Applicability of Selected 3D Printing Materials in Electrochemistry
Abstract
:1. Introduction
2. Experimental
2.1. 3D Printed Tested Objects
2.2. Chemicals
2.3. Apparatus
2.4. Procedures
2.5. Surface Imaging
3. Results and Discussion
3.1. Effect of Organic Solvents and Supporting Electrolytes on Mechanical and Physical Properties
3.2. Effect of Materials Exposure on the Electrochemical Properties of Electrolytes
3.3. Effect of Exposure of Supporting Electrolytes to 3DPMs on Voltammetric Behavior of Selected Redox Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-manufactured (3d-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.G.; Vaneckova, E.; Lachmanova, S.N.; Vivaldi, F.; Heyda, J.; Kubista, J.; Shestivska, V.; Spanel, P.; Schwarzova-Peckova, K.; Rathousky, J.; et al. The development of a fully integrated 3d printed electrochemical platform and its application to investigate the chemical reaction between carbon dioxide and hydrazine. Electrochim. Acta 2020, 360, 136984. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, G.D.; Quero, R.F.; Bressan, L.P.; Bonacin, J.A.; de Jesus, D.P.; da Silva, A.F. Ready-to-use 3d-printed electrochemical cell for in situ voltammetry of immobilized microparticles and Raman spectroscopy. Anal. Chim. Acta 2021, 1141, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Vaneckova, E.; Bousa, M.; Lachmanova, S.N.; Rathousky, J.; Gal, M.; Sebechlebska, T.; Kolivoska, V. 3d printed polylactic acid/carbon black electrodes with nearly ideal electrochemical behaviour. J. Electroanal. Chem. 2020, 857, 113745. [Google Scholar] [CrossRef]
- Sans, V. Emerging trends in flow chemistry enabled by 3d printing: Robust reactors, biocatalysis and electrochemistry. Curr. Opin. Green Sustain. Chem. 2020, 25, 100367. [Google Scholar] [CrossRef]
- Abdalla, A.; Patel, B.A. 3d-printed electrochemical sensors: A new horizon for measurement of biomolecules. Curr. Opin. Electrochem. 2020, 20, 78–81. [Google Scholar] [CrossRef]
- Hamzah, H.H.; Shafiee, S.A.; Abdalla, A.; Patel, B.A. 3d printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem. Commun. 2018, 96, 27–31. [Google Scholar] [CrossRef]
- Vaneckova, E.; Bousa, M.; Sokolova, R.; Moreno-Garcia, P.; Broekmann, P.; Shestivska, V.; Rathousky, J.; Gal, M.; Sebechlebska, T.; Kolivoska, V. Copper electroplating of 3d printed composite electrodes. J. Electroanal. Chem. 2020, 858, 113763. [Google Scholar] [CrossRef]
- Waseem, M.; Salah, B.; Habib, T.; Saleem, W.; Abas, M.; Khan, R.; Ghani, U.; Siddiqi, M.U.R. Multi-response optimization of tensile creep behavior of pla 3d printed parts using categorical response surface methodology. Polymers 2020, 12, 2962. [Google Scholar] [CrossRef]
- Kumar, M.B.; Sathiya, P. Methods and materials for additive manufacturing: A critical review on advancements and challenges. Thin-Walled Struct. 2021, 159, 107228. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; John, S.; Choudhury, N.R.; Dutta, N.K. Additive manufacturing of polymer materials: Progress, promise and challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Junpha, J.; Wisitsoraat, A.; Prathumwan, R.; Chaengsawang, W.; Khomungkhun, K.; Subannajui, K. Electronic tongue and cyclic voltammetric sensors based on carbon nanotube/polylactic composites fabricated by fused deposition modelling 3d printing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 117, 111319. [Google Scholar] [CrossRef] [PubMed]
- Choinska, M.; Hrdlicka, V.; Sestakova, I.; Navratil, T. Voltammetric determination of heavy metals in honey bee venom using hanging mercury drop electrode and pla/carbon conductive filament for 3d printer. Monatsh. Chem. 2021, 152, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Kadimisetty, K.; Rusling, J. 3d-printed miniaturized fluidic tools in chemistry and biology. TrAC-Trends Anal. Chem. 2018, 106, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3d-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and applications of 3d printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Kuhn, S.; Ameloot, R. 3d printing in chemical engineering and catalytic technology: Structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef]
- Xing, D.; Chen, L.; Ma, Q.; Hao, B.; Gutnikov, S.I.; Lazoryak, B.I.; Mader, E.; Ma, P.C. What happens to glass fiber under extreme chemical conditions? J. Non-Cryst. Solids 2020, 548, 120331. [Google Scholar] [CrossRef]
- Gu, H. Tensile behaviours of some high performance filaments after naoh treatment. Mater. Des. 2008, 29, 1893–1896. [Google Scholar] [CrossRef]
- Heikkinen, I.T.S.; Kauppinen, C.; Liu, Z.J.; Asikainen, S.M.; Spoljaric, S.; Seppala, J.V.; Savin, H.; Pearce, J.M. Chemical compatibility of fused filament fabrication-based 3-d printed components with solutions commonly used in semiconductor wet processing. Addit. Manuf. 2018, 23, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Salentijn, G.I.J.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused deposition modeling 3d printing for (bio)analytical device fabrication: Procedures, materials, and applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef] [Green Version]
- IUPAC. Compendium of Analytical Nomenclature. Available online: https://media.iupac.org/publications/analytical_compendium/ (accessed on 26 November 2021).
