Investigation of Conditions for Capture of Live Legionella pneumophila with Polyclonal and Recombinant Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Culture
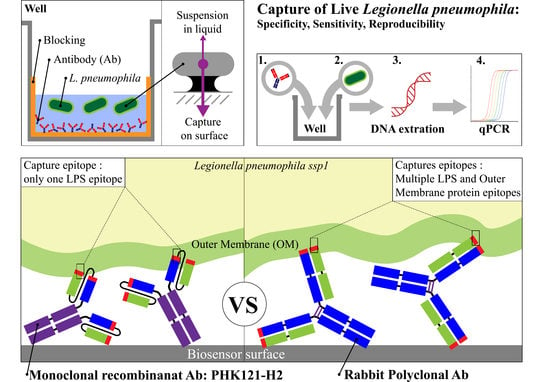
2.3. Immuno-Capture of Live Bacteria on 96 Well Plates
2.4. Effect of Antibody Pre-Sonication on Bacterial Capture
2.5. Direct DNA Extraction
2.6. Polymerase Chain Reaction (qPCR)
2.7. Bacteria Quantification Curve
3. Results and Discussion
3.1. Calibration Curve
3.2. Optimal Bacterial Concentration for Incubation in Ab-Coated Wells
3.3. Effect of Pre-Sonication of Antibodies on Bacterial Capture
3.4. Specificity of Polyclonal and Recombinant Antibodies
3.5. Evaluation of Sensitivity and Reproducibility of Polyclonal and Recombinant Antibodies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Colour of Media and pH to Monitor Bacterial Growth

Appendix A.2. Effect of Detergents on the Viability of L. pneumophila

References
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Interactive effects of corrosion, copper, and chloramines on legionella and mycobacteria in hot water plumbing. Environ. Sci. Technol. 2017, 51, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Schrammel, B.; Petzold, M.; Cervero-Arago, S.; Sommer, R.; Luck, C.; Kirschner, A. Persistent presence of outer membrane epitopes during short- and long-term starvation of five Legionella pneumophila strains. BMC Microbiol. 2018, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Faucher, S.P. Transcriptomic changes of Legionella pneumophila in water. BMC Genom. 2015, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Brown, S.A.; Palmer, K.L.; Whiteley, M. Revisiting the host as a growth medium. Nat. Rev. Microbiol. 2008, 6, 657–666. [Google Scholar] [CrossRef] [Green Version]
- McDade, J.E. Legionella and the Prevention of Legionellosis. Emerg. Infect. Dis. 2008, 14, 1006. [Google Scholar] [CrossRef]
- Diederen, B.M. Legionella spp. and Legionnaires’ disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef]
- Shevchuk, O.; Jager, J.; Steinert, M. Virulence properties of the Legionella pneumophila cell envelope. Front. Microbiol. 2011, 2, 74. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; McDade, J.E.; et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef]
- Centre For Disease Control. Communicable Disease Control Chapter I–Management of Specific Diseases Legionella Outbreak Investigation and Control; National Library of Medicine: Bethesda, MD, USA, 2018; p. 42. [Google Scholar]
- Lapierre, P.; Nazarian, E.; Zhu, Y.; Wroblewski, D.; Saylors, A.; Passaretti, T.; Hughes, S.; Tran, A.; Lin, Y.; Kornblum, J.; et al. Legionnaires’ disease outbreak caused by endemic strain of Legionella pneumophila, New York, NY, USA, 2015. Emerg. Infect. Dis. 2017, 23, 1784–1791. [Google Scholar] [CrossRef]
- Levesque, S.; Plante, P.L.; Mendis, N.; Cantin, P.; Marchand, G.; Charest, H.; Raymond, F.; Huot, C.; Goupil-Sormany, I.; Desbiens, F.; et al. Genomic characterization of a large outbreak of Legionella pneumophila serogroup 1 strains in Quebec City, 2012. PLoS ONE 2014, 9, e103852. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Santé et des Services Sociaux (MSSS). Guide d’Intervention-La Legionellose; Ministère de la Santé et des Services Sociaux: Quebec, QC, Canada, 2015.
- NYC Health. Cooling Tower Requirements: What Building Owners Should Know. 2016. Available online: https://www1.nyc.gov/assets/doh/downloads/pdf/cd/cooling-tower-FAQs.pdf (accessed on 1 November 2021).
