An Artifact-Resistant Feature SKNAER for Quantifying the Burst of Skin Sympathetic Nerve Activity Signal
Abstract
:1. Introduction
- (1)
- To more accurately detect the burst area, we proposed a method based on TKE operator and envelope and integral signal to detect the burst area. In addition, we proposed to discriminate the ECG artifacts based on QRS complexes.
- (2)
- To accurately quantify the signal, we proposed a new feature, SKNAER, for SNA evaluation based on the detected burst area. We compared SKNAER with aSKNA in the hemodialysis clinical experiments. HRV features related to SNA were calculated simultaneously based on ECG for the comprehensive comparison.
2. Materials and Methods
2.1. Experimental Design
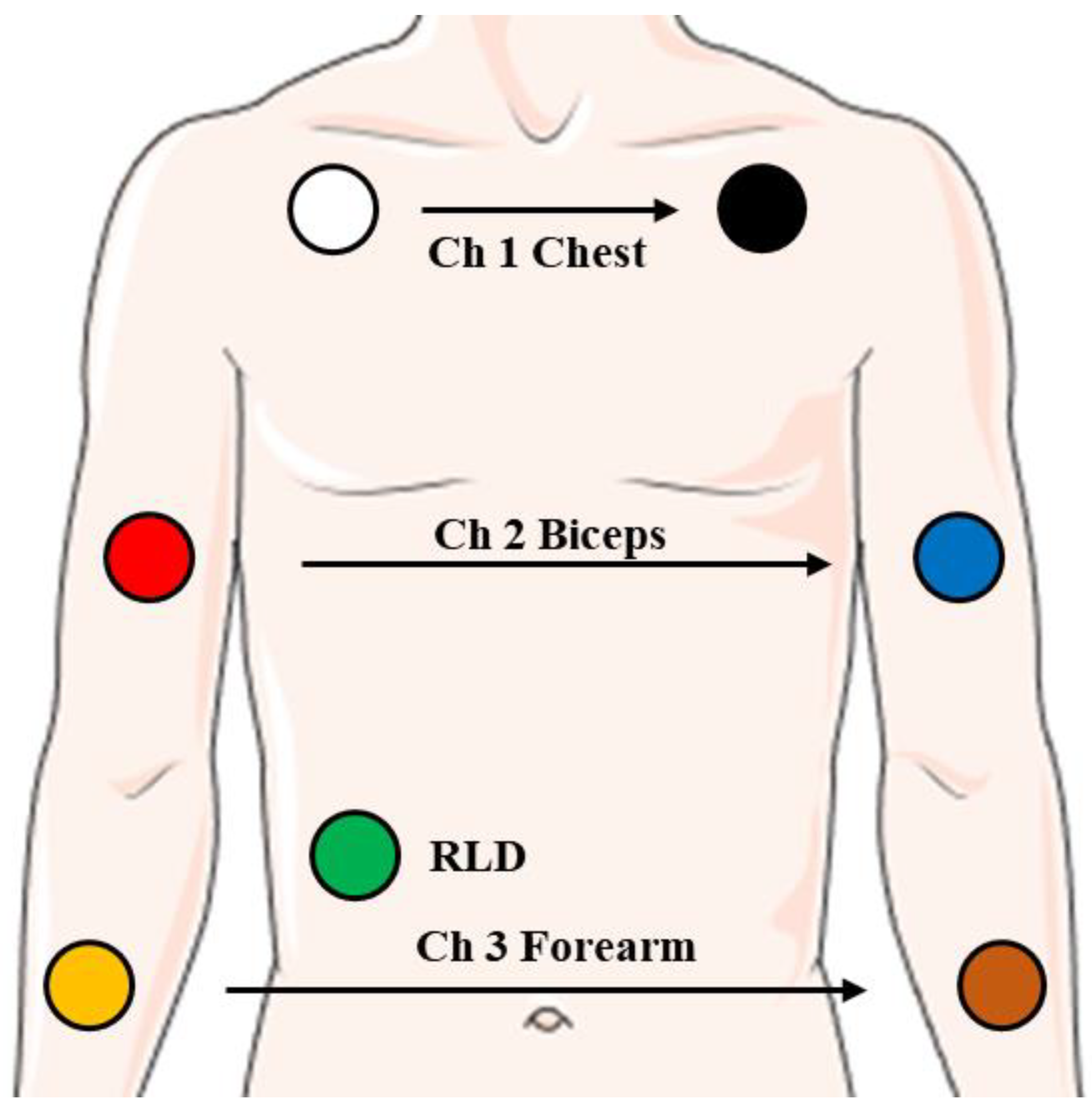
2.1.1. Experimental Setup
2.1.2. Experimental Protocol
- Experiment 1: Standard SKNA signal
- Experiment 2: Clinical SKNA signals
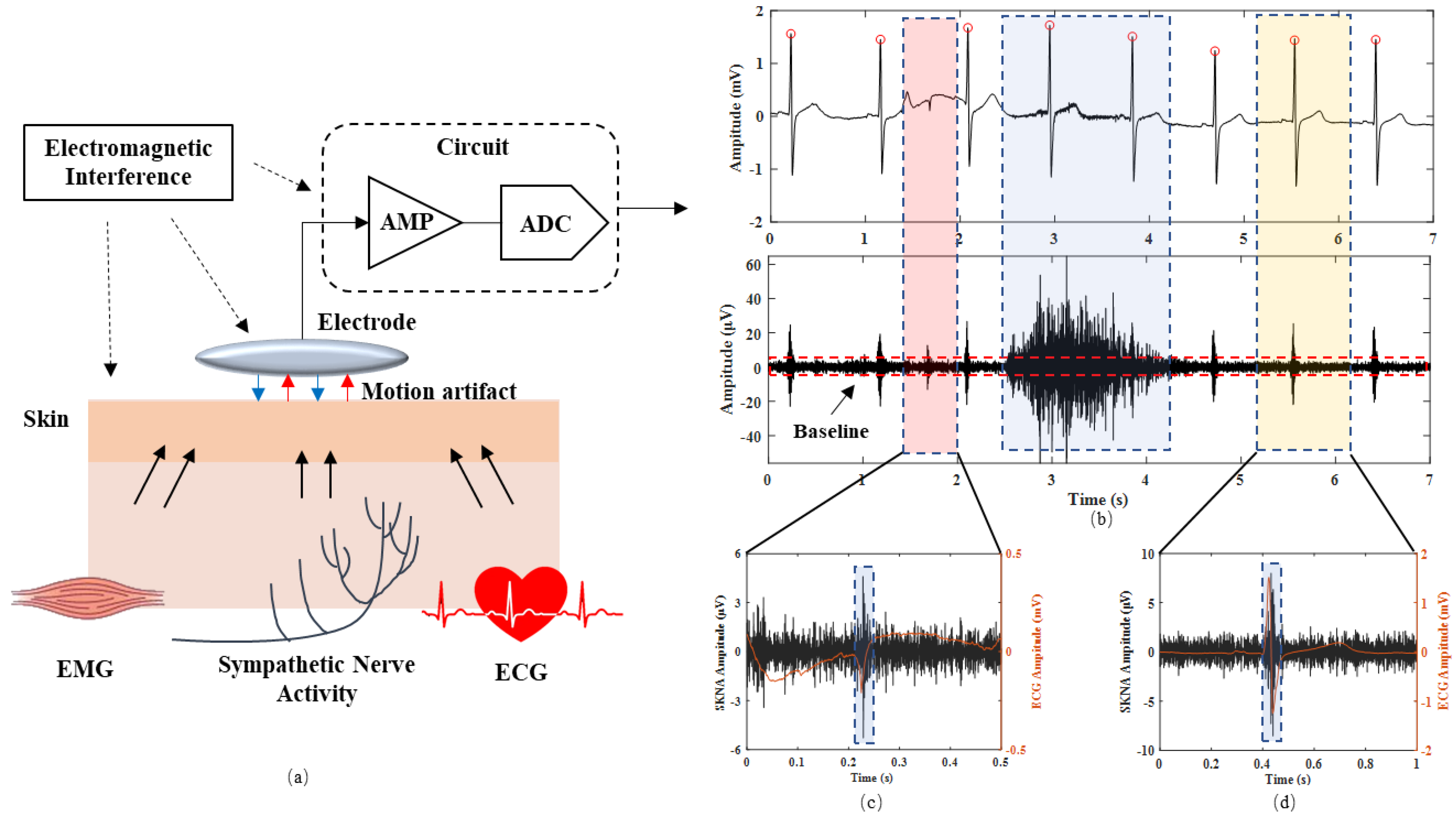
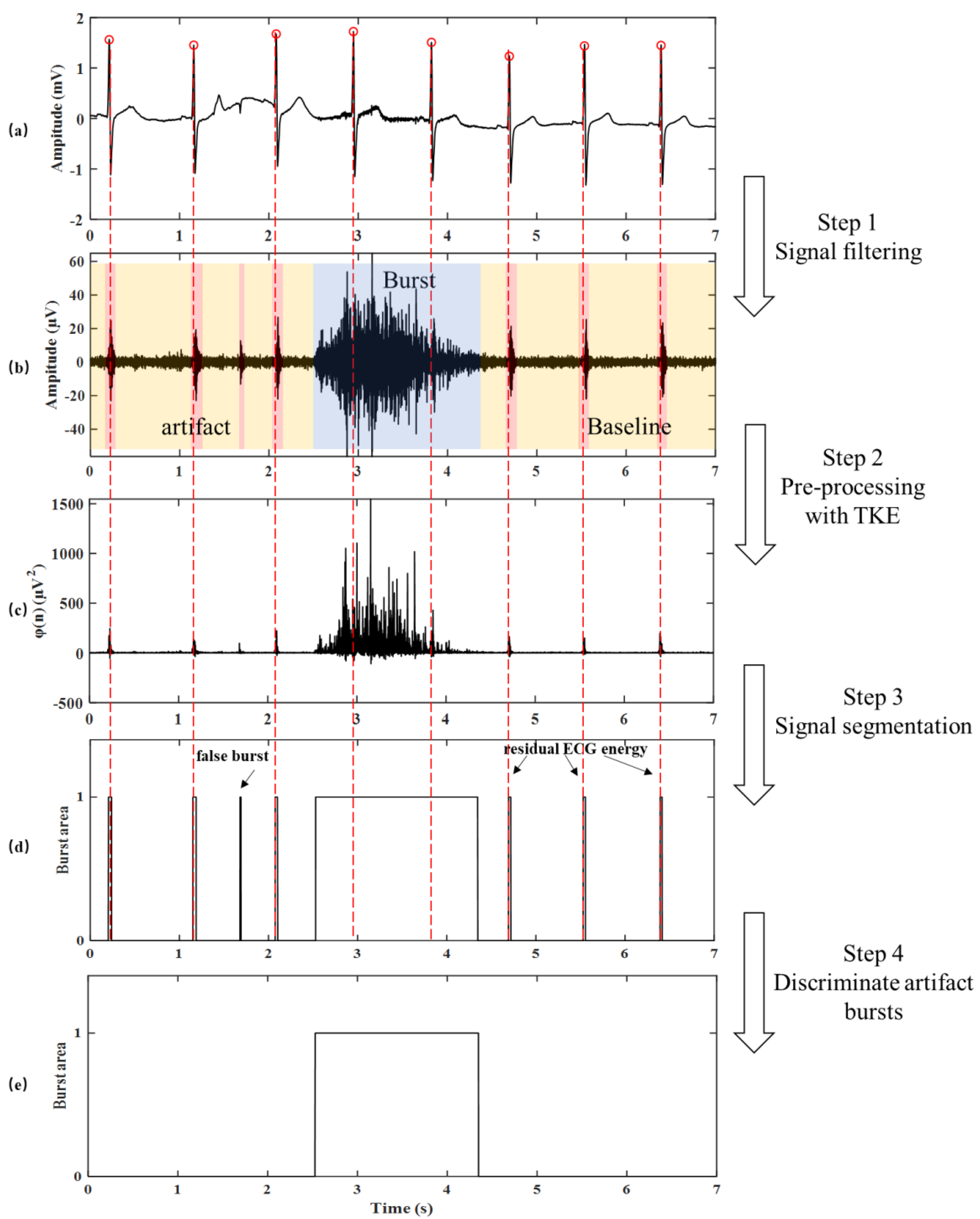
2.2. Burst Detection Method with iSKNA
2.3. Optimized Burst Detection Method
2.3.1. Teager–Kaiser Energy Operator
2.3.2. Signal Segmentation
2.3.3. Discrimination of Artifact Bursts
2.4. SKNA Energy Ratio
2.5. Evaluation Methods
2.6. Reference Features
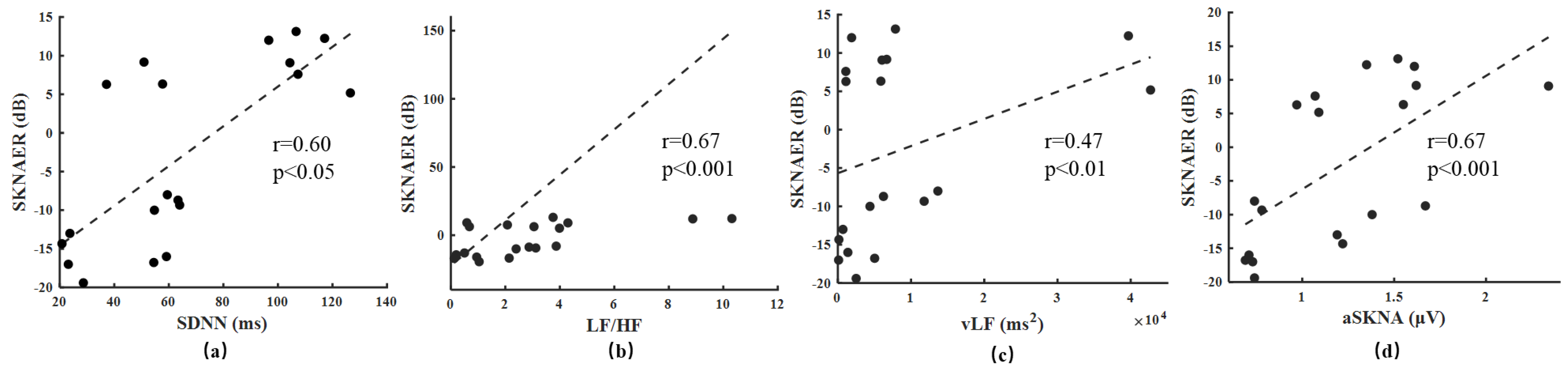
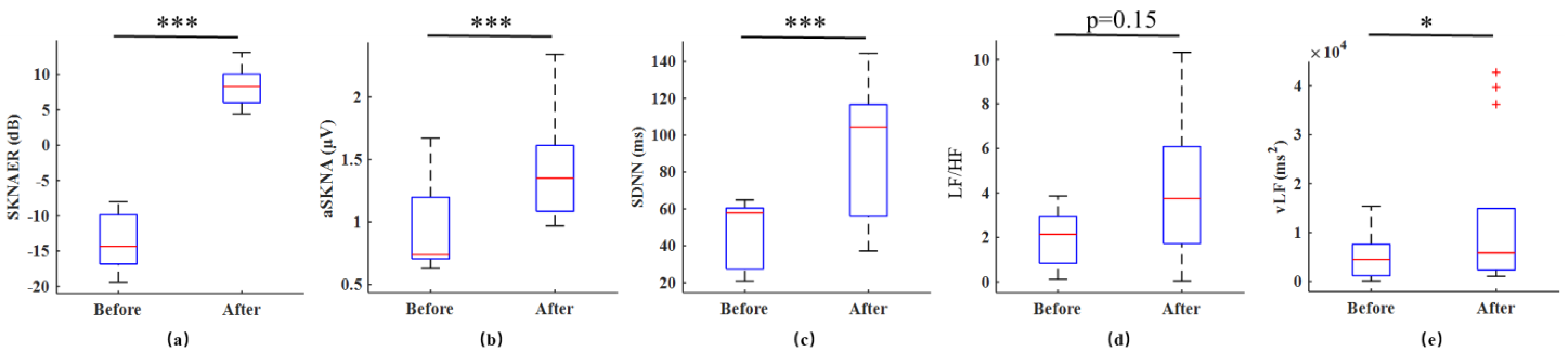
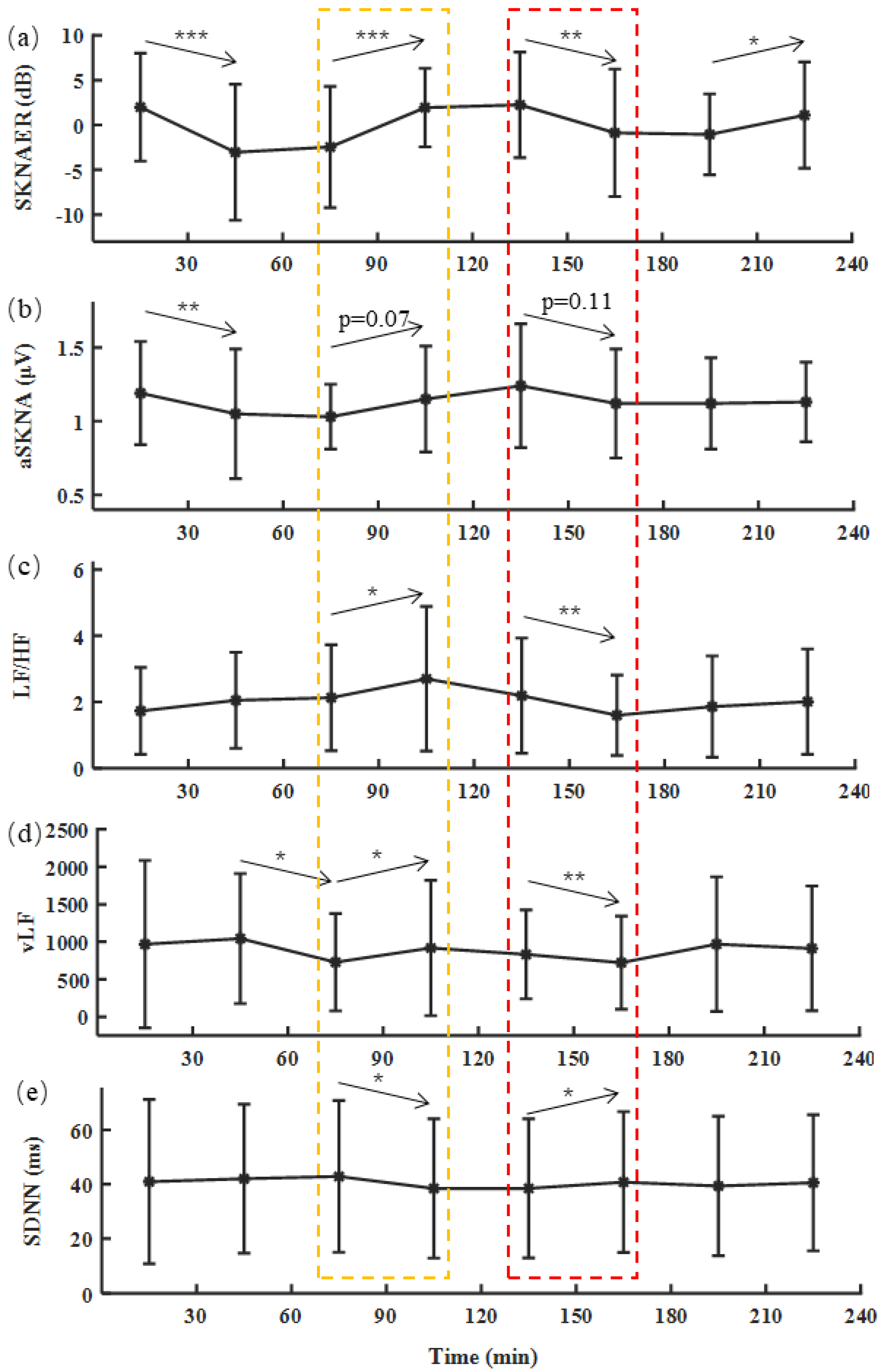
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs); World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- McGill, H.; McMahan, C. Preventing heart disease in the 21st century: Implications of the pathobiological determinants of atherosclerosis