- Novotny, F.; Urbanova, V.; Plutnar, J.; Pumera, M. Preserving fine structure details and dramatically enhancing electron transfer rates in graphene 3d-printed electrodes via thermal annealing: Toward nitroaromatic explosives sensing. ACS Appl. Mater. Interfaces 2019, 11, 35371–35375. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Neumsteir, N.V.; Aparecido, G.D.; Ferraz, T.V.D.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3d printed pla-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Redondo, E.; Munoz, J.; Pumera, M. Green activation using reducing agents of carbon-based 3d printed electrodes: Turning good electrodes to great. Carbon 2021, 175, 413–419. [Google Scholar] [CrossRef]
- Wirth, D.M.; Sheaff, M.J.; Waldman, J.V.; Symcox, M.P.; Whitehead, H.D.; Sharp, J.D.; Doerfler, J.R.; Lamar, A.A.; LeBlanc, G. Electrolysis activation of fused-filament-fabrication 3d-printed electrodes for electrochemical and spectroelectrochemical analysis. Anal. Chem. 2019, 91, 5553–5557. [Google Scholar] [CrossRef]
- Browne, M.P.; Novotny, F.; Sofer, Z.; Pumera, M. 3d printed graphene electrodes’ electrochemical activation. ACS App. Mat. Interf. 2018, 10, 40294–40301. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Hermanova, S.; Sofer, Z.; Pumera, M. Proteinase- sculptured 3d-printed graphene/polylactic acid electrodes as potential biosensing platforms: Towards enzymatic modeling of 3d-printed structures dagger. Nanoscale 2019, 11, 12124–12131. [Google Scholar] [CrossRef]
- Fischer, J.; Barek, J.; Yosypchuk, B.; Navratil, T. Voltammetric determination of trace amounts of 2-methyl-4,6-dinitrophenol at a silver solid amalgam electrode. Electroanalysis 2006, 18, 127–130. [Google Scholar] [CrossRef]
- Erokhin, K.S.; Gordeev, E.G.; Ananikov, V.P. Revealing interactions of layered polymeric materials at solid-liquid interface for building solvent compatibility charts for 3d printing applications. Sci. Rep. 2019, 9, 20177. [Google Scholar] [CrossRef]
- Barek, J.; Fischer, J.; Navratil, T.; Peckova, K.; Yosypchuk, B.; Zima, J. Nontraditional electrode materials in environmental analysis of biologically active organic compounds. Electroanalysis 2007, 19, 2003–2014. [Google Scholar] [CrossRef]
- Navratil, T.; Yosypchuk, B.; Barek, J. A multisensor for electrochemical sequential autonomous automatic measurements. Chem. Anal.-Warsaw 2009, 54, 3–17. [Google Scholar]
