Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled?
Abstract
:1. Introduction: Hesitancy to Embrace LAMP in SARS-CoV-2 Diagnostics
2. Post-Pandemic Surge in LAMP-Diagnostics Research
3. A Look into the Point-of-Care LAMP Market: Research and Commercial
3.1. Leading LAMP Reagents

3.2. Leading LAMP-Detection Technology
3.3. Leading “All-In-One” LAMP Systems
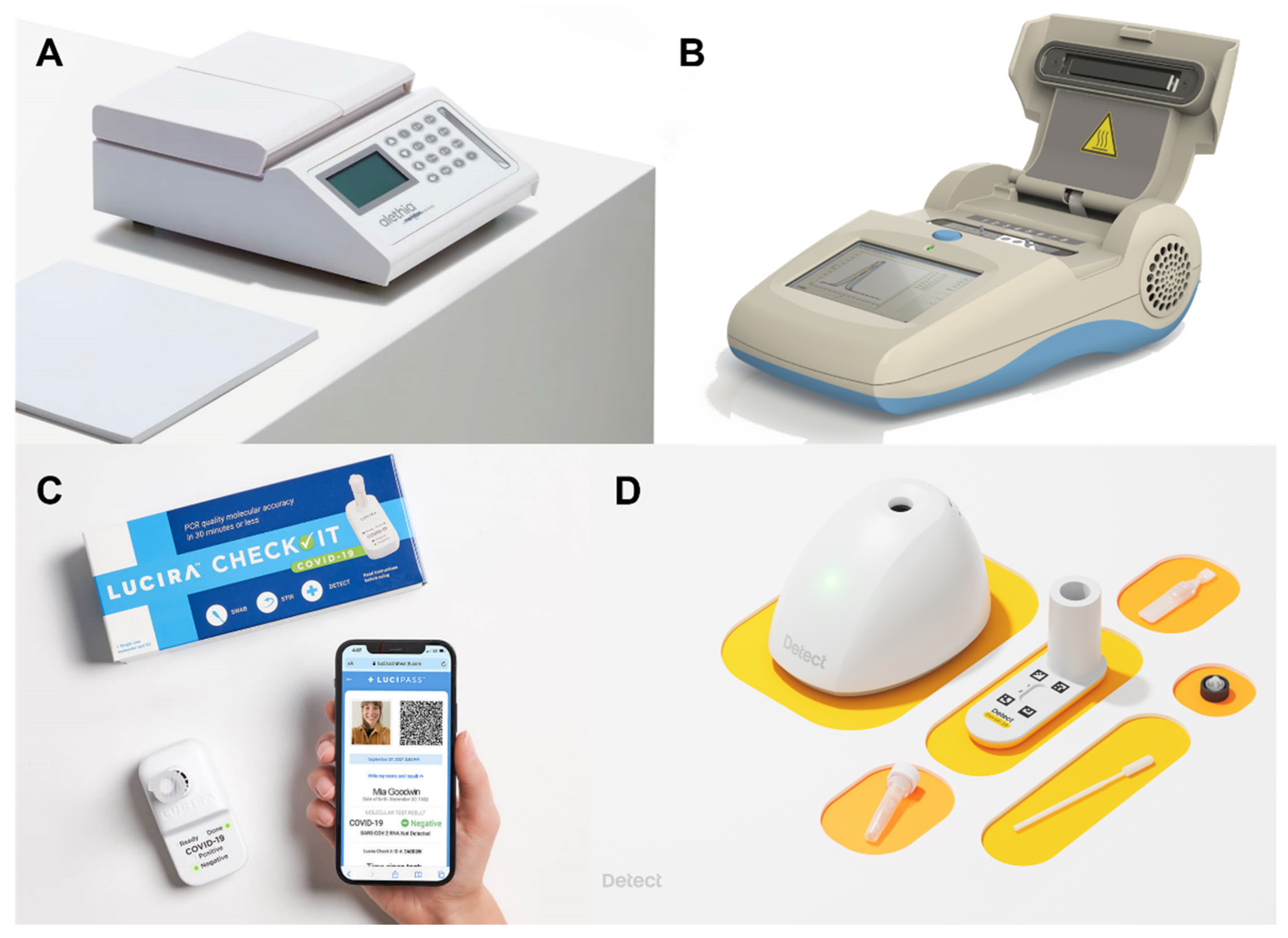
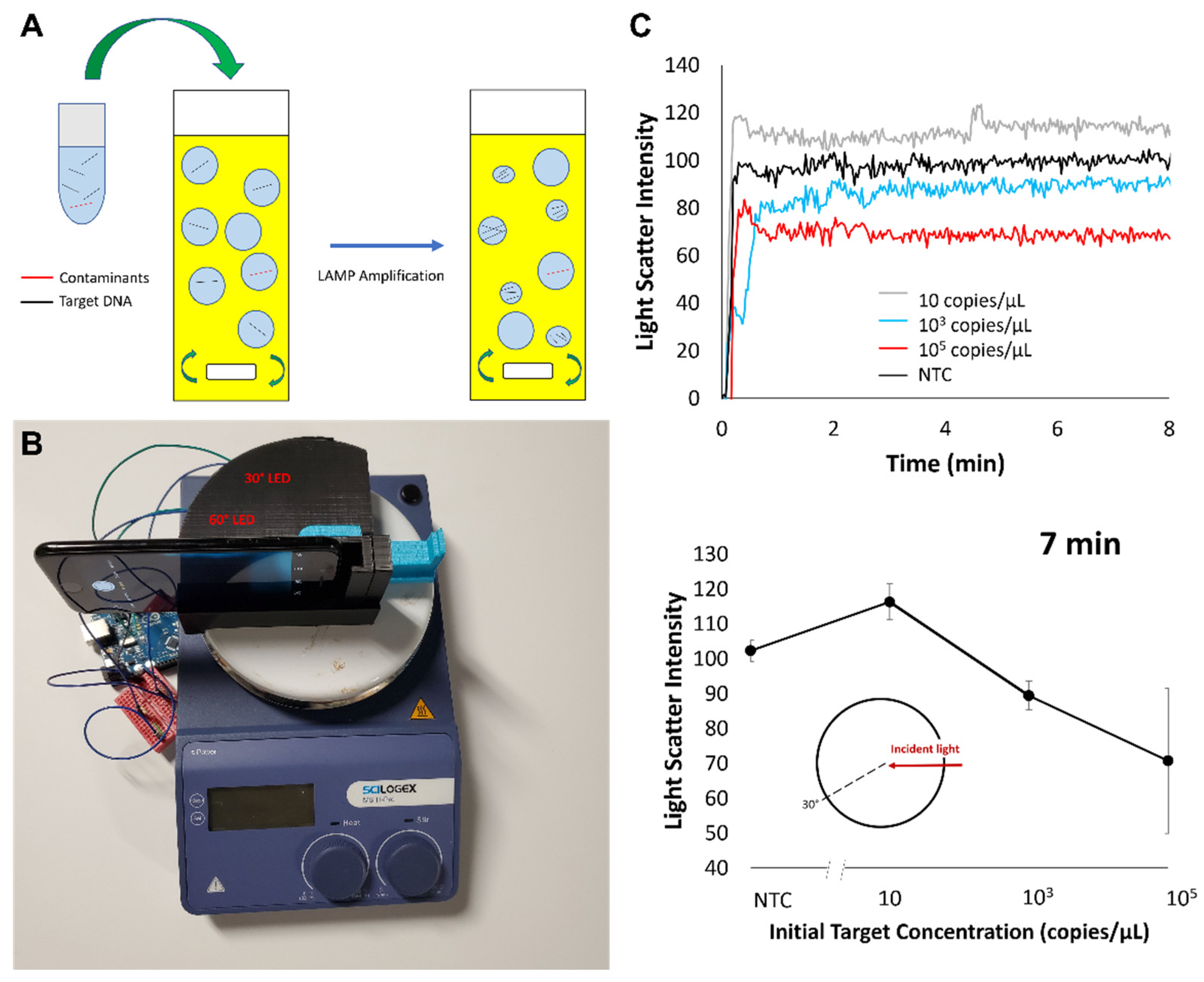
3.4. Recent Research on All-In-One LAMP Systems for SARS-CoV-2
4. LAMP Potentials Disregarded in COVID-19 Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johanna, N.; Citrawijaya, H.; Wangge, G. Mass screening vs. lockdown vs. combination of both to control COVID-19: A systematic review. J. Public Health Res. 2020, 9, 2011. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, M.B. Early Detection of Superspreaders by Mass Group Pool Testing Can Mitigate COVID-19 Pandemic. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.22.20076166v1 (accessed on 1 May 2022). [CrossRef]
- Bergquist, S.; Otten, T.; Sarich, N. COVID-19 pandemic in the United States. Health Policy Technol. 2020, 9, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Lee, H.; Ko, J.; Pittet, M.J. COVID-19 diagnostics in context. Sci. Transl. Med. 2020, 12, eabc1931. [Google Scholar] [CrossRef]