in youth (PDAY) study. Circulation 2008, 117, 1216–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Hanna, P.; Rajendran, P.; Shivkumar, K. Neuromodulation for ventricular tachycardia and atrial fibrillation: A clinical scenario-based review. JACC-Clin. Electrophysiol. 2019, 5, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic nervous system dysfunction. JACC Focus Semin. 2019, 73, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Hagbarth, K.; Vallbo, A. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol. Scand. 1968, 74, 96–108. [Google Scholar] [CrossRef]
- Linz, D.; Mahfoud, F.; Schotten, U.; Ukena, C.; Hohl, M.; Neuberger, H.; Wirth, K.; Böhm, M. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension 2013, 61, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.; Dave, A.; Tan, A.; Gholmieh, G.; Zhou, S.; Wang, D.; Akingba, G.; Fishbein, G.; Montemagno, G.; Lin, S.; et al. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm 2006, 3, 78–85. [Google Scholar] [CrossRef]
- Robinson, E.; Rhee, K.; Tan, A.; Gholmieh, G.; Zhou, S.; Wang, D.; Akingba, G.; Fishbein, G.; Montemagno, C.; Lin, S.; et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. J. Cardiovasc. Electrophysiol. 2015, 26, 70–78. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power spectral density analysis of electrodermal activity for sympathetic function assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Chon, K.H. Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity. Am. J. Physiol. 2016, 311, 582–591. [Google Scholar] [CrossRef] [Green Version]
