Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
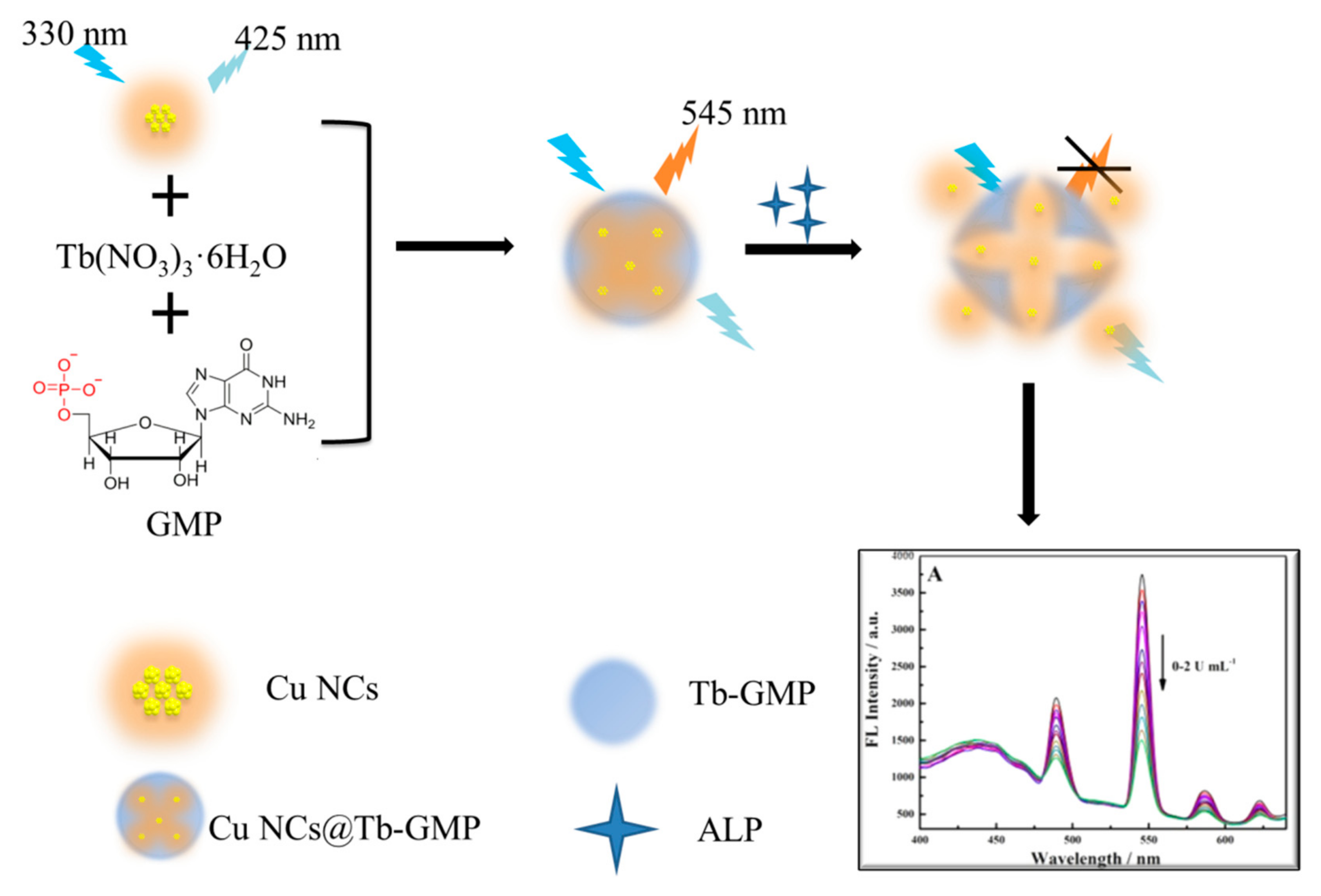
2.3. Synthesis of Nanocomposites
2.3.1. Synthesis of Cu NCs
2.3.2. Synthesis of Tb-GMP and the Cu NCs@Tb-GMP Ratiometric Fluorescent Probe
2.4. Construction of the Ratiometric Fluorescent Sensor
2.5. Fluorescence Assay of ALP in Real Samples
3. Results and Discussion
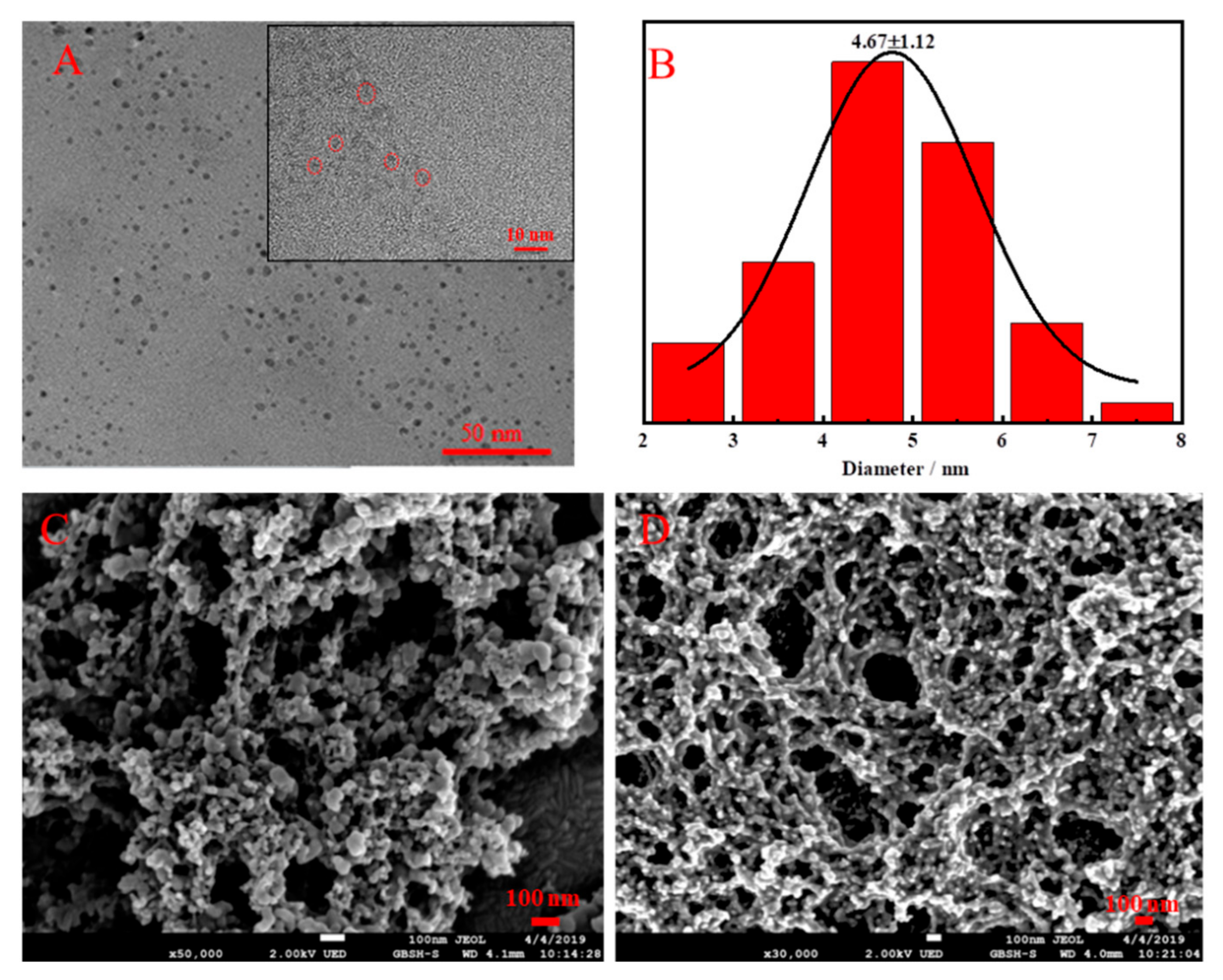
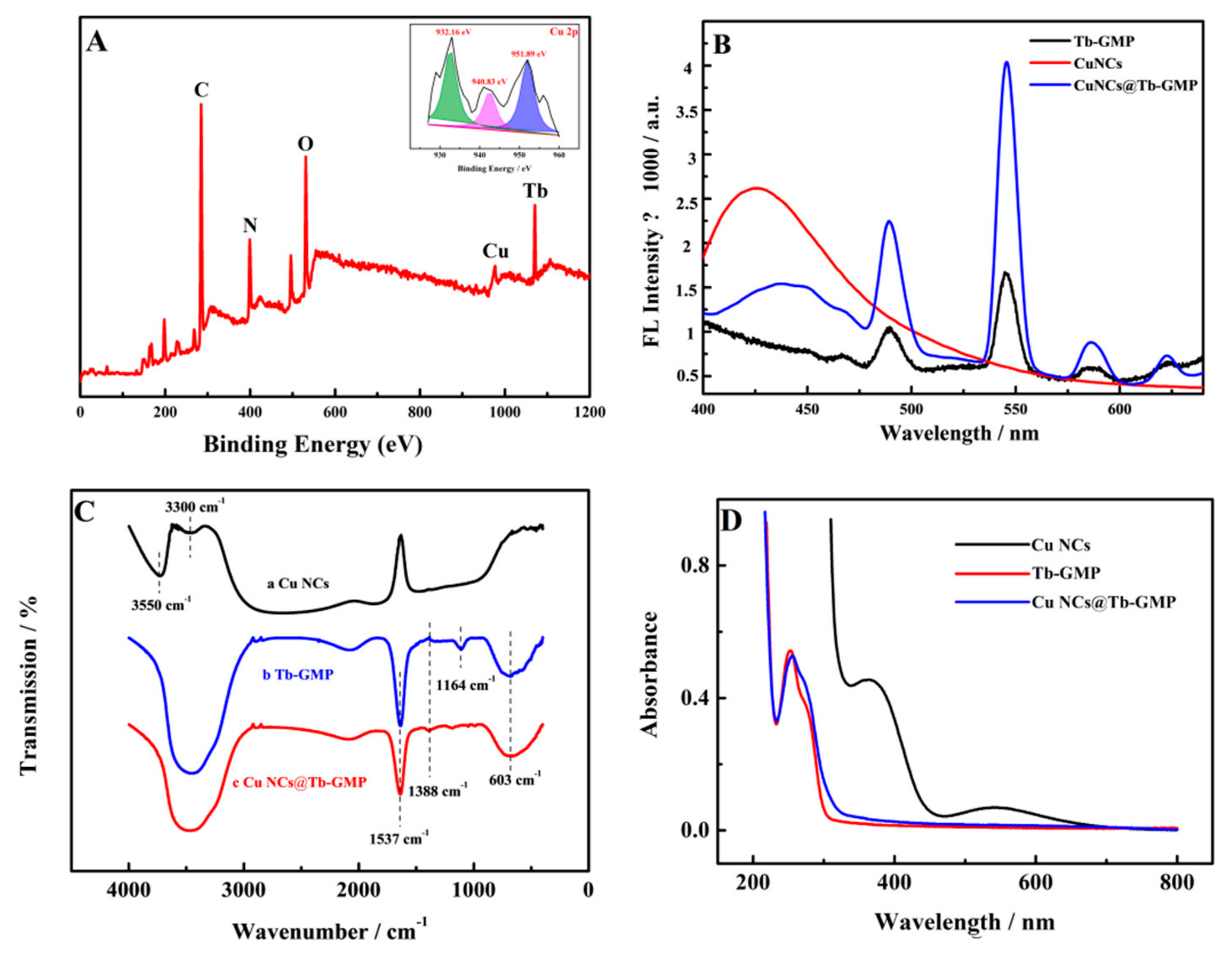
3.1. Characterization of the Cu NCs, Tb-GMP and Cu NCs@Tb-GMP
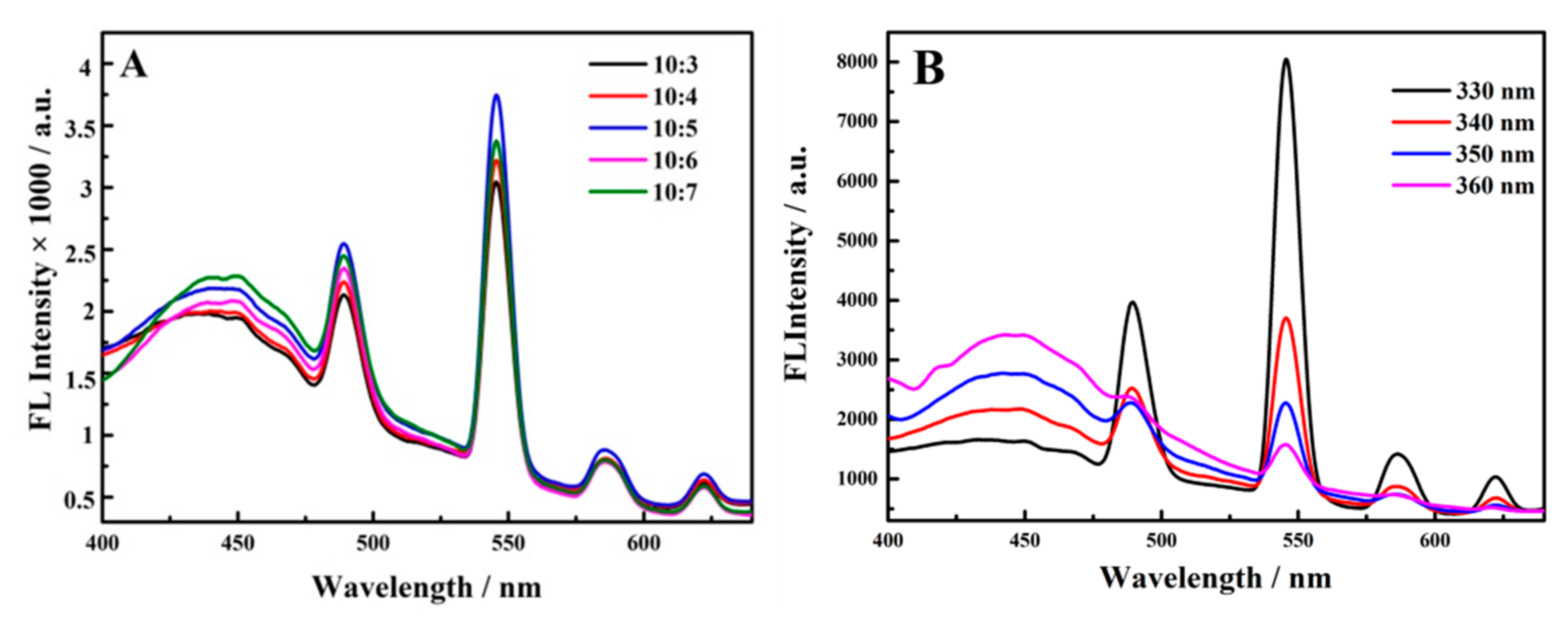
3.2. Optimization Assay
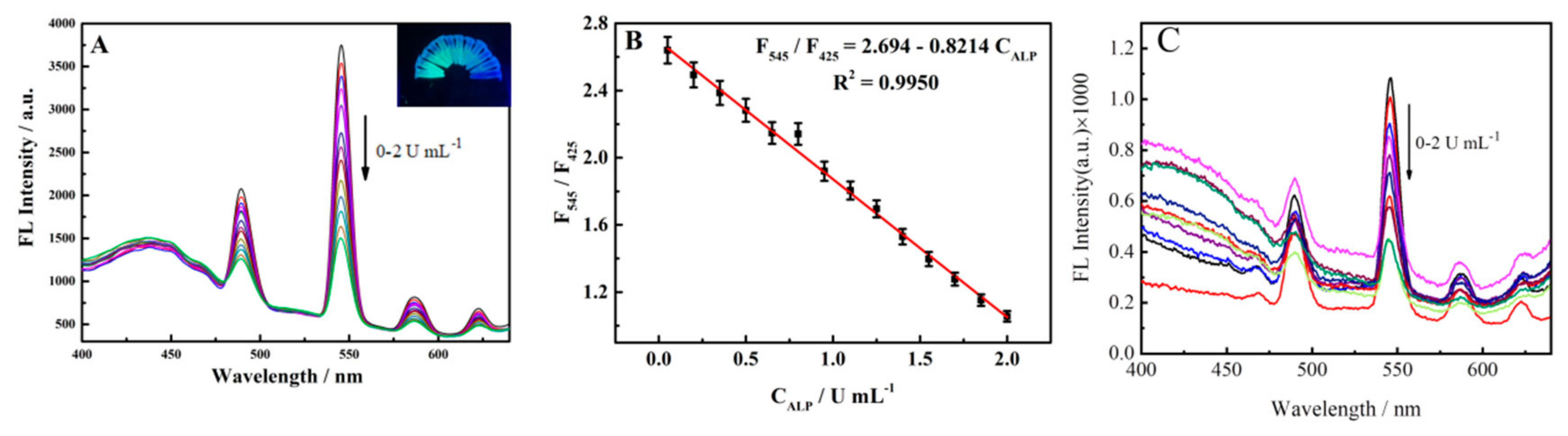
3.3. Linearity of the ALP Ratiometric Fluorescent Sensor in ALP Detection
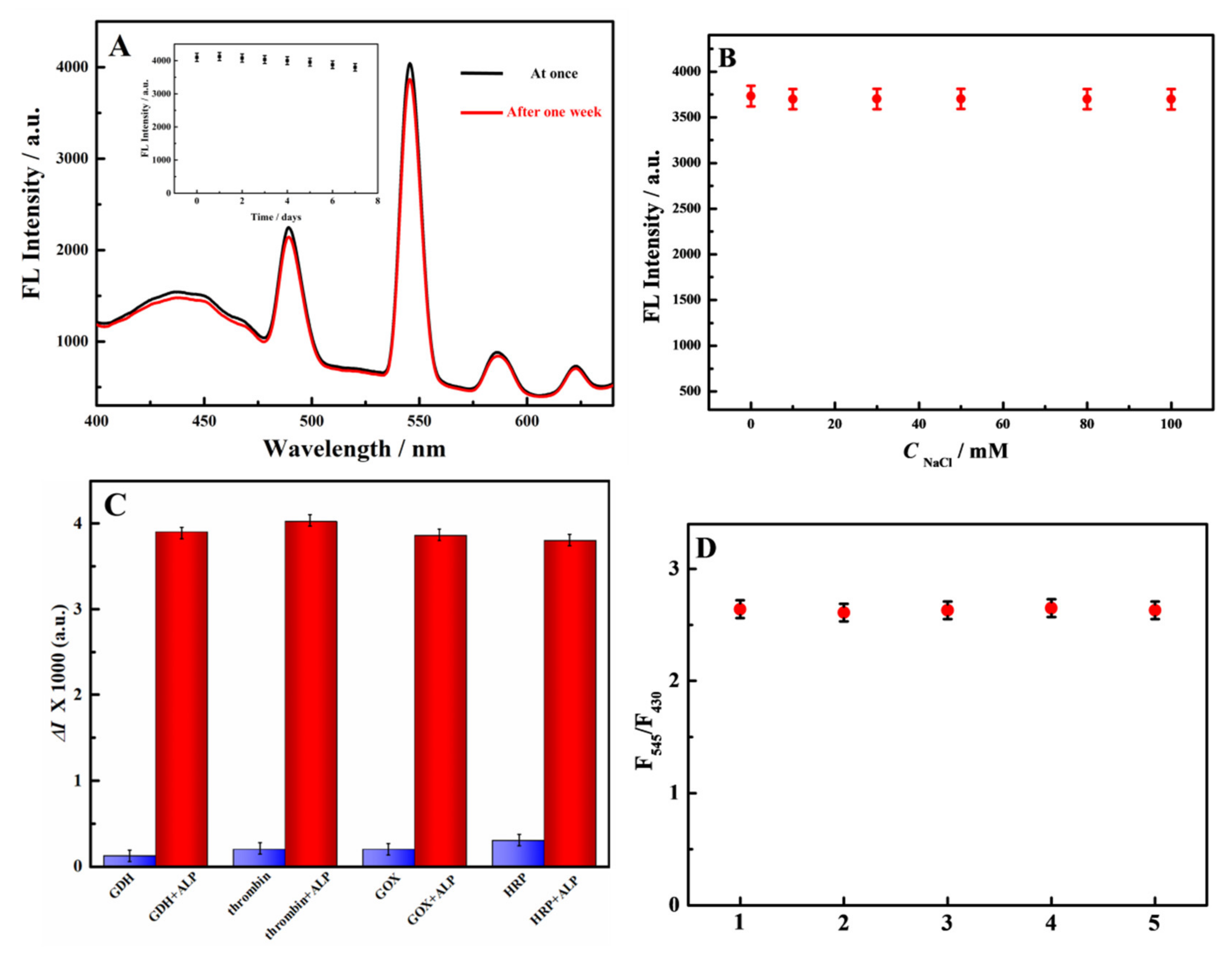
3.4. Stability, Salt Tolerance, Selectivity and Repeatability of the Probe
3.5. Detection of ALP in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budria, A. Beyond troubled waters: The influence of eutrophication on host–parasite interactions. Funct. Ecol. 2017, 31, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Pakhomova, S.; Yakushev, E.; Protsenko, E.; Rigaud, S.; Cossa, D.; Knoery, J.; Couture, R.M.; Radakovitch, O.; Yakubov, S.; Krzeminska, D. Modeling the influence of eutrophication and redox conditions on Mercury cycling at the sediment-water inter cling at the sediment-water interface in the Berr face in the Berre Lagoon. Front. Mar. Sci. 2018, 5, 291. [Google Scholar] [CrossRef]
