Large Area Microfluidic Bioreactor for Production of Recombinant Protein
Abstract
:1. Introduction
2. Materials and Methods
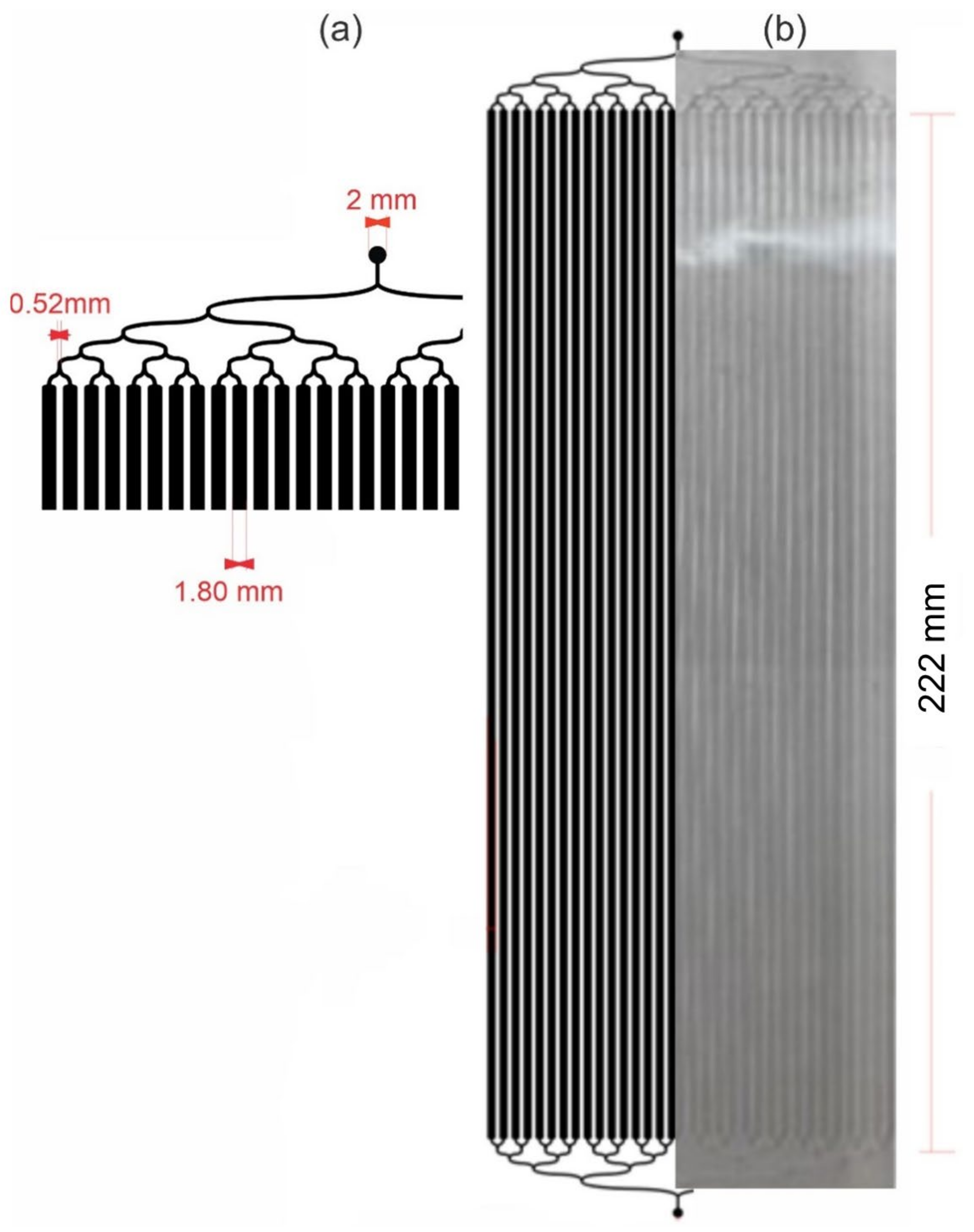
2.1. LM Bioreactor Design and Fabrication
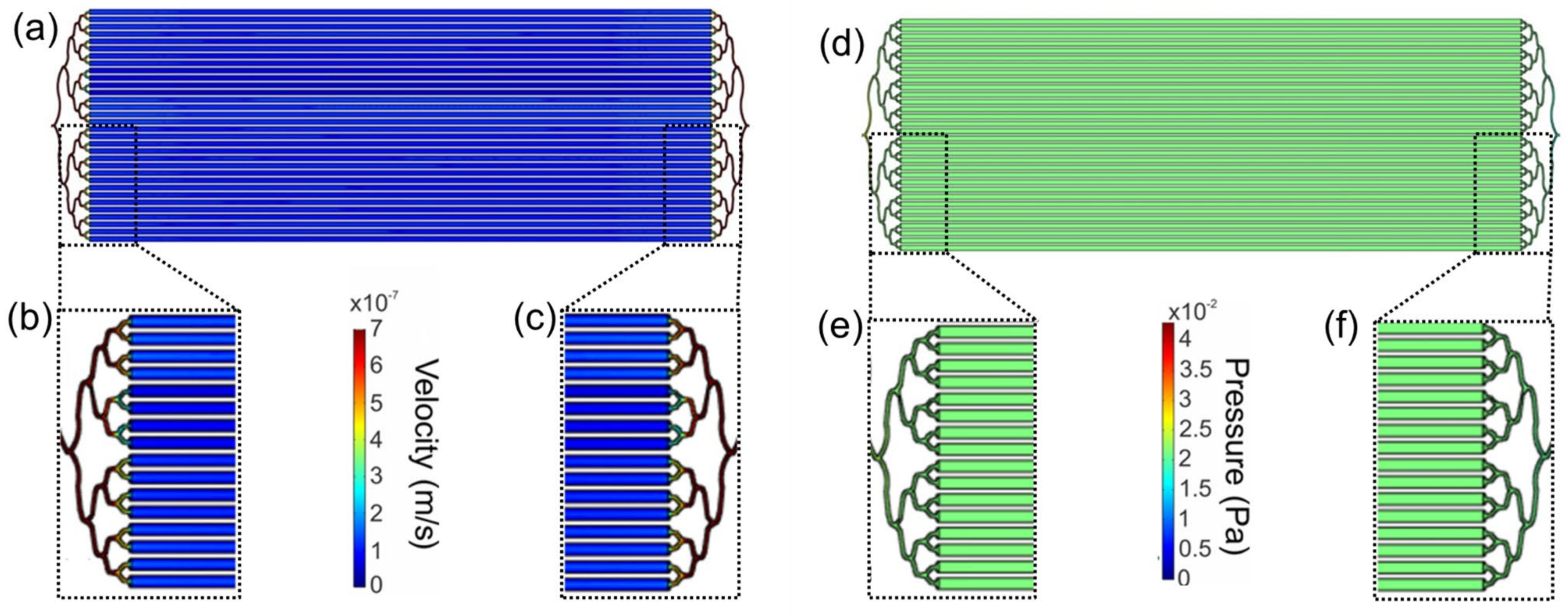
2.2. Computational Fluid Dynamics Characterization
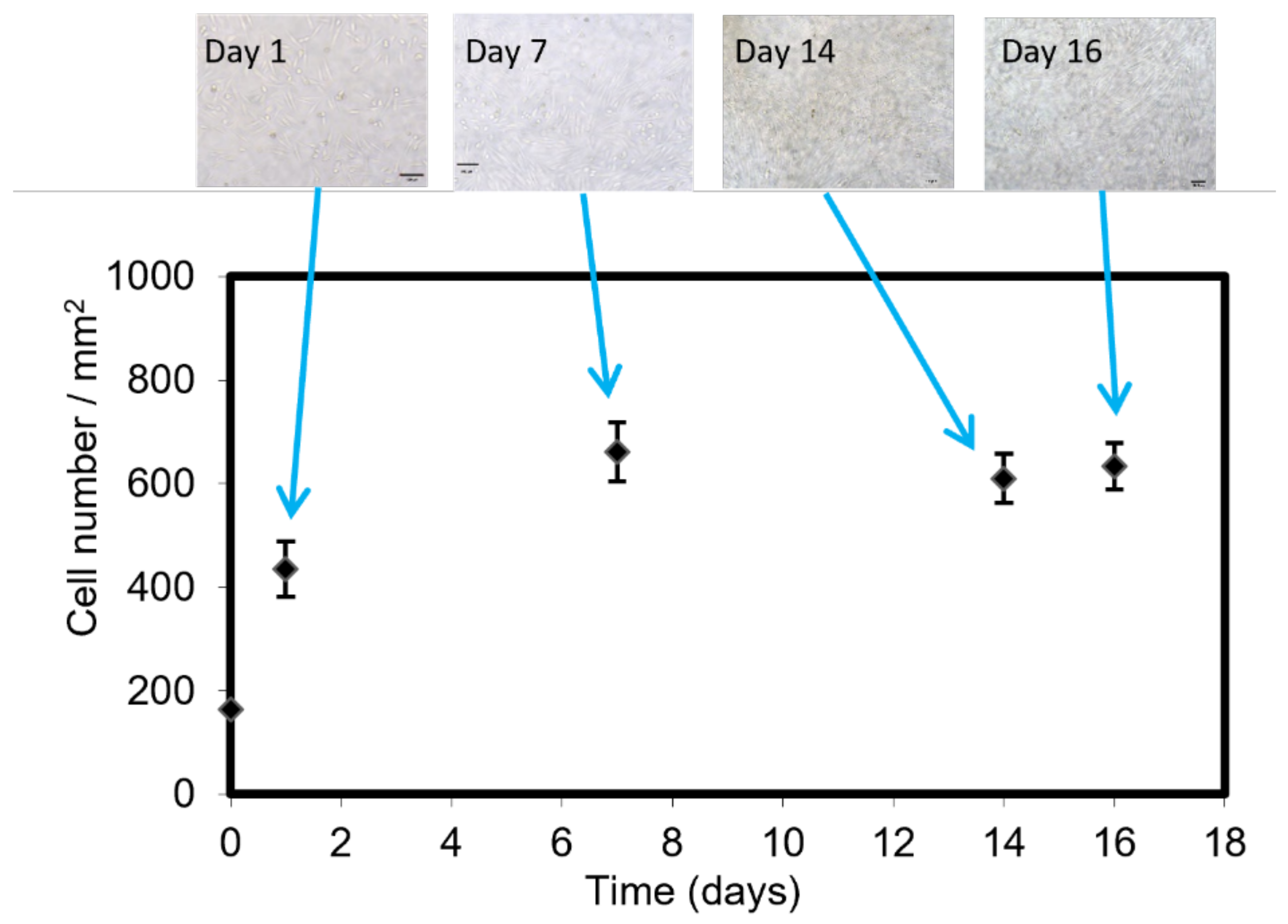
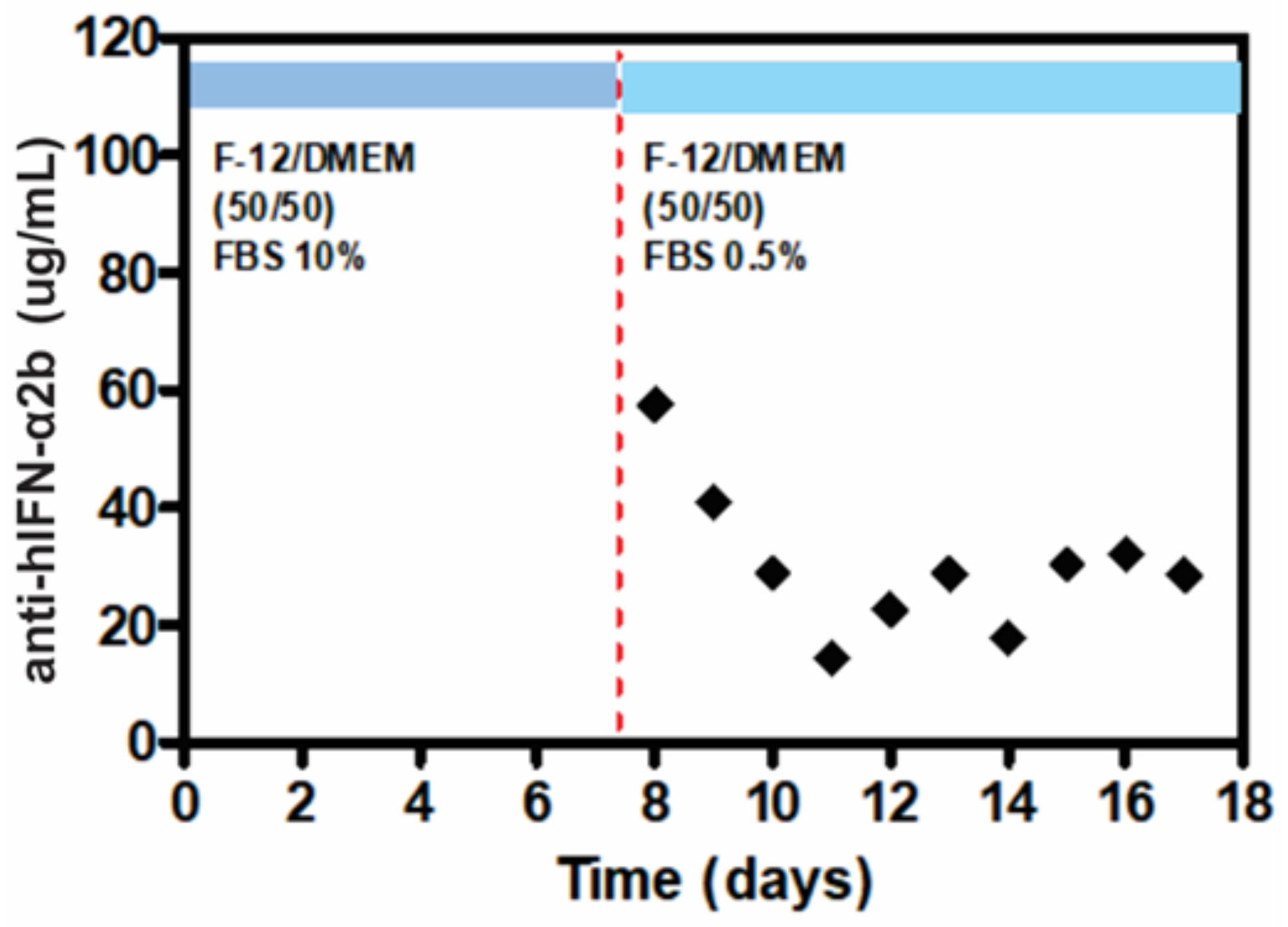
2.3. Cell Culture in the LM Bioreactor
2.4. Quantification of Glucose and Lactate in the Culture Medium
2.5. ELISA Quantification of Anti-hIFN-α2b
2.6. Inhibition of the Antiproliferative Activity of rhIFN-α2b by Anti-hIFN-α2b
2.7. Determination of Affinity Constants of Anti-hIFN-α2b
2.8. Inhibition of rhIFN-α2b Cell Signaling by Anti-hIFN-α2b
2.9. Inhibition of the Antiviral Activity of rhIFN by Anti-hIFN-α2b
2.10. Statistical Analysis
3. Results and Discussion
3.1. LM Bioreactor Manufacturing
3.2. Computational Modeling of the Flow in LM Bioreactor
3.3. Cell Growth in the LM Bioreactor and Glucose/Lactate Quantification
3.4. mAb Production in the LM Bioreactor
3.5. Characterization of the Inhibition of Antiproliferative Activity, Inhibition of Cell Signaling, and Inhibition of the Antiviral Activity of rhIFN-α2b by Antibodies Produced in LM Bioreactor and T-Flasks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Gao, Y.; Hao, Y.; Li, E.; Wang, Y.; Zhang, J.; Wang, W.; Gao, Z.; Wang, Q. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Sakai, Y.; Fujii, T. Cell culture in 3-dimensional microfluidic structure of PDMS (polydimethylsiloxane). Biomed. Microdevices 2003, 5, 109–114. [Google Scholar] [CrossRef]
- Mäki, A.-J.; Peltokangas, M.; Kreutzer, J.; Auvinen, S.; Kallio, P. Modeling carbon dioxide transport in PDMS-based microfluidic cell culture devices. Chem. Engl. Sci. 2015, 137, 515–524. [Google Scholar] [CrossRef]
- Huber, D.; Oskooei, A.; Casadevall i Solvas, X.; Demello, A.; Kaigala, G. V Hydrodynamics in cell studies. Chem. Rev. 2018, 118, 2042–2079. [Google Scholar] [CrossRef] [Green Version]
- Lecault, V.; White, A.K.; Singhal, A.; Hansen, C.L. Microfluidic single cell analysis: From promise to practice. Curr. Opin. Chem. Biol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Albelda, S.M.; Buck, C.A. Integrins and other cell adhesion molecules. FASEB J. 1990, 4, 2868–2880. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131–E138. [Google Scholar] [CrossRef]
- Zhu, X.; Bouffanais, R.; Yue, D.K.P. Interplay between motility and cell-substratum adhesion in amoeboid cells. Biomicrofluidics 2015, 9, 54112. [Google Scholar] [CrossRef] [Green Version]