- Mbwogge, M. Mass testing with contact tracing compared to test and trace for the effective suppression of COVID-19 in the United Kingdom: Systematic review. JMIRx Med. 2021, 2, e27254. [Google Scholar] [CrossRef]
- Benda, A.; Zerajic, L.; Ankita, A.; Cleary, E.; Park, Y.; Pandey, S. COVID-19 testing and diagnostics: A review of commercialized technologies for cost, convenience and quality of tests. Sensors 2021, 21, 6581. [Google Scholar] [CrossRef]
- Patel, R.; Babady, E.; Theel, E.S.; Storch, G.A.; Pinsky, B.A.; George, K.S.; Smith, T.C.; Bertuzzi, S. Report from the American society for microbiology COVID-19 international summit, 23 March 2020: Value of diagnostic testing for SARS-CoV-2/COVID-19. mBio 2020, 11, e00722-20. [Google Scholar] [CrossRef] [Green Version]
- Kierkegaard, P.; McLister, A.; Buckle, P. Rapid point-of-care testing for COVID-19: Quality of supportive information for lateral flow serology assays. BMJ Open 2021, 11, e047163. [Google Scholar] [CrossRef]
- Love, J.; Wimmer, M.T.; Toth, D.J.A.; Chandran, A.; Makhija, D.; Cooper, C.K.; Samore, M.H.; Keegan, L.T. Comparison of antigen- and RT-PCR-based testing strategies for detection of SARS-CoV-2 in two high-exposure settings. PLoS ONE 2021, 16, e0253407. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). COVID-19 Test Basics. Available online: https://www.fda.gov/consumers/consumer-updates/coronavirus-disease-2019-testing-basics (accessed on 1 May 2022).