- Wang, J.L.; Fu, Z.S.; Qiao, H.X.; Liu, F.X. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total. Environ. 2018, 650, 1392. [Google Scholar] [CrossRef]
- Chang, M.Q.; Teurlincx, S.; Angelis, D.L.D.; Janse, J.H.; Troost, T.A.; Wijk, D.; Mooij, W.M.; Janssen, A.B.G. A generically parameterized model of Lake eutrophication (GPLake) that links field-, lab- and model-based knowledge. ACS Appl. Mater. Interf. 2019, 695, 133887. [Google Scholar] [CrossRef]
- Taipale, S.J.; Vuorio, K.; Aalto, S.L.; Peltomaa, E.; Tiirola, M. Eutrophication reduces the nutritional value of phytoplankton in boreal lakes. Environ. Res. 2019, 179, 108836. [Google Scholar] [CrossRef]
- Fitzgerald, G.P.; Nelson, T.C. Extractive and enzymatic analyses for limiting or surplus phosphorus in alage. J. Phycol. 1966, 2, 32–37. [Google Scholar] [CrossRef]
- Holtz, K.M.; Kantrowitz, E.R. The mechanism of the alkaline phosphatase reaction: Insights from NMR, crystallography and site-specific mutagenesis. FEBS Lett. 1999, 462, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.C.; Metcalf, W.W. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc. Natl. Acad. Sci. USA 2004, 101, 7919–7924. [Google Scholar] [CrossRef] [Green Version]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Ho, N.; Tung, C. Sensing Phosphatase Activity by Using Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 707–709. [Google Scholar] [CrossRef]
- Kazakevičienė, B.; Valinčius, G.; Kažemėkaitė, M.; Razumas, V. Self-Assembled Redox System for Bioelectrocatalytic Assay of l-Ascorbylphosphate and Alkaline Phosphatase Activity. Electroanalysis 2010, 20, 2235–2240. [Google Scholar] [CrossRef]