- DeMeester, S.L.; Cobb, J.P.; Hotchkiss, R.S.; Osborne, D.F.; Karl, I.E.; Tinsley, K.W.; Buchman, T.G. Stress-induced fractal rearrangement of the endothelial cell cytoskeleton causes apoptosis. Surgery 1998, 124, 362–371. [Google Scholar] [CrossRef]
- Kim, L.; Toh, Y.-C.; Voldman, J.; Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 2007, 7, 681–694. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Karadimitriou, N.K.; Hassanizadeh, S.M. A Review of Micromodels and Their Use in Two-Phase Flow Studies. Vadose Zone J. 2012, 11, vzj2011.0072. [Google Scholar] [CrossRef]
- Jang, M.; Kwon, Y.J.; Lee, N.Y. Non-photolithographic plastic-mold-based fabrication of cylindrical and multi-tiered poly (dimethylsiloxane) microchannels for biomimetic lab-on-a-chip applications. RSC Adv. 2015, 5, 100905–100911. [Google Scholar] [CrossRef] [Green Version]
- Haswell, S.J.; Middleton, R.J.; O’Sullivan, B.; Skelton, V.; Watts, P.; Styring, P. The application of micro reactors to synthetic chemistry. Chem. Commun. 2001, 5, 391–398. [Google Scholar] [CrossRef]
- Helguera, G.; Penichet, M.L. Antibody-cytokine fusion proteins for the therapy of cancer. Methods Mol. Med. 2005, 109, 347–374. [Google Scholar] [CrossRef]