- Lyu, S.P.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]

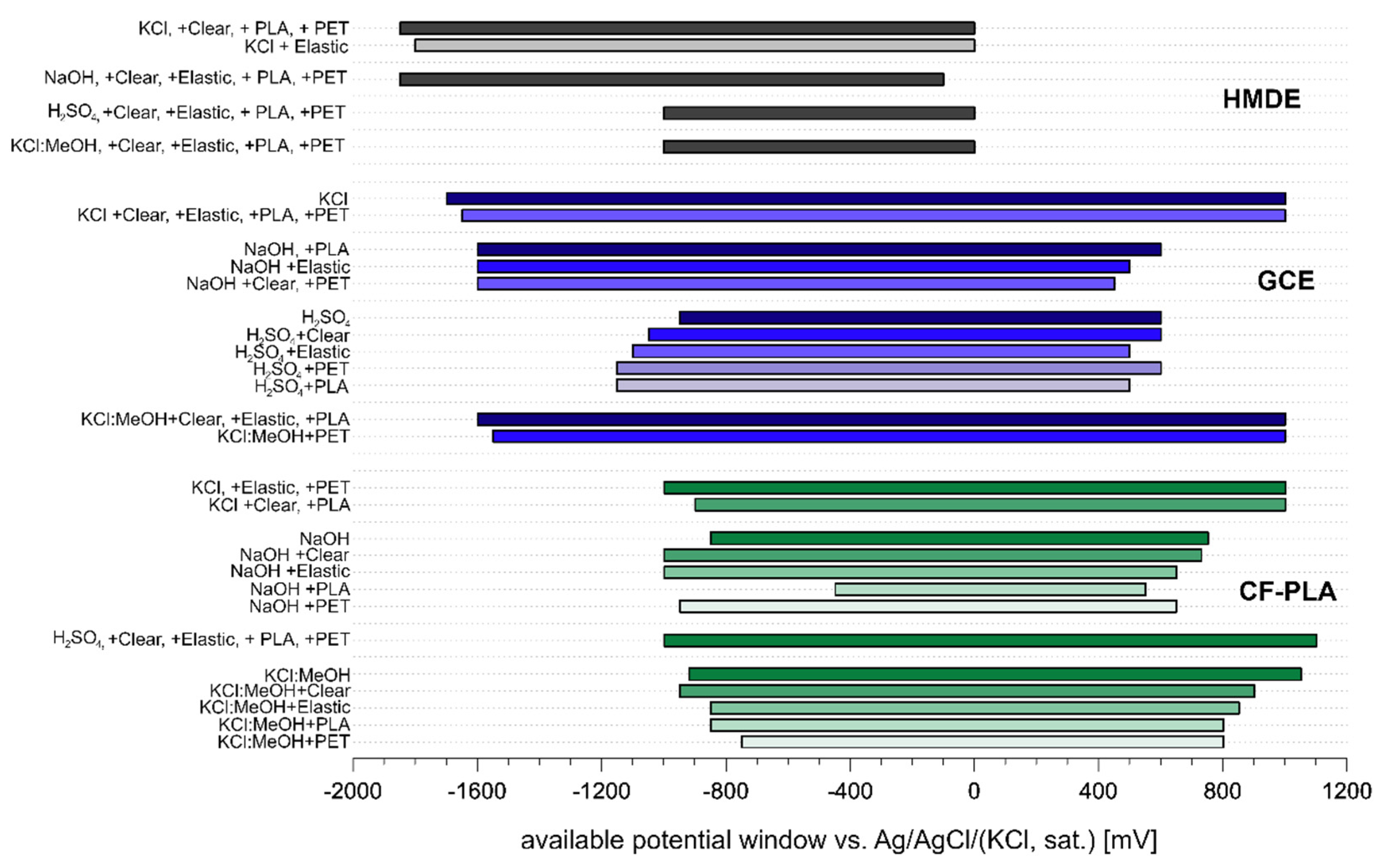
| Electrode: | HMDE | GCE | CF-PLA | |
|---|---|---|---|---|
| Solvent | Accumulation potential (Eacc) [mV] | |||
| 1 mol dm−3 KCl, 1 mol dm−3 NaOH | −1800 | −1600 | −1000 | |
| 1 mol dm−3 KCl: MeOH (9:1, v/v) | −1000 | −1600 | −1000 | |
| 1 mol dm−3 H2SO4 | −1000 | −1000 | −1000 | |
| Solvent | Material | |||
|---|---|---|---|---|
| Elastic | Clear | PET | PLA | |
| Acetone | 25 | 7 cracked | × soft | 13 delamination |
| Acetonitrile | 14 | 2 | × | 17 delamination |
| Chloroform | 53 | --- broken | --- dissolved | --- dissolved |
| Dichloromethane | 45 | 16 broken | --- dissolved | --- dissolved |
| Diethyl ether | 21 | × | × | × |
| Dimethyl sulfoxide | 24 | × | × soft | 5 soft |
| Ethanol | 33 | 2 | × | × |
| Ethyl acetate | 29 | × | × soft | 12 cracked, soft |
| Formaldehyde 38% | 7 | × | × | × |
| Hexane | 5 | × | × | × |
| Isopropanol | 29 | × | × | × |
| Methanol | 24 | 8 cracked | × | × |
| N,N-dimethylformamide | 38 | 3 deformation | 3 soft, deformation | 23 delamination |
| n-penthanol | 32 | × | × | × |
| Tetrahydrofuran | 46 | 4 deformation | --- partly dissolved | --- dissolved |
| Toluene | 33 | × | × | 8 cracked, soft, |
| Aqueous 1M KCl | × | × | × | × |
| Aqueous 1M NaOH | × | × | × | etched |
| Aqueous 1M H2SO4 | × | × | × | × |
| 1M KCl: MeOH (1:9, v/v) | 19 | 6 | × | × |
| Printing Material | Before | Elastic | Clear | PET | PLA | |
|---|---|---|---|---|---|---|
| Supporting electrolyte | 1 mol dm−3 KCl | |||||
| Cd2+; DPV | Ep [mV] | −589 | −584 | −589 | −586 | −589 |
| Jp [µA mm−2] | 1.16 | 1.09 | 1.32 | 1.21 | 1.16 | |
| Cd2+; DPASV | Ep [mV] | −587 | −580 | −584 | −582 | −592 |
| Jp [µA mm−2] | 2.78 | 1.90 | 2.12 | 1.99 | 2.21 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | −154 | −145 | −151 | −148 | −149 |
| Jp [µA mm−2] | 0.18 | 0.19 | 0.17 | 0.18 | 0.22 | |
| Supporting electrolyte | 1 mol dm−3 NaOH | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | −238 | - | −229 | - | - |
| Jp [µA mm−2] | 0.18 | <Cc | 0.04 | <Cc | <Cc | |
| Supporting electrolyte | 1 mol dm−3 H2SO4 | |||||
| Cd2+; DPV | Ep [mV] | −541 | - | −541 | −546 | −540 |
| Jp [µA mm−2] | 0.94 | <Cc | 0.43 | 0.43 | 0.46 | |
| Cd2+; DPASV | Ep [mV] | −587 | −493 | −589 | −589 | −588 |
| Jp [µA mm−2] | 2.15 | 0.28 | 2.10 | 1.79 | 2.85 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | −180 | −173 | −177 | −176 | −176 |