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 test sensitivity—A strategy for containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- New York Times. ‘It’s Like Having No Testing’: Coronavirus Test Results Are Still Delayed. Available online: https://www.nytimes.com/2020/08/04/us/virus-testing-delays.html (accessed on 1 May 2022).
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Ghale-Noie, Z.N.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R.; et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, D.; Lei, Y. Mini review: Recent progress in RT-LAMP enabled COVID-19 detection. Sens. Actuators Rep. 2020, 2, 100017. [Google Scholar] [CrossRef]
- National Laboratory of Enteric Pathogens; Bureau of Microbiology; Laboratory Centre for Disease Control. The polymerase chain reaction: An overview and development of diagnostic PCR protocols at the LCDC. Can. J. Infect. Dis. 1991, 2, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Zhang, H.; Zhou, Y.; Cao, J.; Zhou, J. Comparison of loop-mediated isothermal amplification (LAMP) and real-time PCR method targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Vet. Parasitol. 2012, 185, 296–300. [Google Scholar] [CrossRef]
- Khan, M.; Wang, R.; Li, B.; Liu, P.; Weng, Q.; Chen, Q. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of Alternaria solani. Front. Microbiol. 2018, 9, 2089. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Sethy, K.; Mohapatra, S.; Panda, D. Loop mediated isothermal amplification: An innovative gene amplification technique for animal diseases. Vet. World 2016, 9, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef]
- Schneider, L.; Blakely, H.; Tripathi, A. Mathematical model to reduce loop mediated isothermal amplification (LAMP) false-positive diagnosis. Electrophoresis 2019, 40, 2706–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.S.; Bhadra, S.; Li, B.; Wu, Y.R.; Milligan, J.N.; Ellington, A.D. Robust strand exchange reactions for the sequence-specific, real-time detection of nucleic acid amplicons. Anal. Chem. 2015, 87, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Jiang, Y.S.; Kumar, M.R.; Johnson, R.F.; Hensley, L.E.; Ellington, A.D. Real-time sequence-validated loop-mediated isothermal amplification assays for detection of middle east respiratory syndrome coronavirus (MERS-CoV). PLoS ONE 2015, 10, e0123126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadra, S.; Riedel, T.E.; Saldaña, M.A.; Hegde, S.; Pederson, N.; Hughes, G.L.; Ellington, A.D. Direct nucleic acid analysis of mosquitoes for high fidelity species identification and detection of Wolbachia using a cellphone. PLoS Negl. Trop. Dis. 2018, 12, e0006671. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.S.; Riedel, T.E.; Popoola, J.A.; Morrow, B.R.; Cai, S.; Ellington, A.D.; Bhadra, S. Portable platform for rapid in-field identification of human fecal pollution in water. Water Res. 2018, 131, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)—Review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2018, 60, 201–224. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. Microfluidics-integrated biosensing platforms as emergency tools for on-site field detection of foodborne pathogens. TrAC Trends Anal. Chem. 2020, 125, 115831. [Google Scholar] [CrossRef]
- Sayad, A.; Ibrahim, F.; Uddin, S.M.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 100, 96–104. [Google Scholar] [CrossRef]
- Day, A.S.; Ulep, T.-H.; Budiman, E.; Dieckhaus, L.; Safavinia, B.; Hertenstein, T.; Yoon, J.-Y. Contamination-resistant, rapid emulsion-based isothermal nucleic acid amplification with Mie-scatter inspired light scatter analysis for bacterial identification. Sci. Rep. 2021, 11, 9933. [Google Scholar] [CrossRef]
- Wang, D.-G.; Brewster, J.D.; Paul, M.; Tomasula, P.M. Two methods for increased specificity and sensitivity in loop-mediated isothermal amplification. Molecules 2015, 20, 6048–6059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, A.; Kuentzel, M.; Chittur, S.V. Rapid, affordable, and scalable SARS-CoV-2 detection from saliva. J. Biomol. Tech. JBT 2021, 32, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Jainonthee, C.; Chaisowwong, W.; Ngamsanga, P.; Wiratsudakul, A.; Meeyam, T.; Pichpol, D. A cutoff determination of real-time loop-mediated isothermal amplification (LAMP) for end-point detection of Campylobacter jejuni in chicken meat. Vet. Sci. 2022, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Subali, A.D.; Wiyono, L. Reverse transcriptase loop mediated isothermal amplification (RT-LAMP) for COVID-19 diagnosis: A systematic review and meta-analysis. Pathog. Glob. Health 2021, 115, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Ismail, I.; Mustapa, N.I.; Lai, M.Y.; Soh, T.S.T.; Hassan, A.; Peariasamy, K.M.; Lee, Y.L.; Chong, Y.M.; Sam, I.-C.; et al. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of SARS-CoV-2. PeerJ 2020, 8, e9278. [Google Scholar] [CrossRef]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020, 26, 773–779. [Google Scholar] [CrossRef]