- Helguera, G.; Daniels, T.R.; Rodriguez, J.A.; Penichet, M.L. Monoclonal antibodies, human, engineered. Encycl. Ind. Biotechnol. Bioprocess. Biosep. Cell Technol. 2010, 5, 3526–3542. [Google Scholar]
- Jung, E.H.; Sarpatwari, A.; Kesselheim, A.S.; Sinha, M.S. FDA and EMA Biosimilar Approvals. J. Gen. Intern. Med. 2020, 35, 1908–1910. [Google Scholar] [CrossRef]
- Li, F.; Vijayasankaran, N.; Shen, A.; Kiss, R.; Amanullah, A. Cell culture processes for monoclonal antibody production. MAbs 2010, 2, 466–479. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, A.; McElearney, K.; Kshirsagar, R.; Sinacore, M.S.; Ryll, T. Investigation of metabolic variability observed in extended fed batch cell culture. Biotechnol. Prog. 2013, 29, 1519–1527. [Google Scholar] [CrossRef]
- Hilal-Alnaqbi, A.; Hu, A.Y.C.; Zhang, Z.; Al-Rubeai, M. Growth, metabolic activity, and productivity of immobilized and freely suspended CHO cells in perfusion culture. Biotechnol. Appl. Biochem. 2013, 60, 436–445. [Google Scholar] [CrossRef]
- Kshirsagar, R.; McElearney, K.; Gilbert, A.; Sinacore, M.; Ryll, T. Controlling trisulfide modification in recombinant monoclonal antibody produced in fed-batch cell culture. Biotechnol. Bioeng. 2012, 109, 2523–2532. [Google Scholar] [CrossRef]