| Jp [µA mm−2] | 0.22 | 0.22 | 0.21 | 0.21 | 0.23 | |
| Supporting electrolyte | 1 mol dm−3 KCl: MeOH (1:9, v/v) | |||||
| Cd2+; DPV | Ep [mV] | −714 | - | −704 | −726 | −722 |
| Jp [µA mm−2] | 0.59 | <Cc | 0.68 | 2.98 | 2.59 | |
| Cd2+; DPASV | Ep [mV] | −647 | −639 | −636 | −636 | −659 |
| Jp [µA mm−2] | 1.57 | 0.32 | 0.10 | 0.14 | 1.11 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | −165 | −171 | −153 | −159 | −159 |
| Jp [µA mm−2] | 0.20 | 0.06 | 0.17 | 0.23 | 0.24 | |
| Printing Material | Before | Elastic | Clear | PET | PLA | |
|---|---|---|---|---|---|---|
| Supporting electrolyte | 1 mol dm−3 KCl | |||||
| Cd2+; DPV | Ep [mV] | –589 | –584 | –589 | –586 | –589 |
| Jp [µA mm−2] | 1.16 | 1.09 | 1.32 | 1.21 | 1.16 | |
| Cd2+; DPASV | Ep [mV] | –587 | –580 | –584 | –582 | –592 |
| Jp [µA mm−2] | 2.78 | 1.90 | 2.12 | 1.99 | 2.21 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | –154 | –145 | –151 | –148 | –149 |
| Jp [µA mm−2] | 0.18 | 0.19 | 0.17 | 0.18 | 0.22 | |
| [Fe(CN)6]3− | Ep [mV] | 233 | 233 | 232 | 230 | 229 |
| Jp [µA mm−2] | 2.04 | 2.10 | 2.24 | 2.20 | 2.26 | |
| Supporting electrolyte | 1 mol dm−3 NaOH | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | –238 | - | –229 | - | - |
| Jp [µA mm−2] | 0.18 | <Cc | 0.04 | <Cc | <Cc | |
| Supporting electrolyte | 1 mol dm−3 H2SO4 | |||||
| Cd2+; DPV | Ep [mV] | –541 | - | –541 | –546 | –540 |
| Jp [µA mm−2] | 0.94 | <Cc | 0.43 | 0.43 | 0.46 | |
| Cd2+; DPASV | Ep [mV] | –587 | –493 | –589 | –589 | –588 |
| Jp [µA mm−2] | 2.15 | 0.28 | 2.10 | 1.79 | 2.85 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | –180 | –173 | –177 | –176 | –176 |
| Jp [µA mm−2] | 0.22 | 0.22 | 0.21 | 0.21 | 0.23 | |
| [Fe(CN)6]3− | Ep [mV] | 271 | 263 | 264 | 268 | 268 |
| Jp [µA mm−2] | 2.00 | 1.94 | 2.04 | 1.84 | 1.92 | |
| Supporting electrolyte | 1 mol dm−3 KCl: MeOH (1:9, v/v) | |||||
| Cd2+; DPV | Ep [mV] | –714 | - | –704 | –726 | –722 |
| Jp [µA mm−2] | 0.59 | <Cc | 0.68 | 2.98 | 2.59 | |
| Cd2+; DPASV | Ep [mV] | –647 | –639 | –636 | –636 | –659 |
| Jp [µA mm−2] | 1.57 | 0.32 | 0.10 | 0.14 | 1.11 | |
| [Ru(NH3)6]3+; DPV | Ep [mV] | –165 | –171 | –153 | –159 | –159 |
| Jp [µA mm−2] | 0.20 | 0.06 | 0.17 | 0.23 | 0.24 | |
| [Fe(CN)6]3− | Ep [mV] | 154 | 129 | 179 | 116 | 163 |
| Jp [µA mm−2] | 0.17 | 0.04 | 0.22 | 0.14 | 0.12 | |