- Ingram, A.; Moore, B.D.; Graham, D. Simultaneous detection of alkaline phosphatase and beta-galactosidase activity using SERRS. Bioorg. Med. Chem. Lett. 2009, 19, 1569–1571. [Google Scholar] [CrossRef]
- Liu, Y.; Schanze, K.S. Conjugated Polyelectrolyte-Based Real-Time Fluorescence Assay for Alkaline Phosphatase with Pyrophosphate as Substrate. Anal. Chem. 2008, 80, 8605–8612. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Cao, M.D.; Li, J.K.; Wang, J.B. A Sensitive Ratiometric Fluorescent Sensor for Zinc(II) with High Selectivity. Sensors 2013, 13, 3131. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.B.; Wang, J. Pyrene-based ratiometric and fluorescent sensor for selective Al3+ detection. Inorg. Chim. Acta 2019, 489, 61–66. [Google Scholar] [CrossRef]
- Ma, R.X.; Xu, M.; Liu, C.; Shi, G.Y.; Deng, J.J.; Zhou, T.S. Stimulus Response of GQD-Sensitized Tb/GMP ICP Nanoparticles with Dual-Responsive Ratiometric Fluorescence: Toward Point-ofUse Analysis of Acetylcholinesterase and Organophosphorus Pesticide Poisoning with Acetylcholinesterase as a Biomarker. ACS. Appl. Mater. Inter. 2020, 12, 42119. [Google Scholar] [CrossRef]
- Loas, A.; Lippard, S.J. Direct ratiometric detection of nitric oxide with Cu(II)-based fluorescent probes. J. Mater. Chem. B 2017, 5, 8929–8933. [Google Scholar] [CrossRef]
- Wang, Y.B.; Yang, M.; Ren, Y.K.; Fan, J. Cu-Mn codoped ZnS quantum dots-based ratiometric fluorescent sensor for folic acid. Anal. Chim. Acta 2018, 1040, 136–142. [Google Scholar] [CrossRef]
