Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Enzymes, Isolation, and Purification
2.3. Synthesis of CuCe Nanoparticles and Estimation of Their Pseudo-Peroxidase Activity
2.4. Apparatus
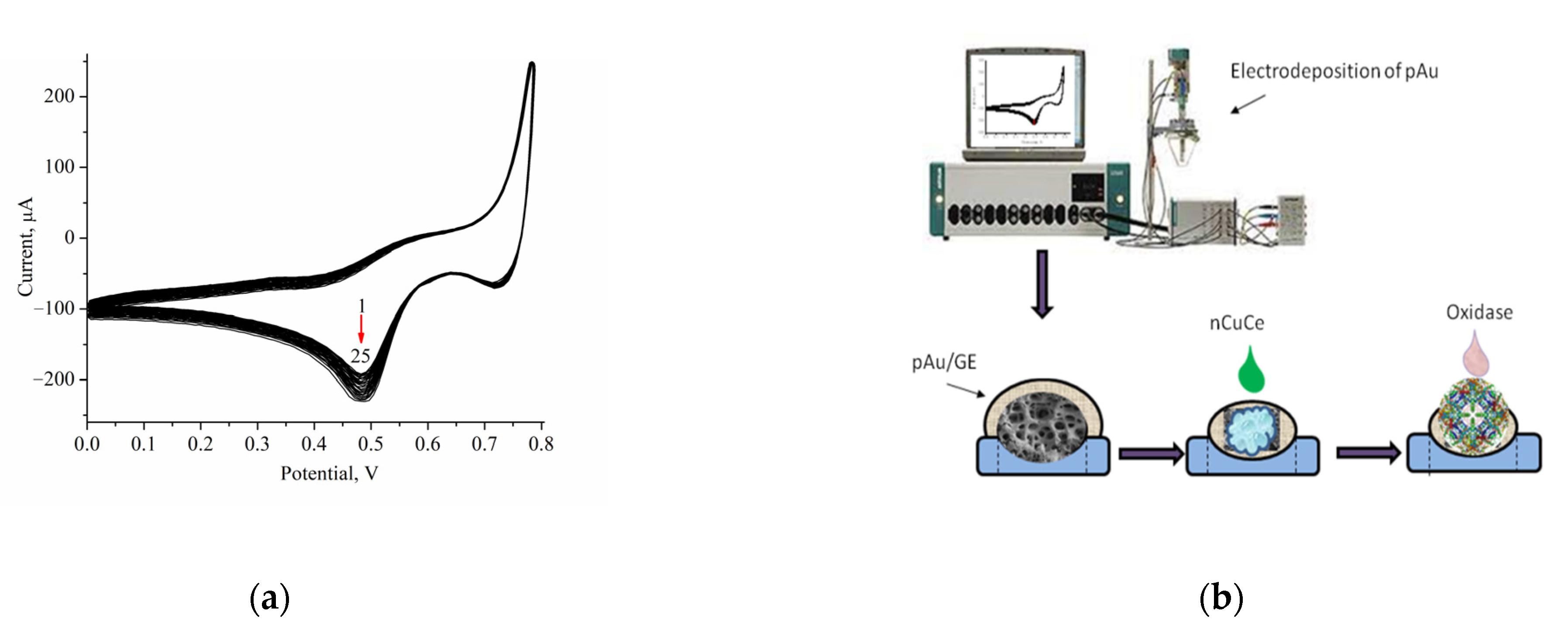
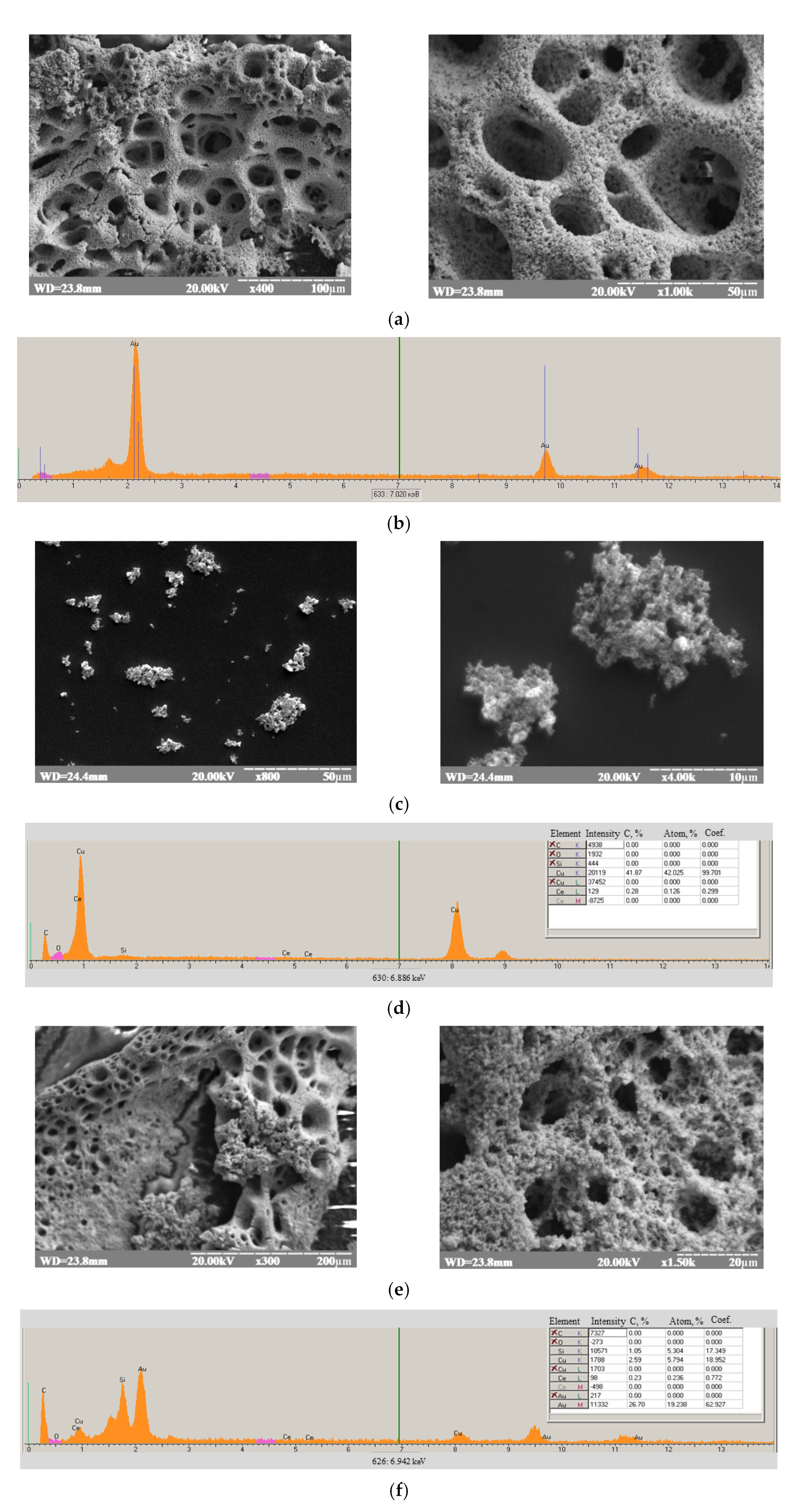
2.5. Electrodeposition of Porous Gold onto Graphite Electrode
2.6. Immobilization of nCuCe and Peroxidase on Electrodes
2.7. Immobilization of Oxidases onto the Modified Electrodes
2.8. Measurements and Calculations
3. Results
3.1. Development of Oxidase-Based Biosensors Using nCuCe and Porous Gold
3.2. Characterization of the Constructed Biosensors
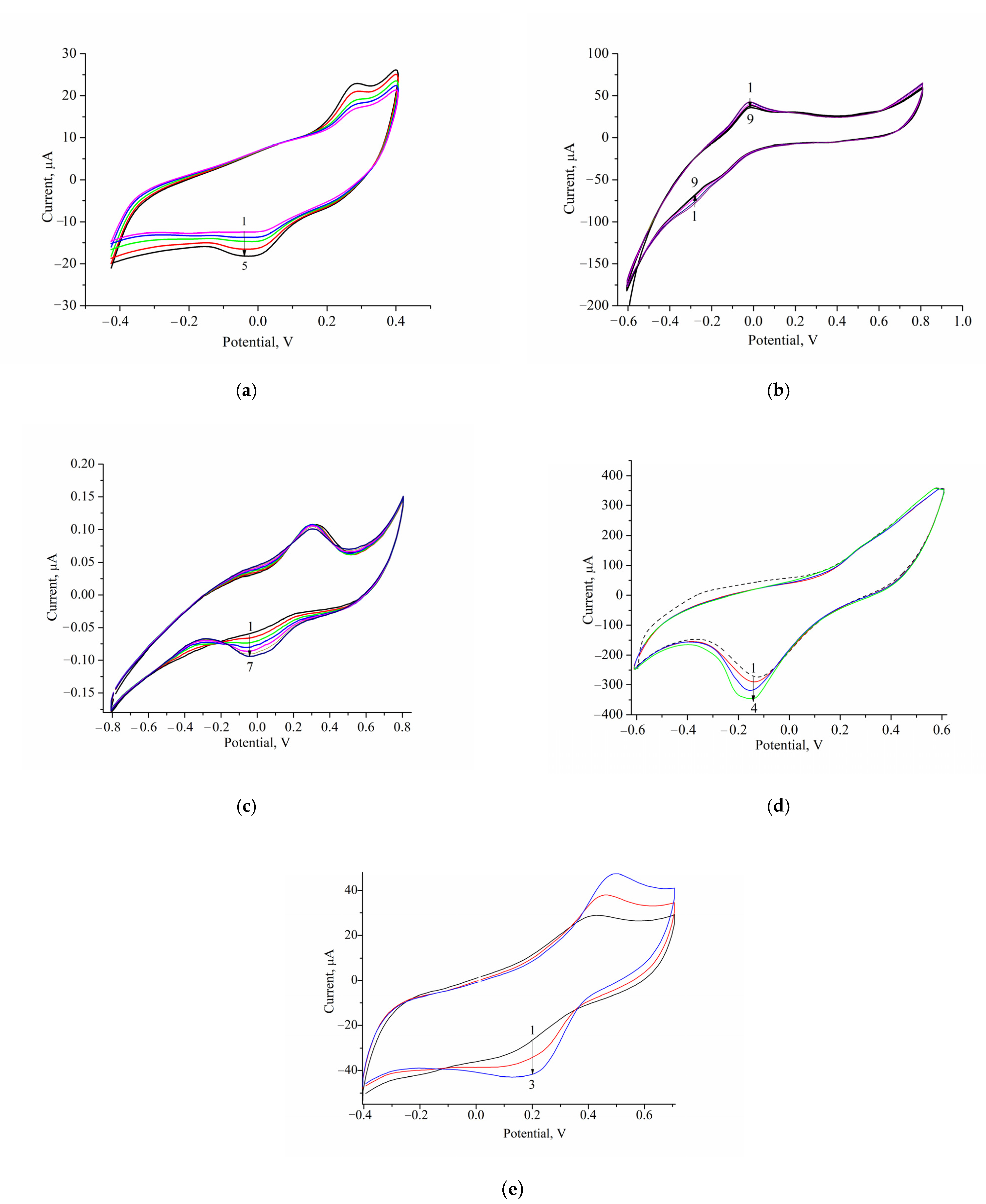
3.2.1. Optimal Working Potential
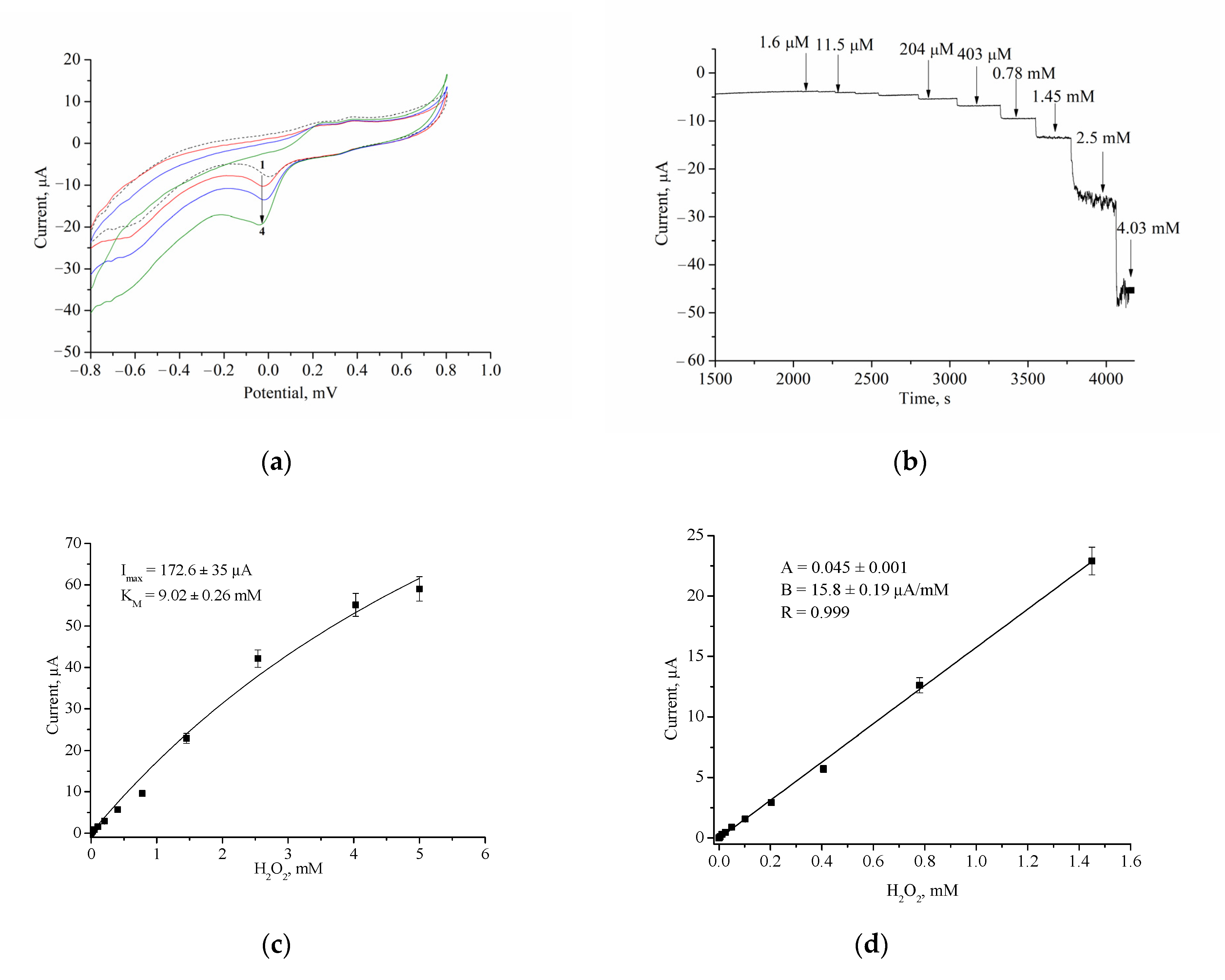
3.2.2. Analytical Properties
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; El-Moghazy, A.Y.; Hussin, M.; Abu-Saied, M.A.; El-Shanshory, A.A.; Solman, H.M.A. The nanomaterials and recent progress in biosensing systems: A review. Trends Environ. Anal. Chem. 2020, 26, e00087. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Bytesnikova, Z.; Barek, J.; Richtera, L.; Adam, V. A critical comparison of natural enzymes and nanozymes in biosensing and bioassays. Biosens. Bioelectron. 2021, 192, 113494. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on metal- and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, catalytic properties and application in biosensorics of nanozymes and electronanocatalysts: A Review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef]
