Development of Nanomaterial-Modified Impedimetric Aptasensor—A Single-Step Strategy for 3,4-Methylenedioxymethylamphetamine Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Apparatus and Procedures
2.3. Synthesis and Characterization of Tin Nanoparticles (SnNPs)
2.4. Preparation of AptMDMASolutions
2.5. Electrochemical Characterization of AptMDMA/SnNPs@Au
2.6. Determination of Aptasensor Response to MDMA Analyte
2.7. Optimization of Analytical Parameters
2.8. MDMA Detection in Real Samples
3. Results and Discussion
3.1. Strategy of Aptasensing for MDMA Detection
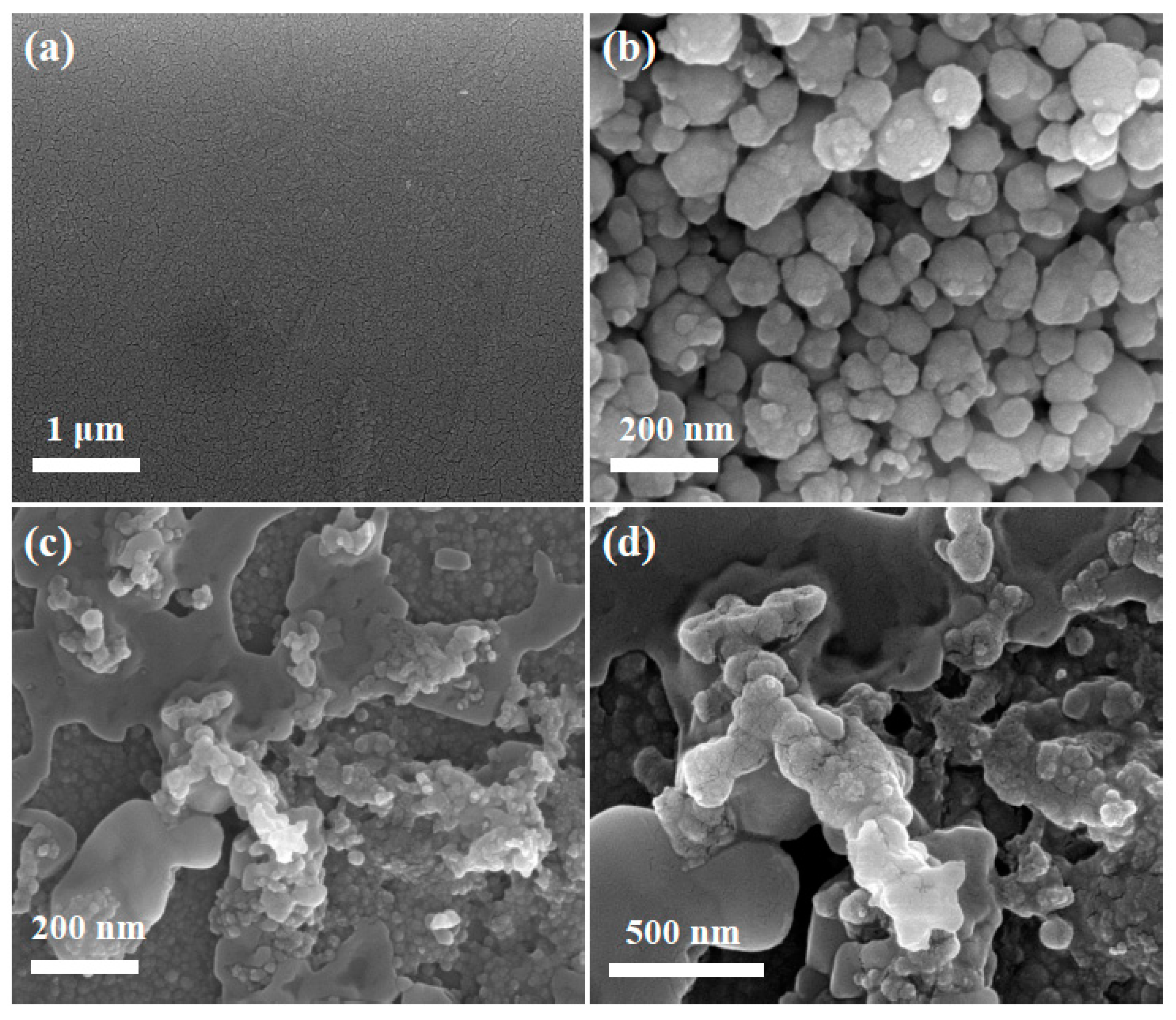
3.2. Characterization of SnNPs
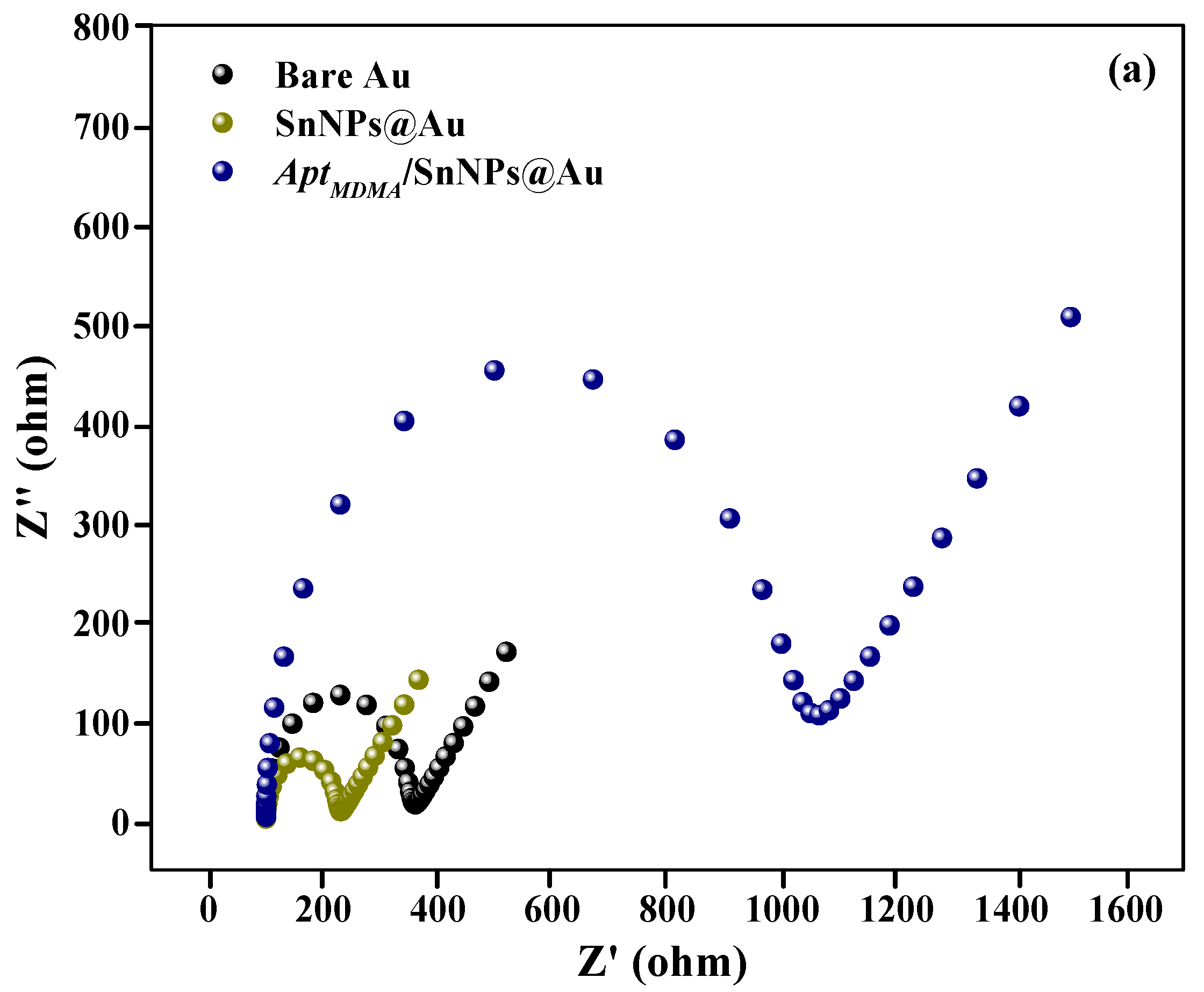