- Mohon, A.N.; Oberding, L.; Hundt, J.; van Marle, G.; Pabbaraju, K.; Berenger, B.M.; Lisboa, L.; Griener, T.; Czub, M.; Doolan, C.; et al. Optimization and clinical validation of dual-target RT-LAMP for SARS-CoV-2. J. Virol. Methods 2020, 286, 113972. [Google Scholar] [CrossRef]
- Chow, F.W.-N.; Chan, T.T.-Y.; Tam, A.R.; Zhao, S.; Yao, W.; Fung, J.; Cheng, F.K.-K.; Lo, G.C.-S.; Chu, S.; Aw-Yong, K.L.; et al. A rapid, simple, inexpensive, and mobile colorimetric assay COVID-19-LAMP for mass on-site screening of COVID-19. Int. J. Mol. Sci. 2020, 21, 5380. [Google Scholar] [CrossRef]
- Arnaout, R.; Lee, R.A.; Lee, G.R.; Callahan, C.; Yen, C.F.; Smith, K.P.; Arora, R.; Kirby, J.E. SARS-CoV2 Testing: The Limit of Detection Matters. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.06.02.131144v1.full.pdf+html (accessed on 1 May 2022). [CrossRef]
- Ali, N.; Rampazzo, R.; Costa, A.D.T.; Krieger, M.A. Current nucleic acid extraction methods and their implications to point-of-care diagnostics. BioMed Res. Int. 2017, 2017, 9306564. [Google Scholar] [CrossRef] [Green Version]
- Dineva, M.A.; Mahilum-Tapay, L.; Lee, H. Sample preparation: A challenge in the development of point-of-care nucleic acid-based assays for resource-limited settings. Analyst 2007, 132, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.P.; Bender, A.T.; Ngyuen, D.N.; Zhang, J.Y.; Posner, J.D. Nucleic acid sample preparation from whole blood in a paper microfluidic device using isotachophoresis. J. Chromatogr. B 2021, 1163, 122494. [Google Scholar] [CrossRef] [PubMed]
- Price, C.W.; Leslie, D.C.; Landers, J.P. Nucleic acid extraction techniques and application to the microchip. Lab Chip 2009, 9, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.M.; Nixon, G.; Ahern, J.; Balachandran, W. A magnetic bead-based DNA extraction and purification microfluidic device. Microfluid. Nanofluid. 2011, 11, 157–165. [Google Scholar] [CrossRef]
- Boehme, C.C.; Nabeta, P.; Hillemann, D.; Nicol, M.P.; Shenai, S.; Krapp, F.; Allen, J.; Tahirli, R.; Blakemore, R.; Rustomjee, R.; et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 2010, 363, 1005–1015. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.C.; Yiap, B.C. DNA, RNA, and protein extraction: The past and the present. J. Biomed. Biotechnol. 2009, 2009, 574398. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Pawlowski, D.R.; Karalus, R.J. Electricity-free, sequential nucleic acid and protein isolation. J. Vis. Exp. 2012, 63, e4202. [Google Scholar] [CrossRef] [Green Version]
- Archer, M.J.; Lin, B.; Wang, Z.; Stenger, D.A. Magnetic bead-based solid phase for selective extraction of genomic DNA. Anal. Biochem. 2006, 355, 285–297. [Google Scholar] [CrossRef]
- Berensmeier, S. Magnetic particles for the separation and purification of nucleic acids. Appl. Microbiol. Biotechnol. 2006, 73, 495–504. [Google Scholar] [CrossRef]
- Adams, N.M.; Bordelon, H.; Wang, K.-K.A.; Albert, L.E.; Wright, D.W.; Haselton, F.R. Comparison of three magnetic bead surface functionalities for RNA extraction and detection. ACS Appl. Mater. Interfaces 2015, 7, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP diagnostics at the point-of-care: Emerging trends and perspectives for the developer community. Expert Rev. Mol. Diagn. 2021, 21, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kohl, E.; Djandji, A.; Morgan, S.; Whittier, S.; Mansukhani, M.; Hod, E.; D’Alton, M.; Suh, Y.; Williams, Z. Direct diagnostic testing of SARS-CoV-2 without the need for prior RNA extraction. Sci. Rep. 2021, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.M.; Cahill, J.; Aidelberg, G.; Aronoff, R.; Bektaş, A.; Bezdan, D.; Butler, D.J.; Chittur, S.V.; Codyre, M.; Federici, F.; et al. Loop-mediated isothermal amplification detection of SARS-CoV-2 and myriad other applications. J. Biomol. Tech. 2021, 32, 228–275. [Google Scholar] [CrossRef]
- Kumar, S.; Gallagher, R.; Bishop, J.; Kline, E.; Buser, J.; Lafleur, L.; Shah, K.; Lutz, B.; Yager, P. Long-term dry storage of enzyme-based reagents for isothermal nucleic acid amplification in a porous matrix for use in point-of-care diagnostic devices. Analyst 2020, 145, 6875–6886. [Google Scholar] [CrossRef]
