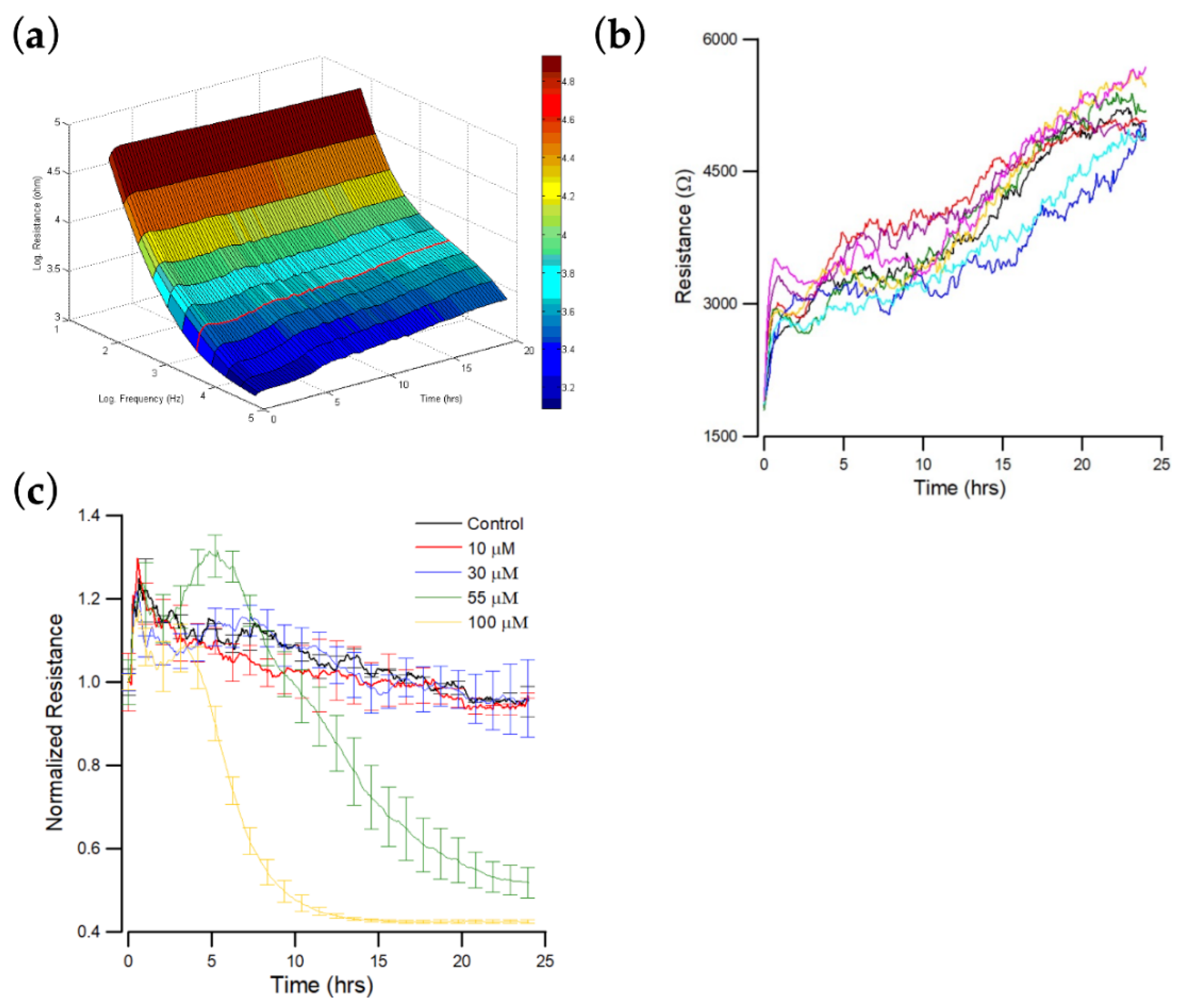
3.2. Real-Time Impedance Monitoring of OEC-M1 Cells Attachment and Spreading
3.3. Effect of Andrographolide on the Time Course of Overall Resistance
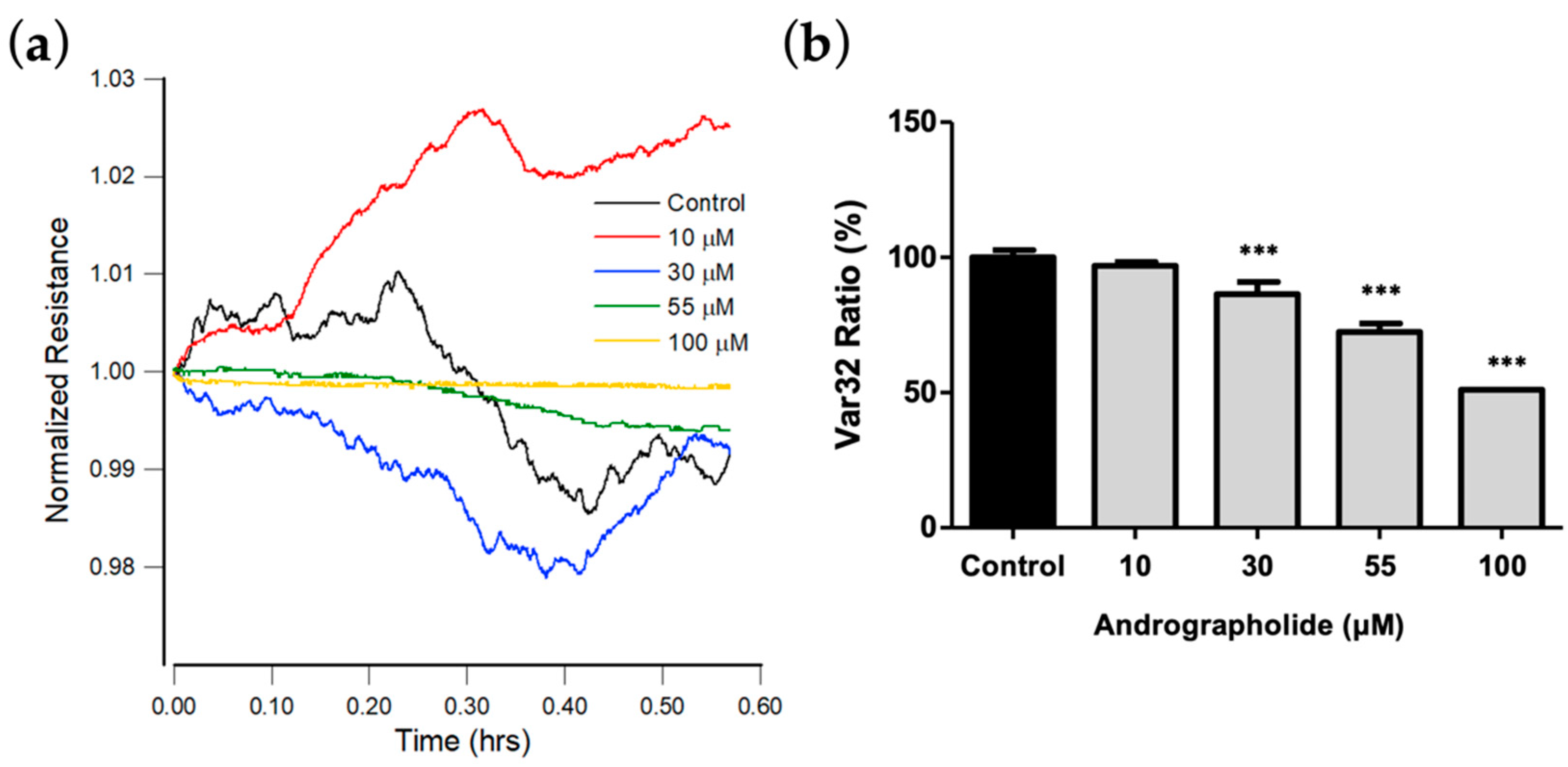
3.4. Effect of Andrographolide on the Morphological Parameters of OEC-M1 Cells
3.5. A Wound Healing Migration Assay Using ECIS
3.6. Effect of Andrographolide on OEC-M1 Micromotion
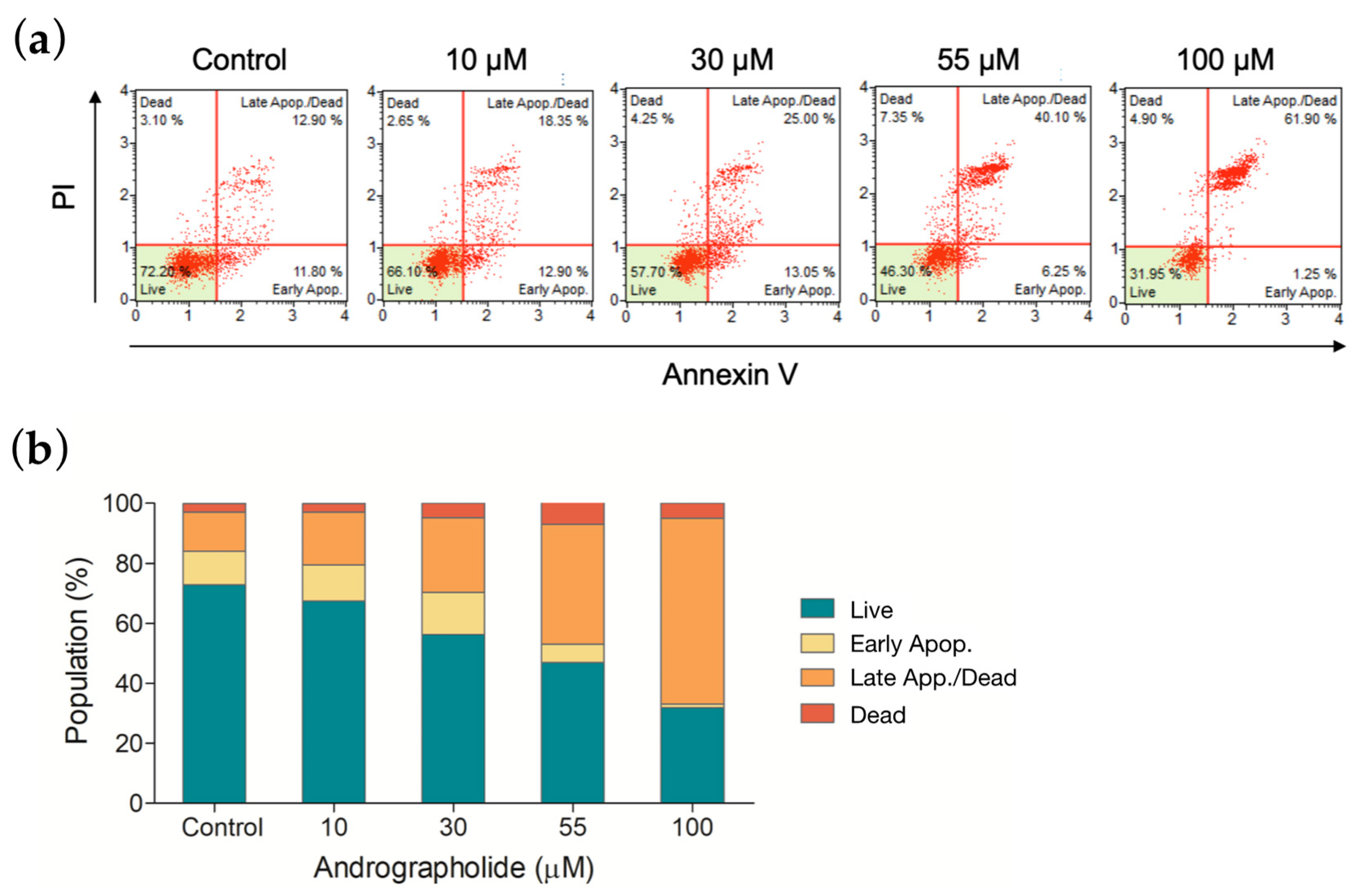
3.7. Apoptotic Profile of OEC-M1 Cells after Drug Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Guo, S.-E.; Huang, T.-J.; Huang, J.-C.; Lin, M.-S.; Hong, R.-M.; Chang, C.-H.; Chen, M.-Y. Alcohol, betel-nut and cigarette consumption are negatively associated with health promoting behaviors in Taiwan: A cross-sectional study. BMC Public Health 2013, 13, 257. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.C.; Menezes-Garcia, Z.; Henriques, M.C.; Soriani, F.M.; Pinho, V.; Faria, A.M.; Santiago, A.F.; Cara, D.C.; Souza, D.G.; Teixeira, M.M. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obesity 2013, 21, E396–E406. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA A Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Huang, S.H. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233. [Google Scholar] [CrossRef]
- Metzstein, M.M.; Stanfield, G.M.; Horvitz, H.R. Genetics of programmed cell death in C. elegans: Past, present and future. Trends Genet. 1998, 14, 410–416. [Google Scholar] [CrossRef]