- Ye, T.; Lu, J.Q.; Yuan, M.; Cao, H.; Yin, F.Q.; Wu, X.X.; Hao, L.L.; Xu, F. Toehold-mediated enzyme-free cascade signal amplification for ratiometric fluorescent detection of kanamycin. Sens. Actuat. B Chem. 2021, 340, 129939. [Google Scholar] [CrossRef]
- Zhang, X.L.; Deng, J.J.; Xue, Y.M.; Shi, G.Y.; Zhou, T.S. Stimulus Response of Au-NPs@GMP-Tb Core−Shell Nanoparticles:Toward Colorimetric and Fluorescent Dual-Mode Sensing of Alkaline Phosphatase Activity in Algal Blooms of a Freshwater Lake. Environ. Sci. Technol. 2016, 50, 847–855. [Google Scholar] [CrossRef]
- Lu, W.; Cao, X.; Tao, L.; Ge, J.; Dong, J.; Qian, W. A novel label-free amperometric immunosensor for carcinoembryonic antigen based on Ag nanoparticle decorated infinite coordination polymer fibres. Biosens. Bioelectron. 2014, 57, 219–225. [Google Scholar] [CrossRef]
- Deng, J.; Fei, W.; Ping, Y.; Mao, L. On-site sensors based on infinite coordination polymer nanoparticles: Recent progress and future challenge. Appl. Mater. Today 2018, 11, 338–351. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, B.; Chen, G.; Tang, D. Redox and catalysis ‘all-in-one’ infinite coordination polymer for electrochemical immunosensor of tumor markers. Biosens. Bioelectron. 2015, 64, 6–12. [Google Scholar] [CrossRef]
- Tan, H.; Yang, C. Ag+-enhanced fluorescence of lanthanide/nucleotide coordination polymers and Ag+ sensing. Chem. Commun. 2011, 47, 12373. [Google Scholar] [CrossRef]