- Magro, L.; Escadafal, C.; Garneret, P.; Jacquelin, B.; Kwasiborski, A.; Manuguerra, J.-C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper microfluidics for nucleic acid amplification testing (NAAT) of infectious diseases. Lab Chip 2017, 17, 2347–2371. [Google Scholar] [CrossRef] [Green Version]
- Mauk, M.G.; Song, J.; Liu, C.; Bau, H.H. Simple approaches to minimally-instrumented, microfluidic-based point-of-care nucleic acid amplification tests. Biosensors 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Magro, L.; Jacquelin, B.; Escadafal, C.; Garneret, P.; Kwasiborski, A.; Manuguerra, J.-C.; Monti, F.; Sakuntabhai, A.; Vanhomwegen, J.; Lafaye, P.; et al. Paper-based RNA detection and multiplexed analysis for Ebola virus diagnostics. Sci. Rep. 2017, 7, 1347. [Google Scholar] [CrossRef]
- Coz, E.; Garneret, P.; Martin, E.; do Nascimento, D.F.; Vilquin, A.; Hoinard, D.; Feher, M.; Grassin, Q.; Vanhomwegen, J.; Manuguerra, J.C.; et al. Clinical Evaluation of a RT-LAMP SARS-CoV-2 Test for the Point-of-Care, Rapid, Low-Cost, Integrating Sample Solid Phase Extraction and on Which Reagents Are Lyophilized. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.10.03.21264480v1 (accessed on 1 May 2022). [CrossRef]
- Nzelu, C.; Kato, H.; Peters, N.C. Loop-mediated isothermal amplification (LAMP): An advanced molecular point-of-care technique for the detection of Leishmania infection. PLoS Negl. Trop. Dis. 2019, 13, e0007698. [Google Scholar] [CrossRef] [Green Version]
- Ganguli, A.; Ornob, A.; Yu, H.; Damhorst, G.; Chen, W.; Sun, F.; Bhuiya, A.; Cunningham, B.T.; Bashir, R. Hands-free smartphone-based diagnostics for simultaneous detection of Zika, Chikungunya, and Dengue at point-of-care. Biomed. Microdevices 2017, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Pandian, V.; Mauk, M.G.; Bau, H.H.; Cherry, S.; Tisi, L.C.; Liu, C. Smartphone-based mobile detection platform for molecular diagnostics and spatiotemporal disease mapping. Anal. Chem. 2018, 90, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.J.; Athamanolap, P.; Chen, L.; Hardick, J.; Lewis, M.; Hsieh, Y.H.; Rothman, R.E.; Gaydos, C.A.; Wang, T.H. Mobile nucleic acid amplification testing (mobiNAAT) for Chlamydia trachomatis screening in hospital emergency department settings. Sci. Rep. 2017, 7, 4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heithoff, D.M.; Barnes, L.; Mahan, S.P.; Fox, G.N.; Arn, K.E.; Ettinger, S.J.; Bishop, A.M.; Fitzgibbons, L.N.; Fried, J.C.; Low, D.A.; et al. Assessment of a smartphone-based loop-mediated isothermal amplification assay for detection of SARS-CoV-2 and influenza viruses. JAMA Netw. Open 2022, 5, e2145669. [Google Scholar] [CrossRef]
- Ning, B.; Yu, T.; Zhang, S.; Huang, Z.; Tian, D.; Lin, Z.; Niu, A.; Golden, N.; Hensley, K.; Threeton, B.; et al. A smartphone-read ultrasensitive and quantitative saliva test for COVID-19. Sci. Adv. 2021, 7, eabe3703. [Google Scholar] [CrossRef]
- Ganguli, A.; Mostafa, A.; Berger, J.; Aydin, M.Y.; Sun, F.; de Ramirez, S.A.S.; Valera, E.; Cunningham, B.T.; King, W.P.; Bashir, R. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 22727–22735. [Google Scholar] [CrossRef]
- Patel, J.C.; Lucchi, N.W.; Srivastava, P.; Lin, J.T.; Sug-Aram, R.; Aruncharus, S.; Bharti, P.K.; Shukla, M.M.; Congpuong, K.; Satimai, W.; et al. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J. Infect. Dis. 2014, 210, 1180–1187. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, H.; González, I.J.; Polley, S.D.; Angutoko, P.; Ategeka, J.; Asiimwe, C.; Agaba, B.; Kyabayinze, D.; Sutherland, C.; Perkins, M.D.; et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: Performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J. Infect. Dis. 2013, 208, 645–652. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; DaCal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Health 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Kudyba, H.M.; Louzada, J.; Ljolje, D.; Kudyba, K.A.; Muralidharan, V.; Oliveira-Ferreira, J.; Lucchi, N.W. Field evaluation of malaria malachite green loop-mediated isothermal amplification in health posts in Roraima state, Brazil. Malar. J. 2019, 18, 98. [Google Scholar] [CrossRef] [Green Version]
- Priye, A.; Bird, S.W.; Light, Y.K.; Ball, C.S.; Negrete, O.A.; Meagher, R. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep. 2017, 7, 44778. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Instrument-free point-of-care molecular detection of zika virus. Anal. Chem. 2016, 88, 7289–7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Future Market Insights. Loop-Mediated Isothermal Amplification (LAMP) Market. Available online: https://www.futuremarketinsights.com/reports/loop-mediated-isothermal-amplification-market (accessed on 16 March 2022).