- Alberts, K.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, W.P.; Walter, P. Chapter 18 Apoptosis: Programmed cell death eliminates unwanted cells. In Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY, USA, 2008; p. 1115. [Google Scholar]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Griffiths, G.J.; Dubrez, L.; Morgan, C.P.; Jones, N.A.; Whitehouse, J.; Corfe, B.M.; Dive, C.; Hickman, J.A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 1999, 144, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Mikhailov, V.; Mikhailova, M.; Pulkrabek, D.J.; Dong, Z.; Venkatachalam, M.A.; Saikumar, P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 2001, 276, 18361–18374. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Holler, N.; Micheau, O.; Martinon, F.; Tinel, A.; Hofmann, K.; Tschopp, J. Bcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J. Biol. Chem. 2001, 276, 19548–19554. [Google Scholar] [CrossRef] [Green Version]
- Marzo, I.; Brenner, C.; Zamzami, N.; Susin, S.A.; Beutner, G.; Brdiczka, D.; Rémy, R.; Xie, Z.-H.; Reed, J.C.; Kroemer, G. The permeability transition pore complex: A target for apoptosis regulation by caspases and Bcl-2–related proteins. J. Exp. Med. 1998, 187, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Harris, M.; Thompson, C. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000, 7, 1182–1191. [Google Scholar] [CrossRef]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c–dependent activation of caspase-3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Virág, L.; Marmer, D.J.; Szabó, C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic. Biol. Med. 1998, 25, 1075–1082. [Google Scholar] [CrossRef]
- Kumar, S.; Harvey, N.L. Role of multiple cellular proteases in the execution of programmed cell death. FEBS Lett. 1995, 375, 169–173. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef] [Green Version]