- Neumann, B.; Wollenberger, U. Electrochemical biosensors employing natural and artificial heme peroxidases on semiconductors. Sensors 2020, 20, 369. [Google Scholar] [CrossRef]
- Demkiv, O.; Stasyuk, N.; Serkiz, R.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Peroxidase-like metal-based nanozymes: Synthesis, catalytic properties, and analytical application. Appl. Sci. 2021, 11, 777. [Google Scholar] [CrossRef]
- Gayda, G.Z.; Demkiv, O.M.; Gurianov, Y.; Serkiz, R.Y.; Klepach, H.M.; Gonchar, M.V.; Nisnevitch, M. “Green” Prussian Blue analogues as peroxidase mimetics for amperometric sensing and biosensing. Biosensors 2021, 11, 193. [Google Scholar] [CrossRef]
- Demkiv, O.; Stasyuk, N.; Gayda, G.; Gonchar, M. Highly sensitive amperometric sensor based on laccase-mimicking metal-based hybrid nanozymes for adrenaline analysis in pharmaceuticals. Catalysts 2021, 11, 1510. [Google Scholar] [CrossRef]
- Keihan, A.H.; Karimi, R.R.; Sajjadi, S. Wide dynamic range and ultrasensitive detection of hydrogen peroxide based on beneficial role of gold nanoparticles on the electrochemical properties of Prussian blue. J. Electroanal. Chem. 2020, 862, 114001. [Google Scholar] [CrossRef]
- Tripathi, A.; Harris, K.D.; Elias, A.L. High surface area nitrogen-functionalized Ni nanozymes for efficient peroxidase-like catalytic activity. PLoS ONE 2021, 16, e0257777. [Google Scholar] [CrossRef]
- Fu, Z.; Zeng, W.; Cai, S.; Li, H.; Ding, J.