- Thermo Fisher Scientific. Invitrogen™ SuperScript™ IV RT-LAMP Master Mix. Available online: https://www.thermofisher.com/order/catalog/product/A51803?SID=srch-srp-A51803 (accessed on 28 April 2022).
- New England BioLabs. WarmStart® LAMP Kit (DNA & RNA). Available online: https://www.neb.com/products/e1700-warmstart-lamp-kit-dna-rna#Product%20Information (accessed on 28 April 2022).
- New England BioLabs. WarmStart® Colorimetric LAMP 2X Master Mix with UDG. Available online: https://www.neb.com/products/m1804-warmstart-colorimetric-lamp-2x-master-mix-with-udg#Product%20Information (accessed on 28 April 2022).
- Meridian BIOSCIENCE®. Lyo-Ready™ RT-LAMP 1-Step Mix, 4x. Available online: https://collateral.meridianlifescience.com/view/159769789/ (accessed on 28 April 2022).
- Meridian BIOSCIENCE®. Air-Dryable™ LAMP/RT-LAMP Mixes. Available online: https://www.meridianbioscience.com/lifescience/products/molecular-reagents/isothermal-amplification/air-dryable-lamp-rt-lamp-mixes/?country=US (accessed on 28 April 2022).
- Eiken Chemical Co., Ltd. LAMP Detection Kit. Available online: https://www.eiken.co.jp/en/products/lamp/detection_kit/ (accessed on 28 April 2022).
- Lau, Y.-L.; Lai, M.-Y.; Teoh, B.-T.; Abd-Jamil, J.; Johari, J.; Sam, S.-S.; Tan, K.-K.; Abubakar, S. Colorimetric detection of dengue by single tube reverse-transcription-loop-mediated isothermal amplification. PLoS ONE 2015, 10, e0138694. [Google Scholar] [CrossRef]
- Cook, J.; Aydin-Schmidt, B.; González, I.J.; Bell, D.; Edlund, E.; Nassor, M.H.; Msellem, M.; Ali, A.; Abass, A.K.; Mårtensson, A.; et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar. J. 2015, 14, 43. [Google Scholar] [CrossRef]
- Nliwasa, M.; MacPherson, P.; Chisala, P.; Kamdolozi, M.; Khundi, M.; Kaswaswa, K.; Mwapasa, M.; Msefula, C.; Sohn, H.; Flach, C.; et al. The sensitivity and specificity of loop-mediated isothermal amplification (LAMP) assay for tuberculosis diagnosis in adults with chronic cough in Malawi. PLoS ONE 2016, 11, e0155101. [Google Scholar] [CrossRef] [Green Version]
- Adams, E.R.; Schoone, G.; Versteeg, I.; Gomez, M.A.; Diro, E.; Mori, Y.; Perlee, D.; Downing, T.; Saravia, N.; Assaye, A.; et al. Development and evaluation of a novel loop-mediated isothermal amplification assay for diagnosis of cutaneous and visceral leishmaniasis. J. Clin. Microbiol. 2018, 56, e00386-18. [Google Scholar] [CrossRef] [Green Version]
- Geisler, J. Choosing the Best Detection Method: Absorbance vs. Fluorescence. Available online: https://www.biocompare.com/Bench-Tips/173963-Choosing-the-Best-Detection-Method-Absorbance-vs-Fluorescence/ (accessed on 15 June 2022).
- Fischbach, J.; Xander, N.C.; Frohme, M.; Glökler, J.F. Shining a light on LAMP assays’ A comparison of LAMP visualization methods including the novel use of berberine. BioTechniques 2015, 58, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.T.; Layne, T.R.; O’Connell, K.C.; Tanner, N.A.; Landers, J.P. Comparative evaluation and quantitative analysis of loop-mediated isothermal amplification indicators. Anal. Chem. 2020, 92, 13343–13353. [Google Scholar] [CrossRef]
- Hixson, J.L.; Ward, A.S. Hardware selection and performance of low-cost fluorometers. Sensors 2022, 22, 2319. [Google Scholar] [CrossRef]
- Hach. 2100Q Portable Turbidimeter. Available online: https://www.hach.com/turbidimeters/2100q-portable-turbidimeter/family?productCategoryId=35547372710# (accessed on 28 April 2022).
- Thermo Fisher Scientific. Qubit 4 Fluorometer. Available online: https://www.thermofisher.com/us/en/home/industrial/spectroscopy-elemental-isotope-analysis/molecular-spectroscopy/fluorometers/qubit/qubit-fluorometer.html (accessed on 28 April 2022).
- Alidans. AssayColor. Available online: http://www.alidans.com/AssayColor/ (accessed on 28 April 2022).