- Jiao, M.X.; Li, Y.; Jia, Y.X.; Xu, L.; Xu, G.Y.; Guo, Y.S.; Luo, X.L. Ligand-modulated aqueous synthesis of color-tunable copper nanoclusters for the photoluminescent assay of Hg(II). Microchim. Acta 2020, 187, 545. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, Z.Q.; Wang, X.B.; Zhuang, X.M.; Tian, C.Y.; Luan, F.; Fu, X.L. Functionalized copper nanoclusters-based fluorescent probe with aggregation-induced emission property for selective detection of sulfide ions in food additives. J. Agric. Food. Chem. 2020, 68, 11301. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, Y.; Chai, Y.; Yuan, R. An Ultrasensitive Electrochemiluminescence Biosensor for Detection of MicroRNA by in-situ Electrochemically Generated Copper Nanoclusters as Luminophore and TiO2 as Coreaction Accelerator. Biosens. Bioelectron. 2018, 114, 10–14. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, H.; Liu, Y.; Diao, Y.; Li, Z. FRET-based fluorometry assay for curcumin detecting using PVP-templated Cu NCs. Talanta 2021, 223, 121741. [Google Scholar] [CrossRef]
- Geng, F.H.; Zou, C.P.; Liu, J.H.; Zhang, Q.C.; Guo, X.Y.; Fan, Y.C.; Yu, H.D.; Yang, S.; Liu, Z.P.; Li, L. evelopment of luminescent nanoswitch for sensing of alkaline phosphatase in human serum based onAl3þ-PPi interaction and CuNCs with AIE properties. Anal. Chim. Acta 2019, 1076, 131–137. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Ou, Y.; Zhang, F.; Meng, J.; Wang, G.; Fang, Z.L.; Li, Y. Red-Emitting GSH-Cu NCs as Triplet Induced Quenched Fluorescent Probe for Fast Detection of Thiol Pollutants. Nanoscale 2020, 12, 19429–19437. [Google Scholar] [CrossRef]