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J. Colloid Interface Sci. 2021, 604, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue modified boron-doped diamond interfaces for advanced H2O2 electrochemical sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Demkiv, O.; Darmohray, L.; Gonchar, M.; Nisnevitch, M. Amperometric biosensors for L-arginine determination based on L-arginine oxidase and peroxidase-like nanozymes. Appl. Sci. 2021, 11, 7024. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, modification, characterization, and biosensing application of nanoporous gold using electrochemical techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef]
- Stine, K.J.; Jefferson, K.; Shulga, O.V. Nanoporous gold for enzyme immobilization. Methods Mol. Biol. 2017, 1504, 37–60. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, Z.; Zhang, P.; Xiao, S.; Wang, L.; Dong, Y.; Yuan, H.; Li, P.; Sun, Y.; Jiang, X.; et al. 3D nanoporous gold scaffold supported on graphene paper: Freestanding and flexible electrode with high loading of ultrafine PtCo alloy nanoparticles for electrochemical glucose sensing. Anal. Chim. Acta 2016, 938, 63–71. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Caballero, D.; Kwon, I.K.; Oliveira, J.M.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. 3D biosensors in advanced medical diagnostics of high mortality diseases. Biosens. Bioelectron. 2019, 130, 20–39. [Google Scholar] [CrossRef]
- Singh, B.K.; Lee, S.; Na, K. An overview on metal-related catalysts: Metal oxides, nanoporous metals and supported metal nanoparticles on metal organic frameworks and zeolites. Rare Met. 2020, 39, 751–766. [Google Scholar] [CrossRef]