- Doytchinova, A.; Hassel, J.; Yuan, Y.; Lin, H.; Yin, D.; Adams, D.; Straka, S.; Wright, K.; Smith, K.; Wagner, D.; et al. Simultaneous non-invasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 2017, 14, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Kusayama, T.; Wong, J.; Liu, X.; He, W.; Doytchinova, A.; Robinson, E.; Adams, D.; Chen, L.; Lin, S.; Davoren, K.; et al. Simultaneous non-invasive recording of electrocardiogram and skin sympathetic nerve activity (neuECG). Nat. Protoc. 2020, 5, 1853–1877. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, J.; Hu, Z.; Li, Y.; Zhang, Y.; Cui, C.; Cai, C.; Liu, C. A portable neuECG monitoring system for cardiac sympathetic nerve activity assessment. In Proceedings of the 2020 International Conference on Sensing, Measurement & Data Analytics in the era of Artificial Intelligence (ICSMD), Xi’an, China, 15–17 October 2020; pp. 407–412. [Google Scholar]
- Liu, X.; Rabin, P.; Yuan, Y.; Kumar, A.; Vasallo, P.; Wong, J.; Mitscher, G.; Everett, T.; Chen, P. Effects of anesthetic and sedative agents on sympathetic nerve activity. Heart Rhythm 2019, 16, 1875–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Zhao, Y.; Doytchinova, A.; Kamp, N.; Tsai, W.; Yuan, Y.; Adams, D.; Wagner, D.; Shen, C.; Chen, L.; et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm 2015, 12, 1324–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaydens, E.; Taylor, J.A.; Michael, A.C.; Uri, T.E. Characterization and modeling of muscle sympathetic nerve spiking. IEEE Trans. Biomed. Eng. 2013, 60, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Beckman, L.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Di, J.; Xing, Y.; Li, Y.; Li, J. Wearable ECG: History, key technologies, and future challenges. Chin. J. Biomed. Eng. 2019, 38, 641–652. [Google Scholar]