- Cadiau, A.; Auguste, S.; Taulelle, F.; Martineau, C.; Adil, K. Hydrothermal synthesis, ab-initio structure determination and NMR study of the first mixed Cu–Al fluorinated MOF. CrystEngComm 2013, 15, 3430–3435. [Google Scholar] [CrossRef]
- Luz, I.; Loiudice, A.; Sun, D.T.; Queen, W.L.; Buonsanti, R. Understanding the Formation Mechanism of Metal Nanocrystal@MOF-74 Hybrids. Chem. Mater. 2016, 28, 3839–3849. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Chen, Y.; Jiang, H.L. Encapsulating Copper Nanocrystals into Metal–Organic Frameworks for Cascade Reactions by Photothermal Catalysis. Small 2021, 17, 2004481. [Google Scholar] [CrossRef]
- Sun, W.D.; Han, X.; Qu, F.L.; Kong, R.M.; Zhao, Z.L. A carbon dot doped lanthanide coordination polymer nanocomposite as the ratiometric fluorescent probe for the sensitive detection of alkaline phosphatase activity. Analyst 2021, 146, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.O.; Li, C.Y.; Li, Y.F.; Yang, B.; Li, S. An infinite coordination polymer nanoparticles-based near-infrared fluorescent probe with high photostability for endogenous alkaline phosphatase in vivo. J. Sensor. Actuat. B Chem. 2018, 225, 3355–3363. [Google Scholar] [CrossRef]
- Kalambet, Y.; Kozmin, Y. Internal standard arithmetic implemented as relative concentration/relative calibration. J. Chemometr. 2019, 33, e3106. [Google Scholar] [CrossRef]