- Hossain, A.; Canning, J.; Ast, S.; Rutledge, P.J.; Yen, T.L.; Jamalipour, A. Lab-in-a-phone: Smartphone-based portable fluorometer for pH measurements of environmental water. IEEE Sens. J. 2015, 15, 5095–5102. [Google Scholar] [CrossRef] [Green Version]
- Koydemir, H.C.; Rajpal, S.; Gumustekin, E.; Karinca, D.; Liang, K.; Göröcs, Z.; Tseng, D.; Ozcan, A. Smartphone-based turbidity reader. Sci. Rep. 2019, 9, 19901. [Google Scholar] [CrossRef] [PubMed]
- Meridian BIOSCIENCE®. Alethia®. Available online: https://www.meridianbioscience.com/diagnostics/platforms/molecular/alethia/?country=US (accessed on 17 June 2022).
- Launch Diagnostics. Alethia LAMP Technology. Available online: https://www.launchdiagnostics.com/product-list/alethia-lamp-technology/ (accessed on 17 June 2022).
- Lucchi, N.W.; Gaye, M.; Diallo, M.A.; Goldman, I.F.; Ljolje, D.; Deme, A.B.; Badiane, A.; Ndiaye, Y.D.; Barnwell, J.W.; Udhayakumar, V.; et al. Evaluation of the illumigene malaria LAMP: A robust molecular diagnostic tool for malaria parasites. Sci. Rep. 2016, 6, 36808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher Scientific. Meridian Bioscience™ Alethia™ Mycoplasma Direct DNA Amplification Assay. Available online: https://www.fishersci.com/shop/products/alethia-myco-test-kit-50tst-ea/23029012 (accessed on 28 April 2022).
- Kurosaki, Y.; Magassouba, N.; Oloniniyi, O.K.; Cherif, M.S.; Sakabe, S.; Takada, A.; Hirayama, K.; Yasuda, J. Development and evaluation of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay coupled with a portable device for rapid diagnosis of Ebola virus disease in Guinea. PLoS Negl. Trop. Dis. 2016, 10, e0004472. [Google Scholar] [CrossRef]
- Pro-Labs Diagnostics. Genie® III Instrument. Available online: https://www.pro-lab-direct.com/category-s/1892.htm (accessed on 17 June 2022).
- OptiGene. Genie® III. Available online: http://www.optigene.co.uk/instruments/instrument-genie-iii/ (accessed on 17 June 2022).
- Lucira™. Lucira’s Molecular COVID-19 Testing Studies. Available online: https://checkit.lucirahealth.com/ (accessed on 17 June 2022).
- Detect™. Detect—PCR-Quality COVID-19 Test Results in 1 Hour, Right at Home. Available online: https://detect.com/press?_gl=1*1o0431l*_ga*NjEyMjYyNzg3LjE2NTY2MTI3MTM.*_ga_0WDWEKG1H8*MTY1NjYxMjcxMy4xLjEuMTY1NjYxMzIzOC4zMg (accessed on 30 June 2022).
- Lee, S.; Khoo, V.S.L.; Medriano, C.A.D.; Lee, T.; Park, S.-Y.; Bae, S. Rapid and in-situ detection of fecal indicator bacteria in water using simple DNA extraction and portable loop-mediated isothermal amplification (LAMP) PCR methods. Water Res. 2019, 160, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Jayawardena, A.; Chan, J.; Tan, S.M.; Alan, T.; Kwan, P. An ultra-portable, self-contained point-of-care nucleic acid amplification test for diagnosis of active COVID-19 infection. Sci. Rep. 2021, 11, 15176. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Umer, M.; Dirr, L.; Bailly, B.; Guillon, P.; von Itzstein, M.; Soda, N.; Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.-T. A portable device for LAMP based detection of SARS-CoV-2. Micromachines 2021, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Malic, L.; Brassard, D.; Da Fonte, D.; Nassif, C.; Mounier, M.; Ponton, A.; Geissler, M.; Shiu, M.; Morton, K.J.; Veres, T. Automated Sample-to-Answer Centrifugal Microfluidic System for Rapid Molecular Diagnostics of SARS-CoV-2. Lab Chip 2022. Available online: https://pubs.rsc.org/en/Content/ArticleLanding/2022/LC/D2LC00242F (accessed on 1 May 2022). [CrossRef]
- Hu, S.; Jie, Y.; Jin, K.; Zhang, Y.; Guo, T.; Huang, Q.; Mei, Q.; Ma, F.; Ma, H. All-in-one digital microfluidics system for molecular diagnosis with loop-mediated isothermal amplification. Biosensors 2022, 12, 324. [Google Scholar] [CrossRef]
- Kortela, E.; Kirjavainen, V.; Ahava, M.J.; Jokiranta, S.T.; But, A.; Lindahl, A.; Jääskeläinen, A.E.; Jääskeläinen, A.J.; Järvinen, A.; Jokela, P.; et al. Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients. PLoS ONE 2021, 16, e0251661. [Google Scholar] [CrossRef]
- Österdahl, M.F.; Lee, K.A.; Ni Lochlainn, M.; Wilson, S.; Douthwaite, S.; Horsfall, R.; Sheedy, A.; Goldenberg, S.D.; Stanley, C.J.; Spector, T.D.; et al. Detecting SARS-CoV-2 at point of care: Preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR). BMC Infect. Dis. 2020, 20, 783. [Google Scholar] [CrossRef] [PubMed]
- Artik, Y.; Coşğun, A.B.; Cesur, N.P.; Hızel, N.; Uyar, Y.; Sur, H.; Ayan, A. Comparison of COVID-19 laboratory diagnosis by commercial kits: Effectivity of RT-PCR to the RT-LAMP. J. Med. Virol. 2022, 94, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Usherwood, T.; Zhang, L.; Tripathi, A. The path forward for COVID-19 diagnostics. Mol. Diagn. Ther. 2020, 24, 637–639. [Google Scholar] [CrossRef]
- Pu, R.; Liu, S.; Ren, X.; Shi, D.; Ba, Y.; Huo, Y.; Zhang, W.; Ma, L.; Liu, Y.; Yang, Y.; et al. The screening value of RT-LAMP and RT-PCR in the diagnosis of COVID-19: Systematic review and meta-analysis. J. Virol. Methods 2022, 300, 114392. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Real-Time Learning Network. Rapid Testing. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/rapid-testing/ (accessed on 17 June 2022).