- Berkelman, R.L.; Pruden, A. Prevention of Legionnaires’ disease in the 21st century by advancing science and public health practice. Emerg. Infect. Dis. 2017, 23, 1905–1907. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Shen, Z.; Mernaugh, R. Recombinant antibodies and their use in biosensors. Anal. Bioanal. Chem. 2012, 402, 3027–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trilling, A.K.; Hesselink, T.; van Houwelingen, A.; Cordewener, J.H.; Jongsma, M.A.; Schoffelen, S.; van Hest, J.C.; Zuilhof, H.; Beekwilder, J. Orientation of llama antibodies strongly increases sensitivity of biosensors. Biosens. Bioelectron. 2014, 60, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, E.; Hassen, W.M.; Frost, E.H.; Dubowski, J.J. Growth of Escherichia coli on the GaAs (001) surface. Talanta 2018, 178, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, E.; Aithal, S.; Hassen, W.M.; Frost, E.H.; Dubowski, J.J. GaAs/AlGaAs heterostructure based photonic biosensor for rapid detection of Escherichia coli in phosphate buffered saline solution. Sens. Actuators B Chem. 2015, 207, 556–562. [Google Scholar] [CrossRef]
- Nazemi, E.; Hassen, W.M.; Frost, E.H.; Dubowski, J.J. Monitoring growth and antibiotic susceptibility of Escherichia coli with photoluminescence of GaAs/AlGaAs quantum well microstructures. Biosens. Bioelectron. 2017, 93, 234–240. [Google Scholar] [CrossRef]
- Aziziyan, M.R.; Hassen, W.M.; Morris, D.; Frost, E.H.; Dubowski, J.J. Photonic biosensor based on photocorrosion of GaAs/AlGaAs quantum heterostructures for detection of Legionella pneumophila. Biointerphases 2016, 11, 019301. [Google Scholar] [CrossRef] [Green Version]
- Aziziyan, M.R.; Hassen, W.M.; Sharma, H.; Shirzaei Sani, E.; Annabi, N.; Frost, E.H.; Dubowski, J.J. Sodium dodecyl sulfate decorated Legionella pneumophila for enhanced detection with a GaAs/AlGaAs nanoheterostructure biosensor. Sens. Actuators B Chem. 2020, 304, 127007. [Google Scholar] [CrossRef]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Le Berre, M.; Kane, M. Biosensor-based assay for domoic acid: Comparison of performance using polyclonal, monoclonal, and recombinant antibodies. Anal. Lett. 2006, 39, 1587–1598. [Google Scholar] [CrossRef]
- Alcocer, M.J.; Doyen, C.; Lee, H.A.; Morgan, M.R. Properties of polyclonal, monoclonal, and recombinant antibodies recognizing the organophosphorus pesticide chlorpyrifos-ethyl. J. Agric. Food Chem. 2000, 48, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Hassen, W.M.; Tayabali, A.F.; Dubowski, J.J. Short Ligand, Cysteine-modified warnericin RK antimicrobial peptides favor highly sensitive detection of Legionella pneumophila. ACS Omega 2021, 6, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Bedard, E.; Levesque, S.; Martin, P.; Pinsonneault, L.; Paranjape, K.; Lalancette, C.; Dolce, C.E.; Villion, M.; Valiquette, L.; Faucher, S.P.; et al. Energy conservation and the promotion of Legionella pneumophila growth: The probable role of heat exchangers in a nosocomial outbreak. Infect. Control Hosp. Epidemiol. 2016, 37, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, P.; Thiem, S.; Steinert, M.; Purvis, D.; Lugmayr, V.; Treutlein, U.; Plobner, L.; Leiser, R.M.; Hust, M.; Dubel, S. Human Anti-Lipopolysaccharid (LPS) antibodies against Legionella with high species specificity. Hum. Antibodies 2017, 26, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Clemente, R.; Igeño, M.I.; Población, A.G.; Guijo, M.I.; Merchán, F.; Blasco, R. Study of pH changes in media during bacterial growth of several environmental strains. Proceedings 2018, 2, 1297. [Google Scholar] [CrossRef] [Green Version]
- Levin, T.C.; Goldspiel, B.P.; Malik, H.S. Density-dependent resistance protects Legionella pneumophila from its own antimicrobial metabolite, HGA. eLife 2019, 8, e46086. [Google Scholar] [CrossRef]
- Chang, Y.C.; Yang, C.Y.; Sun, R.L.; Cheng, Y.F.; Kao, W.C.; Yang, P.C. Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Sci. Rep. 2013, 3, 1863. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Leskiw, B.K.; Kaufman, W.R. Antimicrobial activity in the egg wax of the African cattle tick Amblyomma hebraeum (Acari: Ixodidae). Exp. Appl. Acarol. 2006, 39, 297–313. [Google Scholar] [CrossRef]