- Liu, N.; Hao, J.; Cai, K.Y.; Zeng, M.; Song, Y. Ratiometric fluorescence detection of superoxide anion based on AuNPs-BSA@Tb/GMP nanoscale coordination polymers. Luminescence 2017, 33, 119–124. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.J. Synthesis of Size-Controlled and Shaped Copper Nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef]
- Deng, J.J.; Yu, P.; Wang, Y.X.; Mao, L.Q. Real-time Ratiometric Fluorescent Assay for Alkaline Phosphatase Activity with Stimulus Responsive Infinite Coordination Polymer Nanoparticles. Anal. Chem. 2015, 87, 3080–3086. [Google Scholar] [CrossRef]
- Mahato, K.; Purohit, B.; Kumar, A.; Chandra, P. Clinically comparable impedimetric immunosensor for serum alkaline phosphatase detection based on electrochemically engineered Au-nano-dendroids and graphene oxide nanocomposite. Biosens. Bioelectron. 2019, 148, 111815. [Google Scholar] [CrossRef]
- Huang, H.; Bai, J.; Li, J.; Lei, L.L.; Zhang, W.J.; Yan, S.J.; Li, Y.X. Fluorometric and colorimetric analysis of alkaline phosphatase activity based on a nucleotide coordinated copper ion mimicking polyphenol oxidase. J. Mater. Chem. B 2019, 7, 6508–6514. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Q.; Hu, Q.; Zhou, B.J.; Zhang, Y.H.; He, M.H.; Xu, T.; Li, F.; Kong, J.M. Fluorescence Quenching Based Alkaline Phosphatase Activity Detection. Talanta 2017, 176, 52–58. [Google Scholar] [CrossRef] [PubMed]
| Nanoprobes | Linear Range/U mL−1 | Detection Limit/U mL−1 | Ref. |
|---|---|---|---|