- Bollella, P. Porous Gold: A New Frontier for enzyme-based Electrodes. Nanomaterials 2020, 10, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sondhi, P.; Stine, K.J. Electrodeposition of Nanoporous Gold Thin Films. In Nanofibers—Synthesis, Properties and Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2020; 20p. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Pu, X.; Shen, K.; Zhang, L.; Wang, X.; Qin, J.; Wang, W. Dealloyed porous gold anchored by in situ generated graphene sheets as high activity catalyst for methanol electro-oxidation reaction. RSC Adv. 2020, 10, 1666–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan-Quang, G.C.; Yang, Z.; Koh, C.S.L.; Sim, H.Y.F.; Leong, S.X.; Ling, X.Y. Plasmonic-induced overgrowth of amorphous molybdenum sulfide on nanoporous gold: An ambient synthesis method of hybrid nanoparticles with enhanced electrocatalytic activity. J. Chem. Phys. 2019, 151, 244709. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, J.; Schützendübe, P.; Liang, H.; Huang, Y.; Wang, Z. Beyond dealloying: Development of nanoporous gold via metal-induced crystallization and its electrochemical properties. Nanotechnology 2019, 30, 375601. [Google Scholar] [CrossRef]
- Demirci, C.; Marras, S.; Prato, M.; Pasquale, L.; Manna, L.; Colombo, M. Design of catalytically active porous gold structures from a bottom-up method: The role of metal traces in CO oxidation and oxidative coupling of methanol. J. Catal. 2019, 375, 279–286. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Sukeri, A.; Bertotti, M. Fabrication of nanoporous gold-islands via hydrogen bubble template: An efficient electrocatalyst for oxygen reduction and hydrogen evolution reactions. Int. J. Hydrogen Energy 2019, 44, 15001–15008. [Google Scholar] [CrossRef]