- Dong-Ju, K. Relation of microbial biomass to counting units for Pseudomonas aeruginosa. Afr. J. Microbiol. Res. 2012, 6, 4620–4646. [Google Scholar] [CrossRef]
- Vingataramin, L.; Frost, E.H. A single protocol for extraction of gDNA from bacteria and yeast. Biotechniques 2015, 58, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, M.; Moradzadeh, M.; Ghadiri, A.; Saki, M. Isolation and identification of Legionella spp. in environmental water sources based on macrophage infectivity potentiator (mip) gene sequencing in southwest Iran. AIMS Microbiol. 2019, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mediavilla, J.R.; Endimiani, A.; Rosenthal, M.E.; Zhao, Y.; Bonomo, R.A.; Kreiswirth, B.N. Multiplex Real-Time PCR Detection of Klebsiella pneumoniae Carbapenemase (KPC) and New Delhi metallo-β-lactamase (NDM-1). J. Clin. Microbiol. 2011, 49, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lin, S.; Kelen, G.D.; Quinn, T.C.; Dick, J.D.; Gaydos, C.A.; Rothman, R.E. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J. Clin. Microbiol. 2002, 40, 3449–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro, F.; Gilbert, J.A.; Owens, S.; Trimble, W.; Shuman, H.A. Whole-genome sequence of the human pathogen Legionella pneumophila serogroup 12 strain 570-CO-H. J. Bacteriol. 2012, 194, 1613–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellinghausen, N.; Frost, C.; Marre, R. Detection of legionellae in hospital water samples by quantitative real-time LightCycler PCR. Appl. Environ. Microbiol. 2001, 67, 3985–3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rantakokko-Jalava, K.; Jalava, J. Development of conventional and real-time PCR assays for detection of Legionella DNA in respiratory specimens. J. Clin. Microbiol. 2001, 39, 2904–2910. [Google Scholar] [CrossRef] [Green Version]
- Choiniere, S.; Frost, E.H.; Dubowski, J.J. Binding strategies for capturing and growing Escherichia coli on surfaces of biosensing devices. Talanta 2019, 192, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef] [Green Version]
- Virostat Inc. Legionella pneumophila. 2018. Available online: https://www.virostat-inc.com/products1/legionella-pneumonia (accessed on 5 December 2021).
- Abcam. Anti-Legionella pneumophila Antibody (ab20943). 2021. Available online: https://www.abcam.com/legionella-pneumophila-antibody-ab20943.html (accessed on 10 March 2022).
- Groff, K.; Brown, J.; Clippinger, A.J. Modern affinity reagents: Recombinant antibodies and aptamers. Biotechnol. Adv. 2015, 33, 1787–1798. [Google Scholar] [CrossRef] [Green Version]





| Bacteria | Name | Sequence | Absorption/Emmision | |
|---|---|---|---|---|
| Legionella | Mip | |||
| pneumophila | Forward | 5′-TTGTCTTATAGCATTGGTGCCG-3′ | ||
| Reverse | 5′-CCAATTGAGCGCCACTCATAG-3′ | |||
| Probe | 5′-(6-FAM)-CGGAAGCAA(ZEN)TGGCTAAAGGCATGCA–(IABkFQ)-3′ | 495/520 nm | ||
| Universal | 1 rDNA | |||
| Forward | 5′-TGGAGCATGTGGTTTAATTCGA-3′ | |||
| Reverse | 5′-TGCGGGACTTAACCCAAC A-3′ | |||
| Probe | 5′-(5CY5)-CACGAGCTGACGACARCCATGCA–(3BHQ_2)-3′ | 646/520 nm | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladines, L.; Hassen, W.M.; Chawich, J.; Dübel, S.; Lévesque, S.; Dubowski, J.J.; Frost, E.H. Investigation of Conditions for Capture of Live Legionella pneumophila with Polyclonal and Recombinant Antibodies. Biosensors 2022, 12, 380. https://doi.org/10.3390/bios12060380
Paladines L, Hassen WM, Chawich J, Dübel S, Lévesque S, Dubowski JJ, Frost EH. Investigation of Conditions for Capture of Live Legionella pneumophila with Polyclonal and Recombinant Antibodies. Biosensors. 2022; 12(6):380. https://doi.org/10.3390/bios12060380
Chicago/Turabian StylePaladines, Lucas, Walid M. Hassen, Juliana Chawich, Stefan Dübel, Simon Lévesque, Jan J. Dubowski, and Eric H. Frost. 2022. "Investigation of Conditions for Capture of Live Legionella pneumophila with Polyclonal and Recombinant Antibodies" Biosensors 12, no. 6: 380. https://doi.org/10.3390/bios12060380
APA StylePaladines, L., Hassen, W. M., Chawich, J., Dübel, S., Lévesque, S., Dubowski, J. J., & Frost, E. H. (2022). Investigation of Conditions for Capture of Live Legionella pneumophila with Polyclonal and Recombinant Antibodies. Biosensors, 12(6), 380. https://doi.org/10.3390/bios12060380