- Israels, E.; Israels, L. The cell cycle. Oncologist 2000, 5, 510–513. [Google Scholar] [CrossRef]
- Kirkin, V.; Joos, S.; Zörnig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1644, 229–249. [Google Scholar] [CrossRef]
- Szabo, C. Cell Death: The Role of PARP; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Tang, W.; Eisenbrand, G. Andrographis paniculata (Burm. f.) Nees. In Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992; pp. 97–103. [Google Scholar]
- Xia, Y.-F.; Ye, B.-Q.; Li, Y.-D.; Wang, J.-G.; He, X.-J.; Lin, X.; Yao, X.; Ma, D.; Slungaard, A.; Hebbel, R.P. Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 2004, 173, 4207–4217. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Q.; Ding, Y.; Lei, Y.; Qi, C.-L.; He, X.-D.; Lan, T.; Li, J.-C.; Gong, P.; Yang, X.; Geng, J.-G. Andrographolide suppress tumor growth by inhibiting TLR4/NF-κB signaling activation in insulinoma. Int. J. Biol. Sci. 2014, 10, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef]
- Yu, B.-C.; Hung, C.-R.; Chen, W.-C.; Cheng, J.-T. Antihyperglycemic effect of andrographolide in streptozotocin-induced diabetic rats. Planta Med. 2003, 69, 1075–1079. [Google Scholar]
- Rajagopal, S.; Kumar, R.A.; Deevi, D.S.; Satyanarayana, C.; Rajagopalan, R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 2003, 3, 147–158. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chen, C.-F.; Chiou, W.-F. Suppression of rat neutrophil reactive oxygen species production and adhesion by the diterpenoid lactone andrographolide. Planta Med. 2000, 66, 314–317. [Google Scholar] [CrossRef]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Kemeny, D.M.; Leung, B.P.; Wong, W.F. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Romero, A.; Figueroa, J.; Cortés, P.; Concha, I.I.; Hancke, J.L.; Burgos, R.A. Andrographolide interferes with binding of nuclear factor-κB to DNA in HL-60-derived neutrophilic cells. Br. J. Pharmacol. 2005, 144, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Hu, S.-E.; Tan, S.-H.; Cao, R.; Chen, Y.; Xia, D.; Zhu, X.; Yang, X.-F.; Ong, C.-N.; Shen, H.-M. Andrographolide sensitizes cisplatin-induced apoptosis via suppression of autophagosome-lysosome fusion in human cancer cells. Autophagy 2012, 8, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Bothiraja, C.; Pawar, A.P.; Shende, V.S.; Joshi, P.P. Acute and subacute toxicity study of andrographolide bioactive in rodents: Evidence for the medicinal use as an alternative medicine. Comp. Clin. Pathol. 2013, 22, 1123–1128. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Malik, A.B.; Del Vecchio, P.J.; Keese, C.R.; Giaever, I. Electrical method for detection of endothelial cell shape change in real time: Assessment of endothelial barrier function. Proc. Natl. Acad. Sci. USA 1992, 89, 7919–7923. [Google Scholar] [CrossRef] [Green Version]
- Keese, C.R.; Giaever, I. A biosensor that monitors cell morphology with electrical fields. Eng. Med. Biol. Mag. IEEE 1994, 13, 402–408. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [Green Version]
- Opp, D.; Wafula, B.; Lim, J.; Huang, E.; Lo, J.-C.; Lo, C.-M. Use of electric cell–substrate impedance sensing to assess in vitro cytotoxicity. Biosens. Bioelectron. 2009, 24, 2625–2629. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-M.; Keese, C.R.; Giaever, I. Monitoring motion of confluent cells in tissue culture. Exp. Cell Res. 1993, 204, 102–109. [Google Scholar] [CrossRef]