| coumarin@Tb-GMP a | 0.025–0.2 | 0.01 | [39] |
| AuNPs/GO b | 0.1–1 | 0.009 | [40] |
| ATP-Cu c | 0.03–0.3 | 0.03 | [41] |
| Cu(BCDS d)22− | 0.027–0.220 | 0.027 | [42] |
| Cu NCs@Tb-GMP | 0.002–2 | 0.002 | This work |
| Sample | Add/U mL−1 | Founded/U mL−1 | Recovery/% |
|---|---|---|---|
| 1 | 0.2000 | 0.2164 | 108.2 |
| 0.5000 | 0.5036 | 100.7 | |
| 2 | 0.2000 | 0.2120 | 106.0 |
| 0.5000 | 0.4982 | 99.64 | |
| 3 | 0.2000 | 0.2127 | 106.4 |
| 0.5000 | 0.4835 | 96.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Guo, W.; Wang, Y.; Hua, Q.; Tang, F.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors 2022, 12, 372. https://doi.org/10.3390/bios12060372
Li X, Wang X, Guo W, Wang Y, Hua Q, Tang F, Luan F, Tian C, Zhuang X, Zhao L. Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors. 2022; 12(6):372. https://doi.org/10.3390/bios12060372
Chicago/Turabian StyleLi, Xin, Xiaoling Wang, Wei Guo, Yunfei Wang, Qing Hua, Feiyan Tang, Feng Luan, Chunyuan Tian, Xuming Zhuang, and Lijun Zhao. 2022. "Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe" Biosensors 12, no. 6: 372. https://doi.org/10.3390/bios12060372
APA StyleLi, X., Wang, X., Guo, W., Wang, Y., Hua, Q., Tang, F., Luan, F., Tian, C., Zhuang, X., & Zhao, L. (2022). Selective Detection of Alkaline Phosphatase Activity in Environmental Water Samples by Copper Nanoclusters Doped Lanthanide Coordination Polymer Nanocomposites as the Ratiometric Fluorescent Probe. Biosensors, 12(6), 372. https://doi.org/10.3390/bios12060372