- Abu-Absi, S.F.; Yang, L.; Thompson, P.; Jiang, C.; Kandula, S.; Schilling, B.; Shukla, A.A. Defining process design space for monoclonal antibody cell culture. Biotechnol. Bioeng. 2010, 106, 894–905. [Google Scholar] [CrossRef]
- Abaci, H.E.; Devendra, R.; Smith, Q.; Gerecht, S.; Drazer, G. Design and development of microbioreactors for long-term cell culture in controlled oxygen microenvironments. Biomed. Microdevices 2012, 14, 145–152. [Google Scholar] [CrossRef]
- Lee, J.E.; Fusco, M.L.; Saphire, E.O. An efficient platform for screening expression and crystallization of glycoproteins produced in human cells. Nat. Protoc. 2009, 4, 592. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, N.; Attallah, C.; Karp, P.; Booth, R.; Peñaherrera, A.; Payés, C.; Oggero, M.; Pérez, M.S.; Helguera, G.; Lerner, B. Production of monoclonal antibodies in microfluidic devices. Integr. Biol. 2018, 10, 136–144. [Google Scholar] [CrossRef]
- Bourguignon, N.; Olmos, C.M.; Sierra-Rodero, M.; Peñaherrera, A.; Rosero, G.; Pineda, P.; Vizuete, K.; Arroyo, C.R.; Cumbal, L.; Lasorsa, C.; et al. Accessible and Cost-Effective Method of PDMS Microdevices Fabrication Using a Reusable Photopolymer Mold. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1433–1442. [Google Scholar] [CrossRef]
- KLayout High Performance Layout Viewer and Editor; Free Silicon Foundation: Halifax, UK, 2018.