- Diedrich, A.; Charoensuk, W.; Brychta, W.; Ertl, A.; Shiavi, R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: Wavelet analysis in microneurography. IEEE Trans. Biomed. Eng. 2003, 50, 41–50. [Google Scholar] [CrossRef]
- Okamura, S.; Yonezawa, Y.; Ninomiya, I. A new segmentation method of synchronized sympathetic nerve activity. Eng. Med. Biol. Soc. 1995, 2, 1003–1004. [Google Scholar]
- Brychta, R.J.; Tuntrakool, S.; Appalsamy, M.; Keller, N.; Robertson, D.; Shiavi, R.; Diedrich, A. Wavelet methods for spike detection in mouse renal sympathetic nerve activity. IEEE Trans. Biomed. Eng. 2007, 54, 82–93. [Google Scholar] [CrossRef]
- Liu, C.; Lee, C.; Lin, S.; Tsai, W. Temporal clustering of skin sympathetic nerve activity bursts in acute myocardial infarction patients. Front. Neurosci. 2021, 15, 1501. [Google Scholar] [CrossRef]
- Victor, R.; Leimbach, W.; Seals, D.; Wallin, B.; Mark, A. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 1987, 9, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, A. A simple test of cardiac function based upon the heart rate changes induced by the valsalva maneuver. Am. J. Cardiol. 1966, 18, 90–99. [Google Scholar] [CrossRef]
- Lemyre, C.; Jelinek, M.; Lefebvre, R. New approach to voiced onset detection in speech signal and its application for frame error concealment. In Proceedings of the 2008 IEEE International Conference on Acoustics, Speech and Signal Processing, Las Vegas, NV, USA, 31 March–4 April 2008; pp. 4757–4760. [Google Scholar]