- Sondhi, P.; Stine, K.J. Methods to generate structurally hierarchical architectures in nanoporous coinage metals. Coatings 2021, 11, 1440. [Google Scholar] [CrossRef]
- Xiao, X.; Si, P.; Magner, E. An overview of dealloyed nanoporous gold in bioelectrochemistry. Bioelectrochemistry 2016, 109, 117–126. [Google Scholar] [CrossRef]
- Biener, J.; Biener, M.M.; Madix, R.J.; Friend, C.M. Nanoporous gold: Understanding the origin of the reactivity of a 21st Century catalyst made by pre-columbian technology. ACS Catal. 2015, 5, 6263–6270. [Google Scholar] [CrossRef]
- Saffarini, M.H.; Voyiadjis, G.Z.; Ruestes, C.J.; Yaghoobi, M. Ligament size dependency of strain hardening and ductility in nanoporous gold. Comput. Mater. Sci. 2021, 186, 109920. [Google Scholar] [CrossRef]
- Mie, Y.; Takayama, H.; Hirano, Y. Facile control of surface crystallographic orientation of anodized nanoporous gold catalyst and its application for highly efficient hydrogen evolution reaction. J. Catal. 2020, 389, 476–482. [Google Scholar] [CrossRef]
- Masud, M.K.; Na, J.; Lin, T.-E.; Malgras, V.; Preet, A.; Ibn Sina, A.A.; Wood, K.; Billah, M.; Kim, J.; You, J.; et al. Nanostructured mesoporous gold biosensor for microRNA detection at attomolar level. Biosens. Bioelectron. 2020, 168, 112429. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.B.; Newman, R.C. A novel application of nanoporous gold to humidity sensing: A framework for a general volatile compound sensor. Nanoscale Adv. 2020, 2, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Chen, F.; Li, M.; Yu, R.; Xu, Y.; Jiang, Y.; Liu, C.; Hu, J. Light welding Au nanoparticles assembled at water-air interface for monolayered nanoporous gold films with tunable electrocatalytic activity. Electrochim. Acta 2020, 334, 135626. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Bimetallic nanomaterials-based electrochemical biosensor platforms for clinical applications. Micromachines 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Sarkhosh-Inanlou, R.; Shafiei-Irannejad, V.; Azizi, S.; Jouyban, A.; Ezzati-Nazhad Dolatabadi, J.; Mobed, A.; Adel, B.; Soleymani, J.; Hamblin, M.R. Applications of scaffold-based advanced materials in biomedical sensing. Trends Analyt. Chem. 2021, 143, 116342. [Google Scholar] [CrossRef]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Presutti, D.; Agarwal, T.; Zarepour, A.; Celikkin, N.; Hooshmand, S.; Nayak, C.; Ghomi, M.; Zarrabi, A.; Costantini, M.; Behera, B.; et al. Transition metal dichalcogenides (TMDC)-based nanozymes for biosensing and therapeutic applications. Materials 2022, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Barra, P.; Delogu, F. Porosity effects on nanoporous Au Young’s modulus. Mater. Lett. 2021, 304, 130703. [Google Scholar] [CrossRef]
- Klepach, H.M.; Zakalskiy, A.E.; Zakalska, O.M.; Gayda, G.Z.; Smutok, O.V.; Gonchar, M.V. Alcohol oxidase from the methylotrophic yeast Ogataea polymorpha: Isolation, purification, and bioanalytical application. Methods Mol. Biol. 2021, 