- Peñaherrera, A.; Payés, C.; Sierra-Rodero, M.; Vega, M.; Rosero, G.; Lerner, B.; Helguera, G.; Pérez, M.S.S. Evaluation of cell culture in microfluidic chips for application in monoclonal antibody production. Microelectron. Eng. 2016, 158, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Olmos, C.M.; Vaca, A.; Rosero, G.; Peñaherrera, A.; Perez, C.; de Sá Carneiro, I.; Vizuete, K.; Arroyo, C.R.; Debut, A.; Pérez, M.S.; et al. Epoxy resin mold and PDMS microfluidic devices through photopolymer flexographic printing plate. Sens. Actuators B Chem. 2019, 288, 742–748. [Google Scholar] [CrossRef]
- Pérez-Sosa, C.; Pazmiño Peñaherrera, A.B.; Rosero, G.; Bourguignon, N.; Aravelli, A.; Bhansali, S.; Pérez, M.S.; Lerner, B. Novel Reproducible Manufacturing and Reversible Sealing Method for Microfluidic Devices. Micromachines 2022, 13, 650. [Google Scholar] [CrossRef]
- Kodak Flexcel NX. Available online: https://www.miraclon.com/products-technology/our-technology/ (accessed on 1 October 2019).
- Kodak KODAK_DITR_Film. Available online: https://www.miraclon.com/products-technology/solutions-products/kodak-ditr-film/ (accessed on 1 October 2019).
- Olmos, C.M.; Penãherrera, A.; Rosero, G.; Vizuete, K.; Ruarte, D.; Follo, M.; Vaca, A.; Arroyo, C.R.; Debut, A.; Cumbal, L.; et al. Cost-effective fabrication of photopolymer molds with multi-level microstructures for pdms microfluidic device manufacture. RSC Adv. 2020, 10, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Attallah, C.; Etcheverrigaray, M.; Kratje, R.; Oggero, M. A highly efficient modified human serum albumin signal peptide to secrete proteins in cells derived from different mammalian species. Protein Expr. Purif. 2017, 132, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Friguet, B.; Chaffotte, A.F.; Djavadi-Ohaniance, L.; Goldberg, M.E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 1985, 77, 305–319. [Google Scholar] [CrossRef]
- Stevens, F.J. Modification of an ELISA-based procedure for affinity determination: Correction necessary for use with bivalent antibody. Mol. Immunol. 1987, 24, 1055–1060. [Google Scholar] [CrossRef]
- Nederman, T.; Karlström, E.; Sjödin, L. An in vitro bioassay for quantitation of human interferons by measurements of antiproliferative activity on a continuous human lymphoma cell line. Biologicals 1990, 18, 29–34. [Google Scholar] [CrossRef]
- Ceaglio, N.; Etcheverrigaray, M.; Kratje, R.; Oggero, M. Novel long-lasting interferon alpha derivatives designed by glycoengineering. Biochimie 2008, 90, 437–449. [Google Scholar] [CrossRef]
- Bürgi, M.; Prieto, C.; Oggero, M.; Bollati-Fogolín, M.; Etcheverrigaray, M.; Kratje, R. New reporter cell clones to determine the biological activity of human type I interferons. BMC Proc. 2011, 5, P4. [Google Scholar] [CrossRef] [Green Version]
- Paillusson, A.; Hirschi, N.; Vallan, C.; Azzalin, C.M.; Mühlemann, O. A GFP-based reporter system to monitor nonsense-mediated mRNA decay. Nucleic Acids Res. 2005, 33, e54. [Google Scholar] [CrossRef]
- Chen, C.; Townsend, A.D.; Hayter, E.A.; Birk, H.M.; Sell, S.A.; Martin, R.S. Insert-based microfluidics for 3D cell culture with analysis. Anal. Bioanal. Chem. 2018, 410, 3025–3035. [Google Scholar] [CrossRef]