- Kaiser, J. A simple algorithm to calculate the energy of a signal. In Proceedings of the IEEE International Conference Acoustics, Speech, and Signal Processing, Albuquerque, NM, USA, 3–6 April 1990; pp. 381–384. [Google Scholar]
- Liu, C.; Zhang, X.; Zhao, L.; Liu, F.; Chen, X.; Yao, Y.; Li, J. Signal quality assessment and lightweight QRS detection for wearable ECG SmartVest system. IEEE Internet Things J. 2019, 6, 1363–1374. [Google Scholar] [CrossRef]
- Zong, W.; Moody, G.B.; Jiang, D. A robust open-source algorithm to detect onset and duration of QRS complexes. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003; pp. 737–740. [Google Scholar]
- Liu, C.; Li, P.; Maria, C.; Zhao, L.; Zhang, H.; Chen, Z. A multi-step method with signal quality assessment and fine-tuning procedure to locate maternal and fetal QRS complexes from abdominal ECG recordings. Physiol. Meas. 2014, 35, 1665–1683. [Google Scholar] [CrossRef]
- Rashid, U.; Niazi, I.K.; Signal, N.; Farina, D.; Taylor, D. Optimal automatic detection of muscle activation intervals. J. Electromyogr. Kinesiol. 2019, 48, 103–111. [Google Scholar] [CrossRef]
- Bonato, P.; D’Alessio, T.; Knaflitz, M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans. Biomed. Eng. 1998, 45, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, T.; Douglas, A.; Wan, J.; Doytchinova, A.; Wong, J.; Mitscher, G.; Straka, S.; Shen, C.; Everett, T.; Chen, P. Skin sympathetic nerve activity and ventricular rate control during atrial fibrillation. Heart Rhythm 2020, 17, 544–552. [Google Scholar] [CrossRef]