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 2021, CD013705. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Potential for False Positive Results with Antigen Tests for Rapid Detection of SARS-CoV-2—Letter to Clinical Laboratory Staff and Health Care Providers. Available online: https://www.fda.gov/medical-devices/letters-health-care-providers/potential-false-positive-results-antigen-tests-rapid-detection-sars-cov-2-letter-clinical-laboratory (accessed on 1 May 2022).
- Gans, J.S.; Goldfarb, A.; Agrawal, A.K.; Sennik, S.; Stein, J.; Rosella, L. False-positive results in rapid antigen tests for SARS-CoV-2. JAMA 2022, 327, 485–486. [Google Scholar] [CrossRef]
- Makarova, J.A.; Fomicheva, K.A.; Osipyants, A.I.; Shkurnikov, M.Y.; Pokryshchenko, A.A.; Tonevitsky, E.A.; Vechorko, V.I. Loop-mediated isothermal amplification as a promising method for mass COVID-19 diagnostics. Appl. Biochem. Microbiol. 2021, 57, 845–850. [Google Scholar] [CrossRef]
- Park, G.-S.; Ku, K.; Baek, S.-H.; Kim, S.-J.; Kim, S.I.; Kim, B.-T.; Maeng, J.-S. Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J. Mol. Diagn. 2020, 22, 729–735. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Das, S.; Ahmed, R.; Mori, Y.; Notomi, T.; Kevadiya, B.D.; Thakor, A.S. Loop-mediated isothermal amplification (LAMP): A rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 2020, 9, 182. [Google Scholar] [CrossRef]
- Nicolini, A.M.; Toth, T.D.; Kim, S.Y.; Mandel, M.A.; Galbraith, D.W.; Yoon, J. Mie scatter and interfacial tension based real-time quantification of colloidal emulsion nucleic acid amplification. Adv. Biosyst. 2017, 1, 1700098. [Google Scholar] [CrossRef]
- Day, A.S.; Ulep, T.-H.; Safavinia, B.; Hertenstein, T.; Budiman, E.; Dieckhaus, L.; Yoon, J.-Y. Emulsion-based isothermal nucleic acid amplification for rapid SARS-CoV-2 detection via angle-dependent light scatter analysis. Biosens. Bioelectron. 2021, 179, 113099. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Research Group | Clinical Sensitivity (%) | Clinical Specificity (%) | Total Samples Tested | LoD (Copies/mL) |
|---|---|---|---|---|
| Lau et al. [37] | 100 | 100 | 89 | 1 |
| Yan et al. [38] | 100 | 100 | 130 | 20 |
| Mohon et al. [39] | 98.5 | 100 | 124 | 25 |
| Chow et al. [40] | 98.2 | 100 | 366 | 42 |
| Alethia | Genie III | Lucira | Detect | |
|---|---|---|---|---|
| Dimension | A4 paper | 250 × 165 × 85 mm | A cup | A cup |
| Sample type | Extracted DNA | Extracted DNA | Nasal swab | Nasal swab |
| Assay time | 1 h | 15 min | 30 min | 1 h |
| Clinical sensitivity | 96.0% | 97.9% | 92% | 90.9% |
| Clinical specificity | - | 100% | 97% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannier, C.; Yoon, J.-Y. Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled? Biosensors 2022, 12, 492. https://doi.org/10.3390/bios12070492
Mannier C, Yoon J-Y. Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled? Biosensors. 2022; 12(7):492. https://doi.org/10.3390/bios12070492
Chicago/Turabian StyleMannier, Cassidy, and Jeong-Yeol Yoon. 2022. "Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled?" Biosensors 12, no. 7: 492. https://doi.org/10.3390/bios12070492
APA StyleMannier, C., & Yoon, J.-Y. (2022). Progression of LAMP as a Result of the COVID-19 Pandemic: Is PCR Finally Rivaled? Biosensors, 12(7), 492. https://doi.org/10.3390/bios12070492