- Andreadis, C.; Vahtsevanos, K.; Sidiras, T.; Thomaidis, I.; Antoniadis, K.; Mouratidou, D. 5-Fluorouracil and cisplatin in the treatment of advanced oral cancer. Oral Oncol. 2003, 39, 380–385. [Google Scholar] [CrossRef]
- Lee, K.-C.; Chang, H.-H.; Chung, Y.-H.; Lee, T.-Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264. 7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, J.; Xiao, L.; Tang, J.C.O.; Zhang, Y.; Wang, P.; Yang, M. Impedance studies of bio-behavior and chemosensitivity of cancer cells by micro-electrode arrays. Biosens. Bioelectron. 2009, 24, 1305–1310. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.-Y.; Huang, C.-C.; Chao, S.-C.; Chiang, C.-P.; Tang, B.-H.; Lee, S.-P.; Wang, J.-K. Real-Time Monitoring the Cytotoxic Effect of Andrographolide on Human Oral Epidermoid Carcinoma Cells. Biosensors 2022, 12, 304. https://doi.org/10.3390/bios12050304
Liao H-Y, Huang C-C, Chao S-C, Chiang C-P, Tang B-H, Lee S-P, Wang J-K. Real-Time Monitoring the Cytotoxic Effect of Andrographolide on Human Oral Epidermoid Carcinoma Cells. Biosensors. 2022; 12(5):304. https://doi.org/10.3390/bios12050304
Chicago/Turabian StyleLiao, Heng-Yi, Chun-Chung Huang, Shih-Chi Chao, Chien-Ping Chiang, Bo-Hsuan Tang, Shiao-Pieng Lee, and Jehng-Kang Wang. 2022. "Real-Time Monitoring the Cytotoxic Effect of Andrographolide on Human Oral Epidermoid Carcinoma Cells" Biosensors 12, no. 5: 304. https://doi.org/10.3390/bios12050304
APA StyleLiao, H.-Y., Huang, C.-C., Chao, S.-C., Chiang, C.-P., Tang, B.-H., Lee, S.-P., & Wang, J.-K. (2022). Real-Time Monitoring the Cytotoxic Effect of Andrographolide on Human Oral Epidermoid Carcinoma Cells. Biosensors, 12(5), 304. https://doi.org/10.3390/bios12050304