- Christoffersson, J.; Mandenius, C.-F. Using a Microfluidic Device for Culture and Drug Toxicity Testing of 3D Cells. Methods Mol. Biol. 2019, 1994, 235–241. [Google Scholar] [CrossRef]
- Komen, J.; Westerbeek, E.Y.; Kolkman, R.W.; Roesthuis, J.; Lievens, C.; van den Berg, A.; van der Meer, A.D. Controlled pharmacokinetic anti-cancer drug concentration profiles lead to growth inhibition of colorectal cancer cells in a microfluidic device. Lab. Chip 2020, 20, 3167–3178. [Google Scholar] [CrossRef]
- Rahman, S.M.; Campbell, J.M.; Coates, R.N.; Render, K.M.; Byrne, C.E.; Martin, E.C.; Melvin, A.T. Evaluation of intercellular communication between breast cancer cells and adipose-derived stem cells via passive diffusion in a two-layer microfluidic device. Lab Chip 2020, 20, 2009–2019. [Google Scholar] [CrossRef]
- Yang, F.; Carmona, A.; Stojkova, K.; Huitron, E.I.G.; Goddi, A.; Bhushan, A.; Cohen, R.N.; Brey, E.M. A 3D human adipose tissue model within a microfluidic device. Lab Chip 2021, 21, 435–446. [Google Scholar] [CrossRef]
- Huang, M.; Fan, S.; Xing, W.; Liu, C. Microfluidic cell culture system studies and computational fluid dynamics. Math. Comput. Model. 2010, 52, 2036–2042. [Google Scholar] [CrossRef]
- Avraham-Chakim, L.; Elad, D.; Zaretsky, U.; Kloog, Y.; Jaffa, A.; Grisaru, D. Fluid-Flow Induced Wall Shear Stress and Epithelial Ovarian Cancer Peritoneal Spreading. PLoS ONE 2013, 8, e60965. [Google Scholar] [CrossRef] [Green Version]
- Brás, E.J.S.; Chu, V.; Aires-Barros, M.R.; Conde, J.P.; Fernandes, P. A microfluidic platform for physical entrapment of yeast cells with continuous production of invertase. J. Chem. Technol. Biotechnol. 2017, 92, 334–341. [Google Scholar] [CrossRef]
- Su, X.; Theberge, A.B.; January, C.T.; Beebe, D.J. Effect of microculture on cell metabolism and biochemistry: Do cells get stressed in microchannels? Anal. Chem. 2013, 85, 1562–1570. [Google Scholar] [CrossRef] [Green Version]
- Garza-García, L.D.; García-López, E.; Camacho-León, S.; del Refugio Rocha-Pizaña, M.; López-Pacheco, F.; López-Meza, J.; Araiz-Hernández, D.; Tapia-Mejía, E.J.; Trujillo-de Santiago, G.; Rodríguez-González, C.A.; et al. Continuous flow micro-bioreactors for the production of biopharmaceuticals: The effect of geometry, surface texture, and flow rate. Lab Chip 2014, 14, 1320. [Google Scholar] [CrossRef]
- Ballesteros Hernando, J.; Ramos Gómez, M.; Díaz Lantada, A. Modeling Living cells Within Microfluidic Systems Using Cellular Automata Models. Sci. Rep. 2019, 9, 14886. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Hong, S.; Jung, B.; Jeong, S.Y.; Byeon, J.H.; Jeong, G.S.; Choi, J.; Hwang, C. In vitro lung cancer multicellular tumor spheroid formation using a microfluidic device. Biotechnol. Bioeng. 2019, 116, 3041–3052. [Google Scholar] [CrossRef]
- Visone, R.; Ugolini, G.S.; Vinarsky, V.; Penati, M.; Redaelli, A.; Forte, G.; Rasponi, M. A Simple Vacuum-Based Microfluidic Technique to Establish High-Throughput Organs-On-Chip and 3D Cell Cultures at the Microscale. Adv. Mater. Technol. 2019, 4, 1800319. [Google Scholar] [CrossRef] [Green Version]