- Kusayama, T.; Wan, J.; Doytchinova, A.; Wong, J.; Kabir, R.; Mitscher, G.; Straka, S.; Shen, C.; Everett, T.; Chen, P. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI Insight 2019, 4, e125853. [Google Scholar] [CrossRef] [Green Version]
- Vinik, A.; Maser, R.; Mitchell, B.; Freeman, R. Diabetic autonomic neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [Green Version]
- Moak, J.; Goldstein, D.; Eldadah, B.; Saleem, A.; Holmes, C.; Pechnik, S.; Sharabi, Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 2007, 4, 1523–1529. [Google Scholar] [CrossRef]
- Sundlöf, G.; Wallin, B. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J. Physiol. 1978, 274, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Sundlöf, G.; Wallin, B. Muscle-nerve sympathetic activity in man. Relationship to blood pressure in resting normo- and hyper-tensive subjects. Clin. Sci. Mol. Med. 1978, 4, 387–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, Y.; Matsukawa, T.; Suzuki, H.; Iwase, S.; Mano, T. A new method of quantifying human muscle sympathetic nerve activity for frequency domain analysis. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 121–128. [Google Scholar] [CrossRef]
- Federico, L.; Heikki, H.; Scgnudt, G.; Malik, M. Short-term heart rate variability: Easy to measure, difficult to interpret. Heart Rhythm 2018, 15, 1559–1560. [Google Scholar]
- Fred, S.; Zachary, M.; Christopher, L. A critical review of ultra-short-term heart rate variability norms research. Front. Neurosci. 2020, 14, 594880. [Google Scholar]