| Printing Material | Before | Elastic | Clear | PET | PLA | |
|---|---|---|---|---|---|---|
| Supporting electrolyte | 1 mol dm−3 KCl | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | –158 | –163 | –158 | –158 | –158 |
| Jp [µA mm−2] | 0.138 | 0.093 | 0.097 | 0.129 | 0.130 | |
| [Fe(CN)6]3–; DPV | Ep [mV] | 519 | 439 | 439 | 413 | 435 |
| Jp [µA mm−2] | 0.002 | 0.004 | 0.002 | 0.002 | 0.002 | |
| Supporting electrolyte | 1 mol dm−3 NaOH | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | –189 | –192 | –197 | –203 | –180 |
| Jp [µA mm−2] | 0.059 | 0.030 | 0.052 | 0.066 | 0.051 | |
| [Fe(CN)6]3–; DPV | Ep [mV] | 339 | 240 | 234 | 242 | 229 |
| Jp [µA mm−2] | 0.007 | 0.131 | 0.133 | 0.069 | 0.111 | |
| Supporting electrolyte | 1 mol dm−3 H2SO4 | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | –198 | –204 | –201 | –201 | –223 |
| Jp [µA mm−2] | 0.037 | 0.023 | 0.022 | 0.023 | 0.022 | |
| [Fe(CN)6]3–; DPV | Ep [mV] | 237 | 304 | 303 | 256 | 296 |
| Jp [µA mm−2] | 0.014 | 0.011 | 0.009 | 0.011 | 0.010 | |
| Supporting electrolyte | 1 mol dm−3 KCl: MeOH (1:9, v/v) | |||||
| [Ru(NH3)6]3+; DPV | Ep [mV] | –141 | –250 | –149 | –170 | –170 |
| Jp [µA mm−2] | 0.517 | 0.631 | 0.502 | 0.577 | 0.415 | |
| [Fe(CN)6]3–; DPV | Ep [mV] | 299 | 53 | 107 | - | - |
| Jp [µA mm−2] | 0.005 | 0.183 | 0.048 | <Cc | <Cc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choińska, M.; Hrdlička, V.; Dejmková, H.; Fischer, J.; Míka, L.; Vaněčková, E.; Kolivoška, V.; Navrátil, T. Applicability of Selected 3D Printing Materials in Electrochemistry. Biosensors 2022, 12, 308. https://doi.org/10.3390/bios12050308
Choińska M, Hrdlička V, Dejmková H, Fischer J, Míka L, Vaněčková E, Kolivoška V, Navrátil T. Applicability of Selected 3D Printing Materials in Electrochemistry. Biosensors. 2022; 12(5):308. https://doi.org/10.3390/bios12050308
Chicago/Turabian StyleChoińska, Marta, Vojtěch Hrdlička, Hana Dejmková, Jan Fischer, Luděk Míka, Eva Vaněčková, Viliam Kolivoška, and Tomáš Navrátil. 2022. "Applicability of Selected 3D Printing Materials in Electrochemistry" Biosensors 12, no. 5: 308. https://doi.org/10.3390/bios12050308
APA StyleChoińska, M., Hrdlička, V., Dejmková, H., Fischer, J., Míka, L., Vaněčková, E., Kolivoška, V., & Navrátil, T. (2022). Applicability of Selected 3D Printing Materials in Electrochemistry. Biosensors, 12(5), 308. https://doi.org/10.3390/bios12050308