- Wacker, C.; Berger, C.N.; Girard, P.; Meier, R. Glycosylation profiles of therapeutic antibody pharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 79, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Spearman, M.; Doering, J.; Lattová, E.; Perreault, H.; Butler, M. The availability of glucose to CHO cells affects the intracellular lipid-linked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J. Biotechnol. 2014, 170, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Jimenez Del Val, I.; Müller, C.; Wagtberg Sen, J.; Rasmussen, S.K.; Kontoravdi, C.; Weilguny, D.; Andersen, M.R. Amino acid and glucose metabolism in fed-batch CHO cell culture affects antibody production and glycosylation. Biotechnol. Bioeng. 2015, 112, 521–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.S.; Min, B.S.; Kim, Y.J.; Cho, J.M.; Baek, E.; Cho, M.S.; Lee, G.M. Effect of glucose feeding on the glycosylation quality of antibody produced by a human cell line, F2N78, in fed-batch culture. Appl. Microbiol. Biotechnol. 2014, 98, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Attallah, C.; Aguilar, M.F.; Forno, G.; Etcheverrigaray, M.; Brigido, M.D.M.; Maranhão, A.Q.; Oggero, M. The glycosylation of anti-rhIFN-α2b recombinant antibodies influences the antigen-neutralizing activity. Biotechnol. Lett. 2020, 42, 1369–1381. [Google Scholar] [CrossRef]
- Torres, M.; Fernández-Fuentes, N.; Fiser, A.; Casadevall, A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 2007, 282, 13917–13927. [Google Scholar] [CrossRef] [Green Version]
- Hovenden, M.; Hubbard, M.A.; Aucoin, D.P.; Thorkildson, P.; Reed, D.E.; Welch, W.H.; Lyons, C.R.; Lovchik, J.A.; Kozel, T.R. IgG subclass and heavy chain domains contribute to binding and protection by mAbs to the poly γ-D-glutamic acid capsular antigen of Bacillus anthracis. PLoS Pathog. 2013, 9, e1003306. [Google Scholar] [CrossRef] [Green Version]
- Pritsch, O.; Magnac, C.; Dumas, G.; Bouvet, J.P.; Alzari, P.; Dighiero, G. Can isotype switch modulate antigen-binding affinity and influence clonal selection? Eur. J. Immunol. 2000, 30, 3387–3395. [Google Scholar] [CrossRef]



| Culture condition | Ka(109M−1) |
| T-flask | 1.8 ± 0.1 * |
| LM Bioreactor | 3.6 ± 0.3 * |
| Ratio LM Bioreactor/T-flask = 2 | |
| * Significant differences (p < 0.05) determined by Student’s t-test | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourguignon, N.; Karp, P.; Attallah, C.; Chamorro, D.A.; Oggero, M.; Booth, R.; Ferrero, S.; Bhansali, S.; Pérez, M.S.; Lerner, B.; et al. Large Area Microfluidic Bioreactor for Production of Recombinant Protein. Biosensors 2022, 12, 526. https://doi.org/10.3390/bios12070526
Bourguignon N, Karp P, Attallah C, Chamorro DA, Oggero M, Booth R, Ferrero S, Bhansali S, Pérez MS, Lerner B, et al. Large Area Microfluidic Bioreactor for Production of Recombinant Protein. Biosensors. 2022; 12(7):526. https://doi.org/10.3390/bios12070526
Chicago/Turabian StyleBourguignon, Natalia, Paola Karp, Carolina Attallah, Daniel A. Chamorro, Marcos Oggero, Ross Booth, Sol Ferrero, Shekhar Bhansali, Maximiliano S. Pérez, Betiana Lerner, and et al. 2022. "Large Area Microfluidic Bioreactor for Production of Recombinant Protein" Biosensors 12, no. 7: 526. https://doi.org/10.3390/bios12070526
APA StyleBourguignon, N., Karp, P., Attallah, C., Chamorro, D. A., Oggero, M., Booth, R., Ferrero, S., Bhansali, S., Pérez, M. S., Lerner, B., & Helguera, G. (2022). Large Area Microfluidic Bioreactor for Production of Recombinant Protein. Biosensors, 12(7), 526. https://doi.org/10.3390/bios12070526