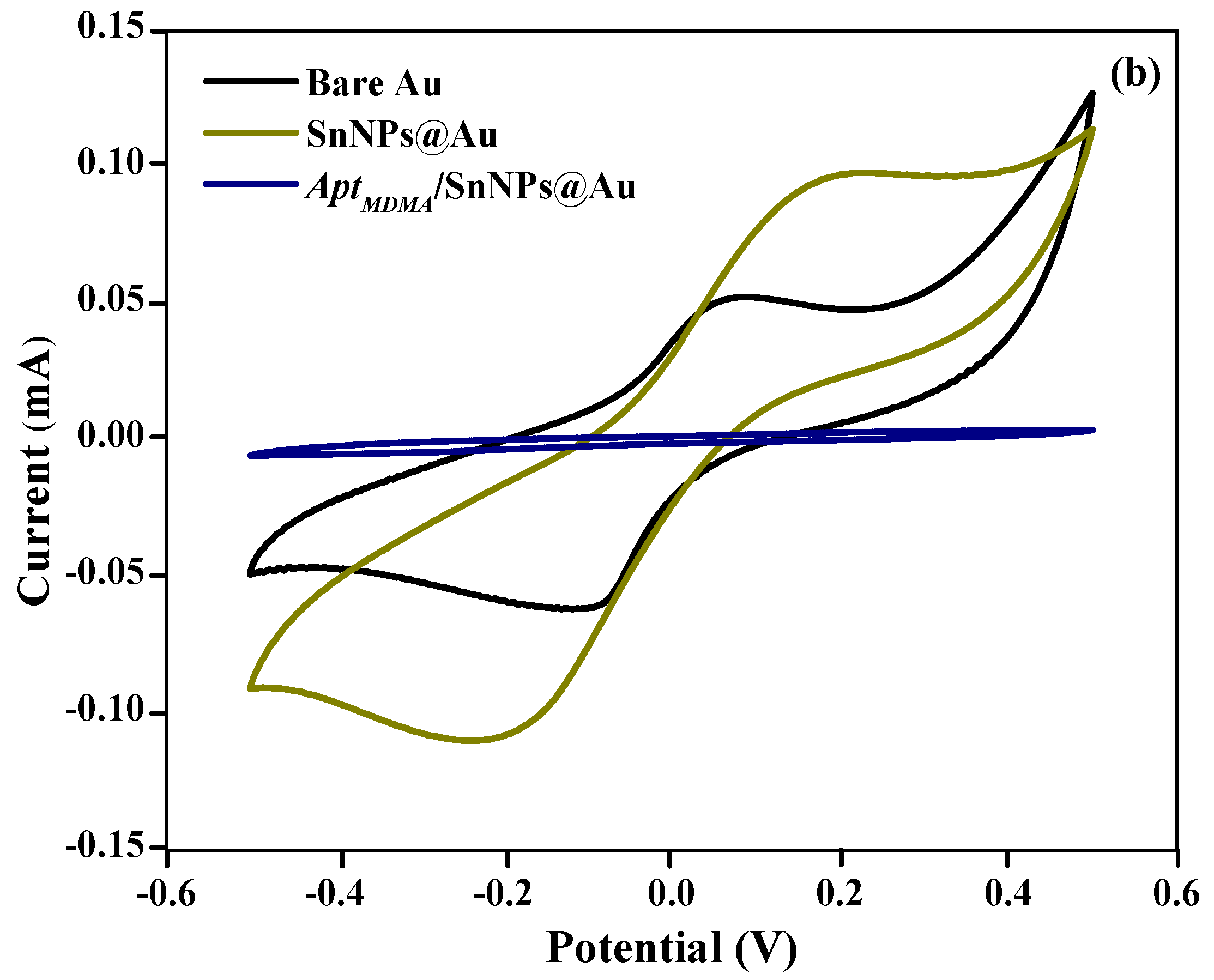
3.3. Electrochemical Characterization of Aptasensor
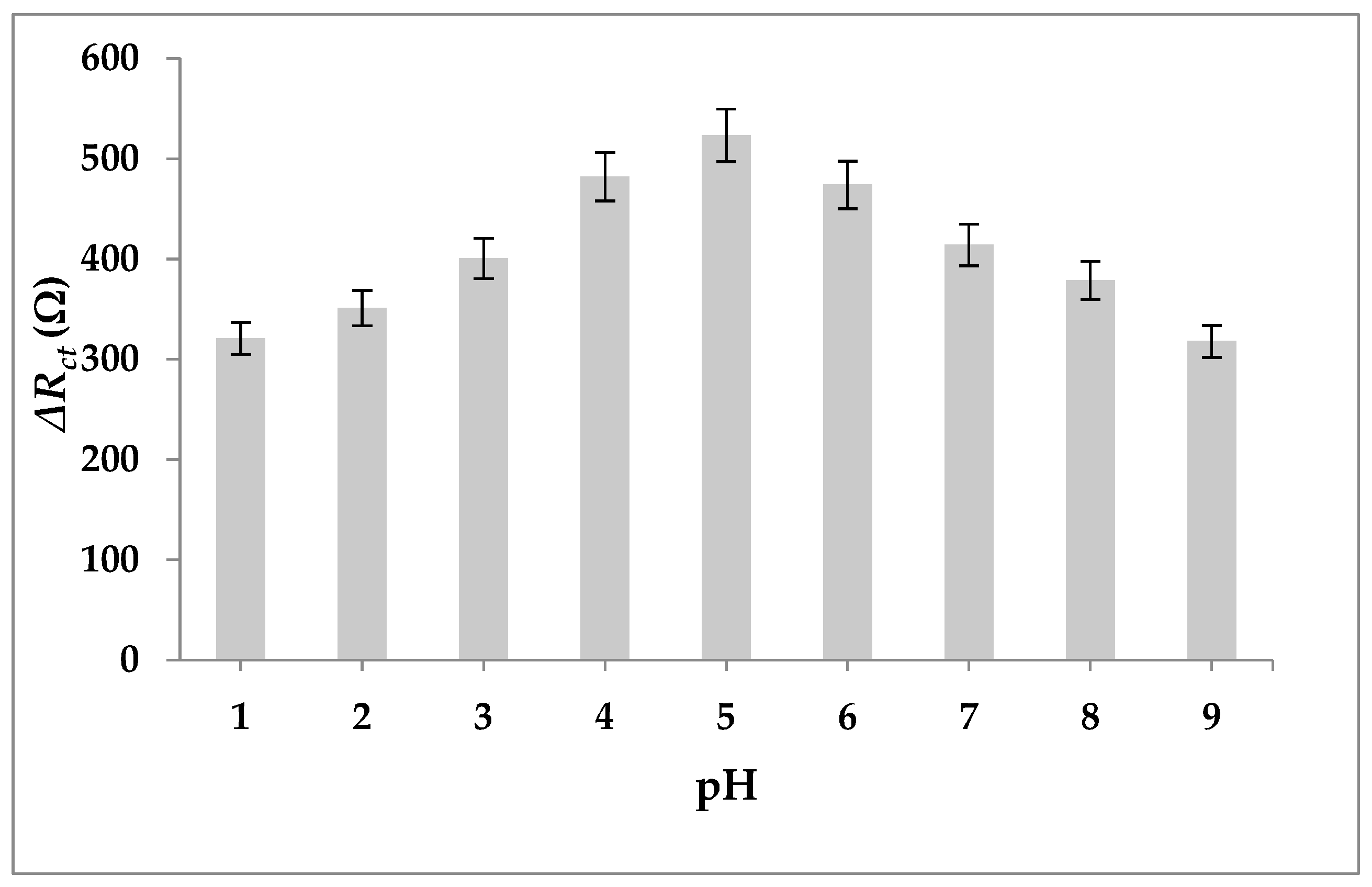