- Zhang, Y.; Wang, J.; Xing, Y.; Cui, C.; Cheng, H.; Chen, Z.; Chen, H.; Liu, C.; Wang, N.; Chen, M. Dynamics of cardiac autonomic response during hemodialysis measured by heart rate variability and skin sympathetic nerve activity: The impact of interdialytic weight gain. Front. Physiol. 2022, 13, 890536. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Y.; Yang, C.; Li, J.; Li, Y.; Cui, C.; Li, J.; Cheng, H.; Fang, Y.; Cai, C.; et al. Design and evaluation of an autonomic nerve monitoring system based on skin sympathetic nerve activity. Biomed. Signal Process. Control 2022, 76, 103681. [Google Scholar] [CrossRef]
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |
| Age/years | 25.1 | 4.6 | 58.9 | 14.6 |
| Height/cm | 173.2 | 6.5 | 170.2 | 10.3 |
| Weight/kg | 71.0 | 13.6 | 70.5 | 13.9 |
| Weight/kg (after dialysis) | 67.9 | 13.6 | ||
| Cohort size | 10 | 20 | ||
| Position | TP | FN | FP | DR (%) | p-Value | CO (%) | p-Value | P+ (%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ch1 Chest | Proposed method | 159 | 0 | 9 | 100.0 ± 0 [100.0 100.0] | 0.18 | 96.4 ± 1.2 [95.6 97.2] | *** | 94.2 ± 5.0 [91.9 97.9] | *** |
| With iSKNA [12] | 157 | 2 | 103 | 98.8 ± 2.6 [97.2 100.2] | 92.2 ± 1.7 [91.4 92.8] | 59.9 ± 3.6 [58.2 62.4] | ||||
| Ch2 Biceps | Proposed method | 111 | 3 | 18 | 96.4 ± 5.5 [95.6 99.3] | * | 92.3 ± 2.1 [91.6 92.9] | *** | 87.3 ± 7.4 [85.3 89.4] | *** |
| With iSKNA [12] | 100 | 14 | 47 | 87.1 ± 11.0 [84.5 92.5] | 87.6 ± 2.4 [87.0 88.6] | 67.6 ± 9.3 [63.9 70.5] | ||||