2280, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Stasyuk, N.E.; Smutok, O.V.; Zakalskiy, A.E.; Zakalska, O.M.; Gonchar, M.V. Methylamine-sensitive amperometric biosensor based on (His)6-tagged Hansenula polymorpha methylamine oxidase immobilized on the gold nanoparticles. Biomed Res. Int. 2014, 2014, 480498. [Google Scholar] [CrossRef] [Green Version]
- Demkiv, O.M.; Gayda, G.Z.; Broda, D.; Gonchar, M.V. Extracellular laccase from Monilinia fructicola: Isolation, primary characterization and application. Cell Biol. Intern. 2021, 45, 536–548. [Google Scholar] [CrossRef]
- Lutz, G. Semiconductor Radiation Detectors; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-71679-2. [Google Scholar]
- Tobin, J.G.; Yu, S.-W.; Sokaras, D. The X-ray Emission Spectroscopy of Cerium Oxide. J. Electron Spectrosc. Relat. Phenom. 2020, 1, 26. [Google Scholar]

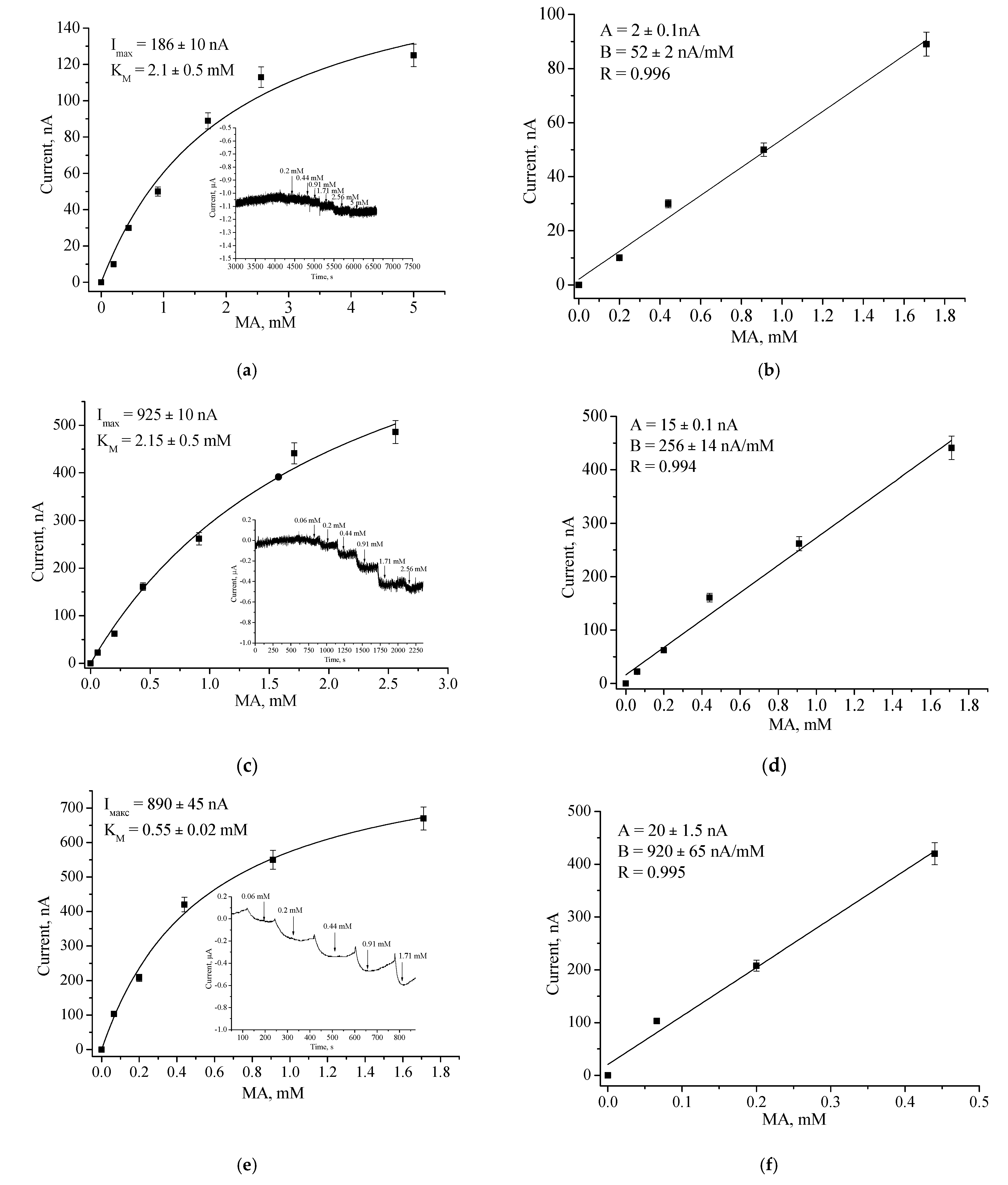

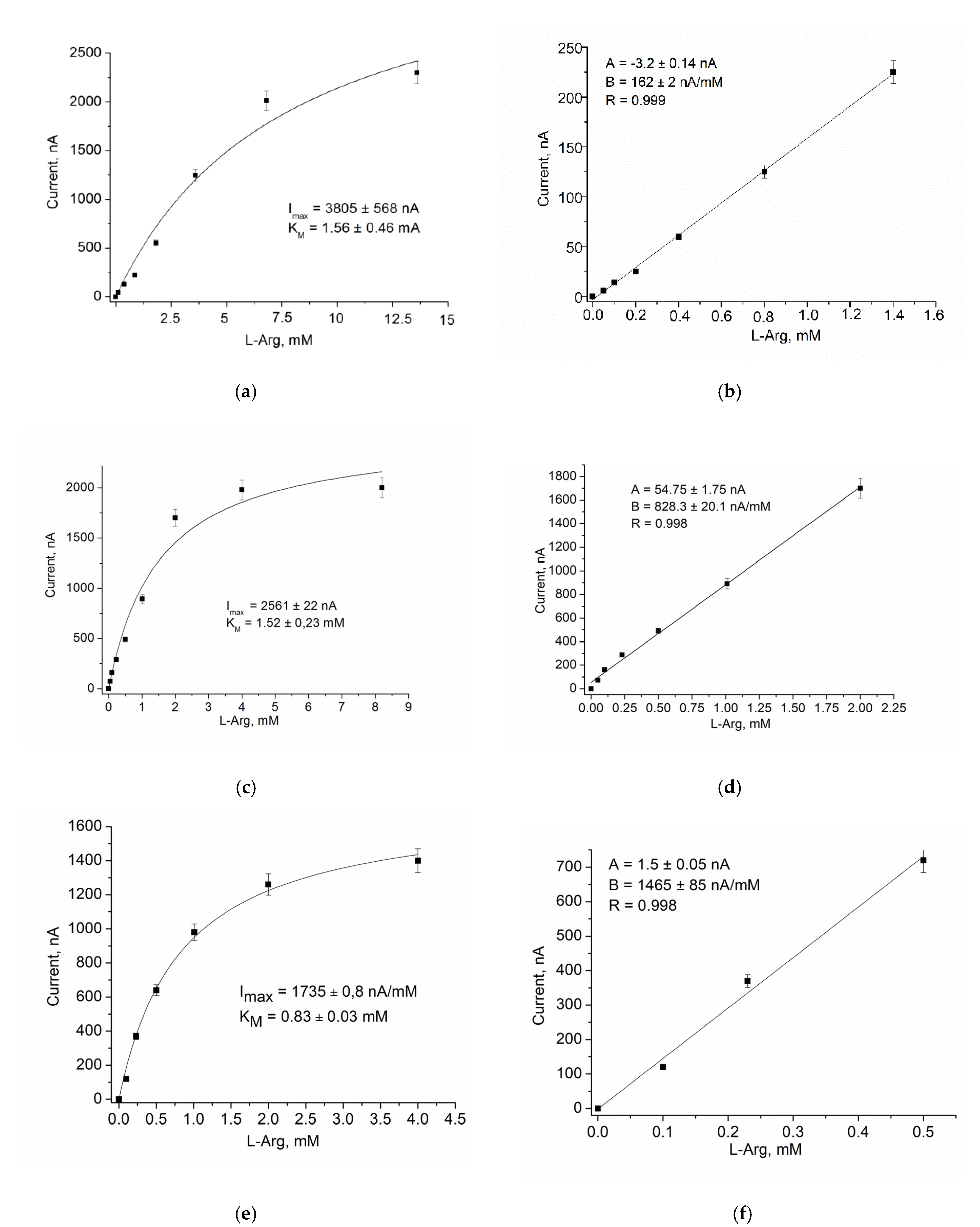
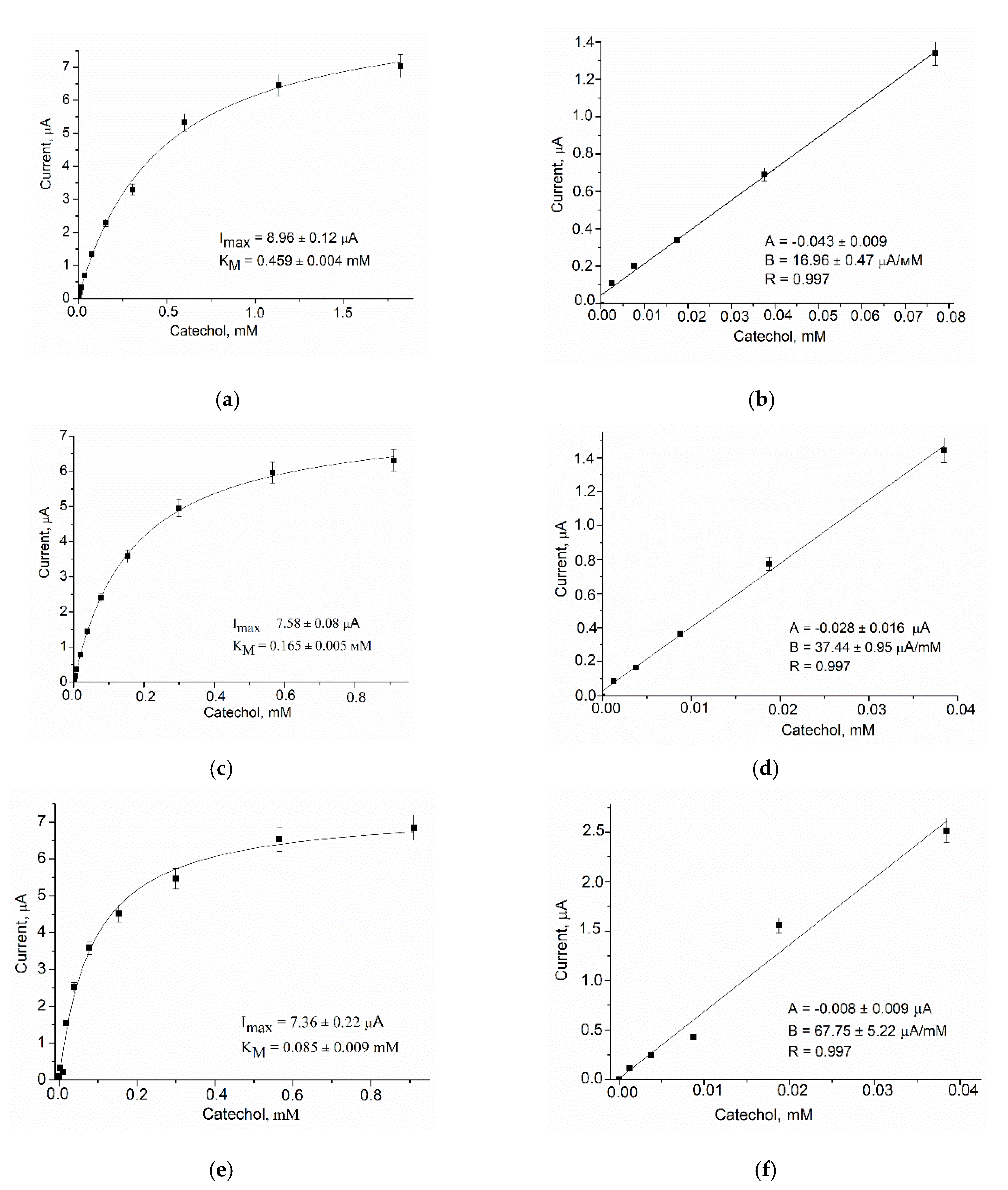
| Bioelectrode | Potential, mV | Sensitivity (S) | Linear Range, µM | LOD, µM | KMapp, mM | ||||
|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Mr, kDa | Sensor for H2O2 | N | A·M−1·m−2 | KS | ||||
| AO | 640 | PO | 1 | –50 | 22 | S2/S1 = 1.5 | 130–900 | 39 | 0.70 |
| nCuCe | 2 | 32 | S3/S2 = 3.2 | 50–2100 | 15 | 0.70 | |||
| nCuCe/pAu | 3 | 102 | S3/S1 = 4.6 | 33–500 | 10 | 1.22 | |||
| AMO | 160 | PO | 1 | –250 | 7 | S2/S1 = 5 | 200–1700 | 61/130 | 2.1 |
| nCuCe | 2 | 35 | S3/S2 = 3.6 | 60–1700 | 18 | 2.2 | |||
| nCuCe/pAu | 3 | 125 | S3/S1 = 17.9 | 60–450 | 18 | 0.55 | |||
| ArgO | 500 | PO | 1 | –150 | 22 | S2/S1 = 5.1 | 75–1400 | 35 | 1.56 |