3.4. Optimization of Experimental Conditions
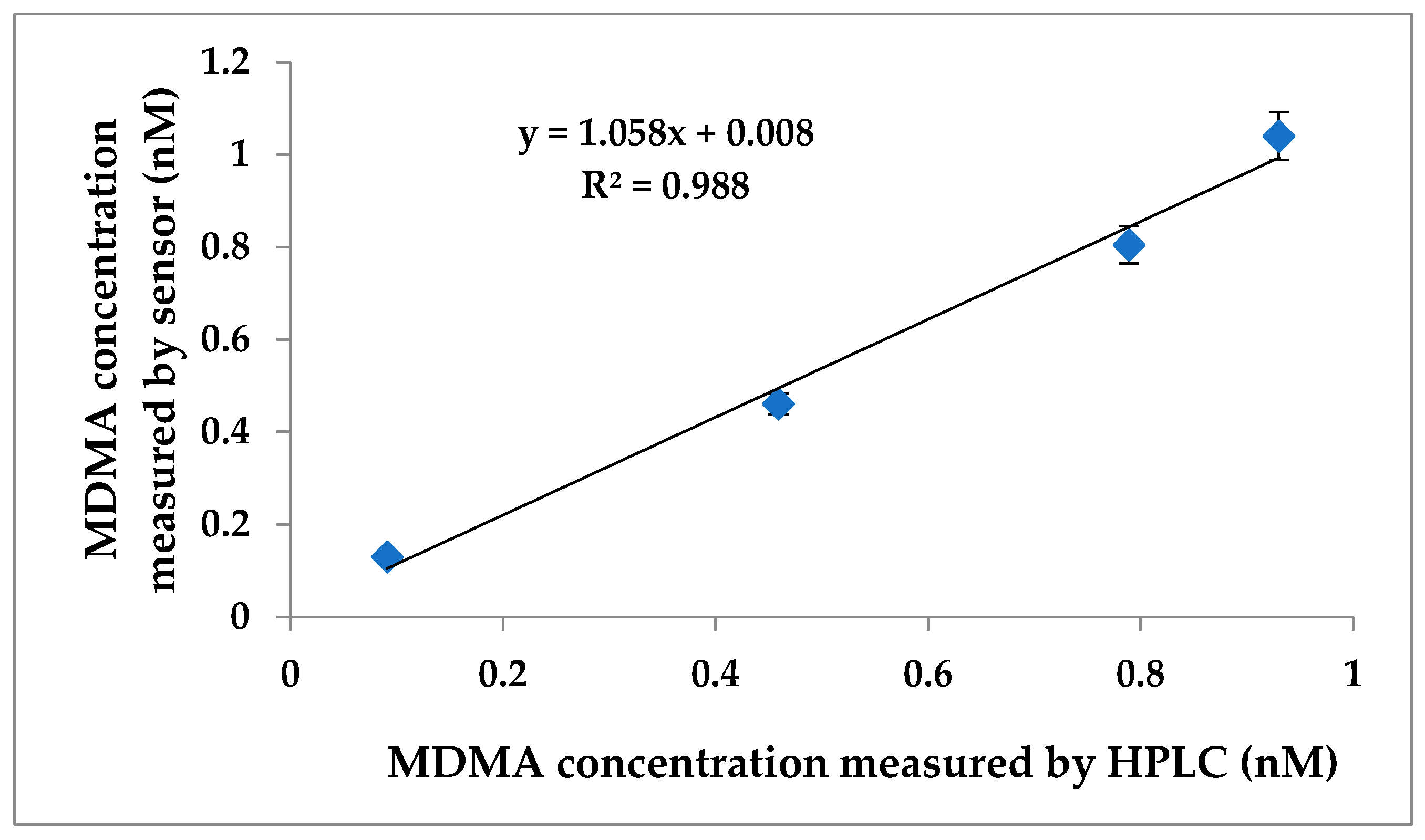
3.5. Analytical Performance of Aptasensor
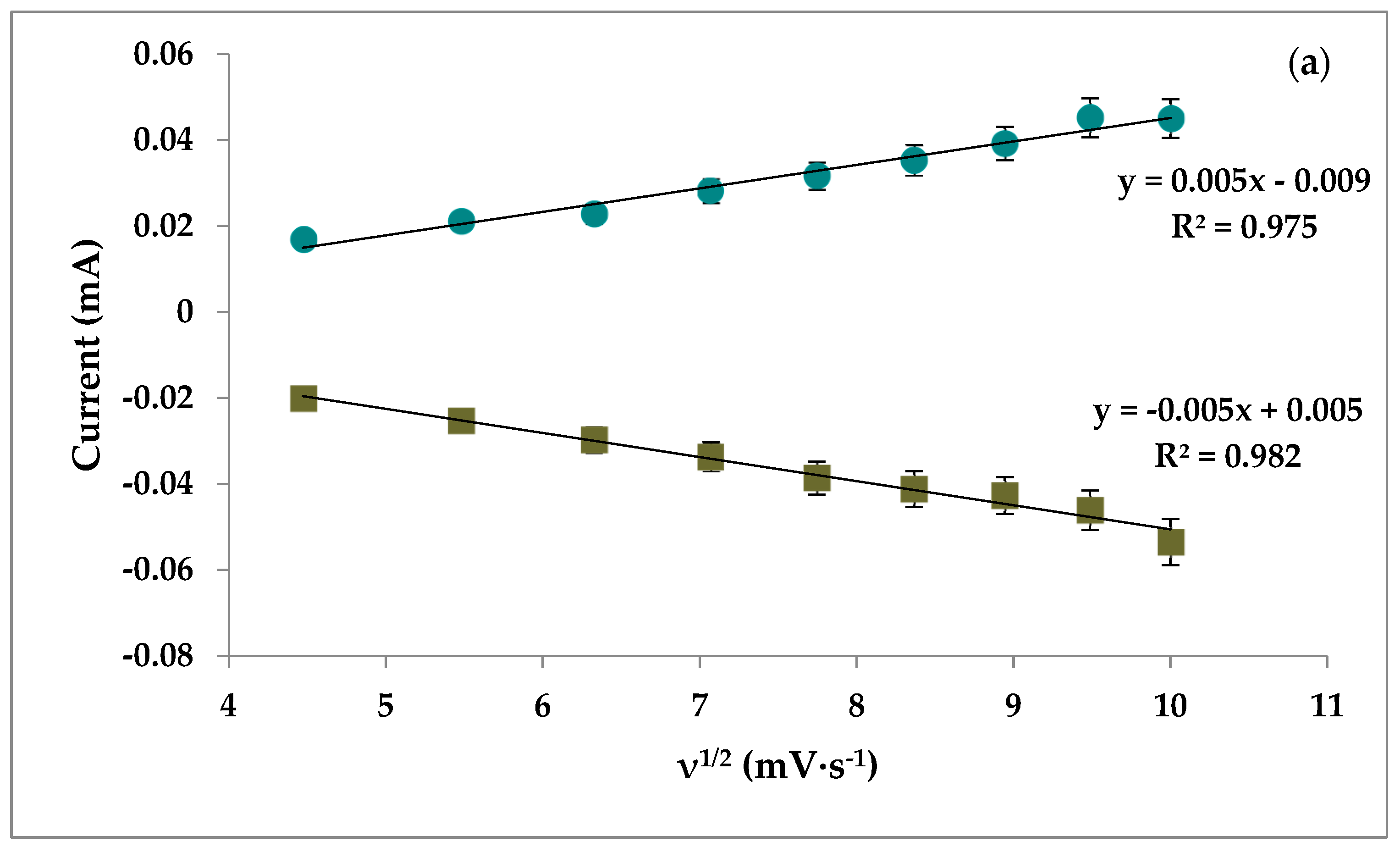