| Ch3 Forearm | Proposed method | 147 | 2 | 10 | 98.7 ± 3.2 [97.0 100.3] | 0.34 | 94.2 ± 1.3 [93.3 94.9] | *** | 93.7 ± 2.6 [92.3 95.4] | *** |
| With iSKNA [12] | 146 | 3 | 94 | 97.8 ± 5.8 [94.2 101.0] | 91.0 ± 0.9 [90.5 91.5] | 60.5 ± 5.8 [57.1 63.8] | ||||
| Summary | Proposed method | 417 | 4 | 46 | 98.2 ± 3.9 [97.7 99.6] | ** | 94.3 ± 2.3 [93.6 95.0] | *** | 91.8 ± 6.2 [89.9 93.9] | *** |
| With iSKNA [12] | 403 | 18 | 244 | 94.5 ± 8.9 [91.7 97.2] | 90.3 ± 2.6 [89.5 91.0] | 62.8 ± 7.3 [60.4 65.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Zhang, Y.; Xiao, Z.; Yang, C.; Li, J.; Cui, C.; Wang, J.; Chen, H.; Li, J.; Liu, C. An Artifact-Resistant Feature SKNAER for Quantifying the Burst of Skin Sympathetic Nerve Activity Signal. Biosensors 2022, 12, 355. https://doi.org/10.3390/bios12050355
Xing Y, Zhang Y, Xiao Z, Yang C, Li J, Cui C, Wang J, Chen H, Li J, Liu C. An Artifact-Resistant Feature SKNAER for Quantifying the Burst of Skin Sympathetic Nerve Activity Signal. Biosensors. 2022; 12(5):355. https://doi.org/10.3390/bios12050355
Chicago/Turabian StyleXing, Yantao, Yike Zhang, Zhijun Xiao, Chenxi Yang, Jiayi Li, Chang Cui, Jing Wang, Hongwu Chen, Jianqing Li, and Chengyu Liu. 2022. "An Artifact-Resistant Feature SKNAER for Quantifying the Burst of Skin Sympathetic Nerve Activity Signal" Biosensors 12, no. 5: 355. https://doi.org/10.3390/bios12050355
APA StyleXing, Y., Zhang, Y., Xiao, Z., Yang, C., Li, J., Cui, C., Wang, J., Chen, H., Li, J., & Liu, C. (2022). An Artifact-Resistant Feature SKNAER for Quantifying the Burst of Skin Sympathetic Nerve Activity Signal. Biosensors, 12(5), 355. https://doi.org/10.3390/bios12050355