| nCuCe | 2 | 113 | S3/S2 = 1.8 | 50–2250 | 15 | 1.52 | |||
| nCeCu/pAu | 3 | 200 | S3/S1 = 9.1 | 100–500 | 33 | 0.83 | |||
| GO | 150–190 | PO | 1 | –50 | 44 | S2/S1 = 1.7 | 500–5000 | 150 | 5.23 |
| nCuCe | 2 | 73 | S3/S2 = 5.5 | 500–7300 | 150 | 18 | |||
| nCeCu/pAu | 3 | 400 | S3/S1 = 9.1 | 250–2000 | 76 | 5.81 | |||
| Laccase | 100 | bulk | 1 | +200 | 2300 | S2/S1 = 2.2 | 8–160 | 2 | 0.46 |
| * nCuCe | 2 | 5055 | S3/S2 = 1.8 | 3–40 | 2 | 0.17 | |||
| nCuCe/pAu | 3 | 9280 | S3/S1 = 4.0 | 2–40 | 1 | 0.09 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasyuk, N.; Demkiv, O.; Gayda, G.; Zakalskiy, A.; Klepach, H.; Bisko, N.; Gonchar, M.; Nisnevitch, M. Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles. Biosensors 2022, 12, 472. https://doi.org/10.3390/bios12070472
Stasyuk N, Demkiv O, Gayda G, Zakalskiy A, Klepach H, Bisko N, Gonchar M, Nisnevitch M. Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles. Biosensors. 2022; 12(7):472. https://doi.org/10.3390/bios12070472
Chicago/Turabian StyleStasyuk, Nataliya, Olha Demkiv, Galina Gayda, Andriy Zakalskiy, Halyna Klepach, Nina Bisko, Mykhailo Gonchar, and Marina Nisnevitch. 2022. "Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles" Biosensors 12, no. 7: 472. https://doi.org/10.3390/bios12070472
APA StyleStasyuk, N., Demkiv, O., Gayda, G., Zakalskiy, A., Klepach, H., Bisko, N., Gonchar, M., & Nisnevitch, M. (2022). Highly Porous 3D Gold Enhances Sensitivity of Amperometric Biosensors Based on Oxidases and CuCe Nanoparticles. Biosensors, 12(7), 472. https://doi.org/10.3390/bios12070472