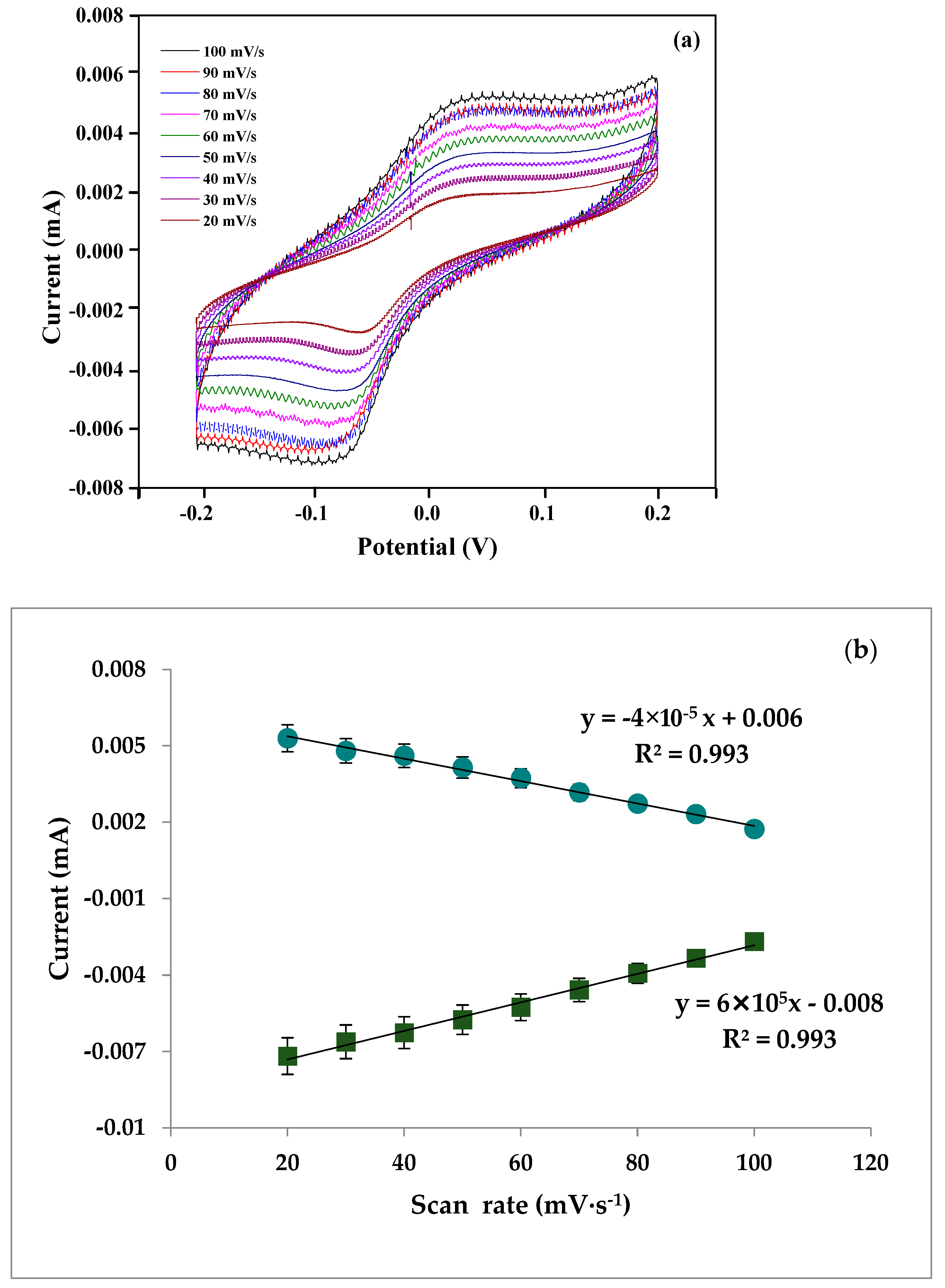
3.6. pH and Scan Rate of Aptasensor
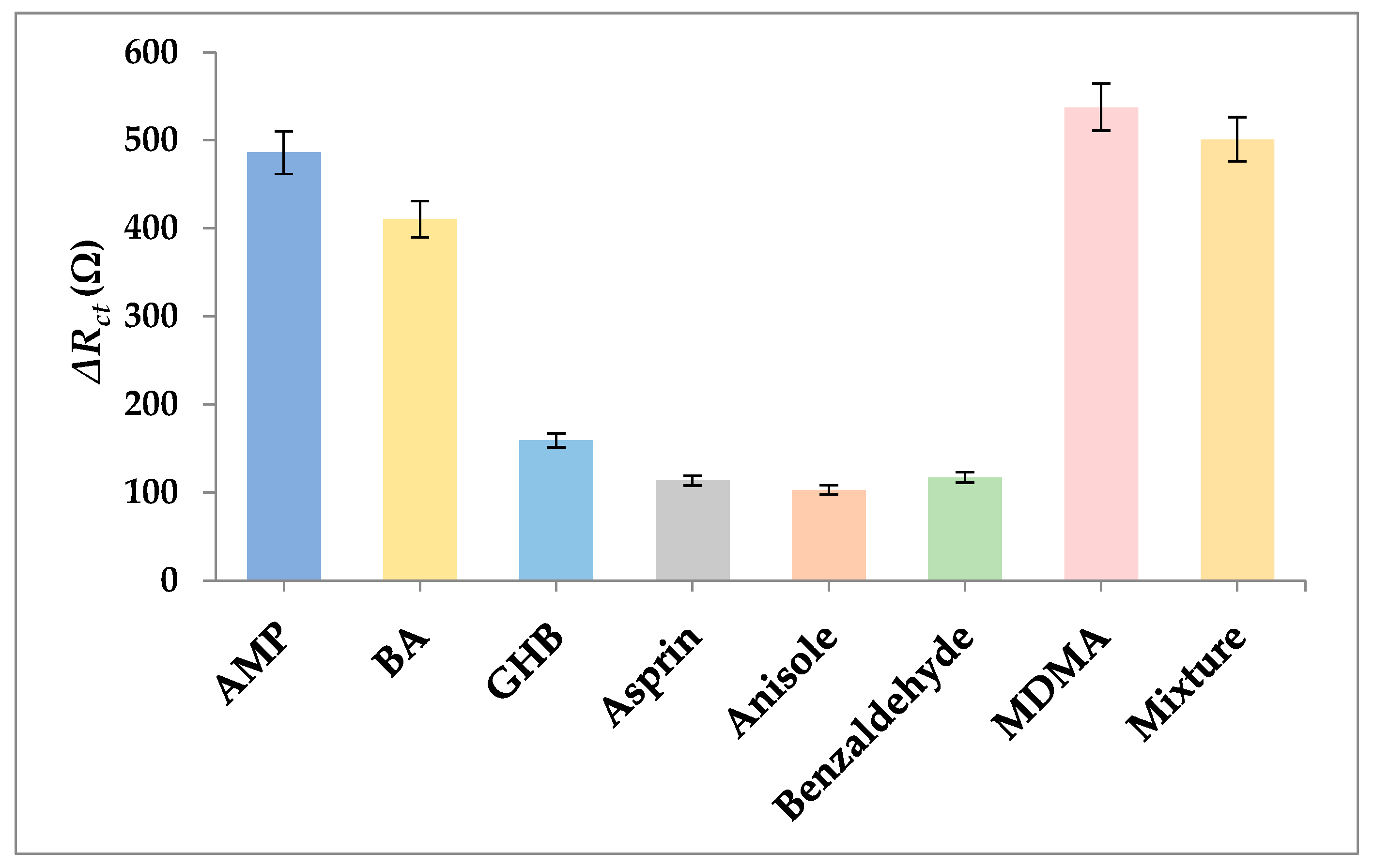
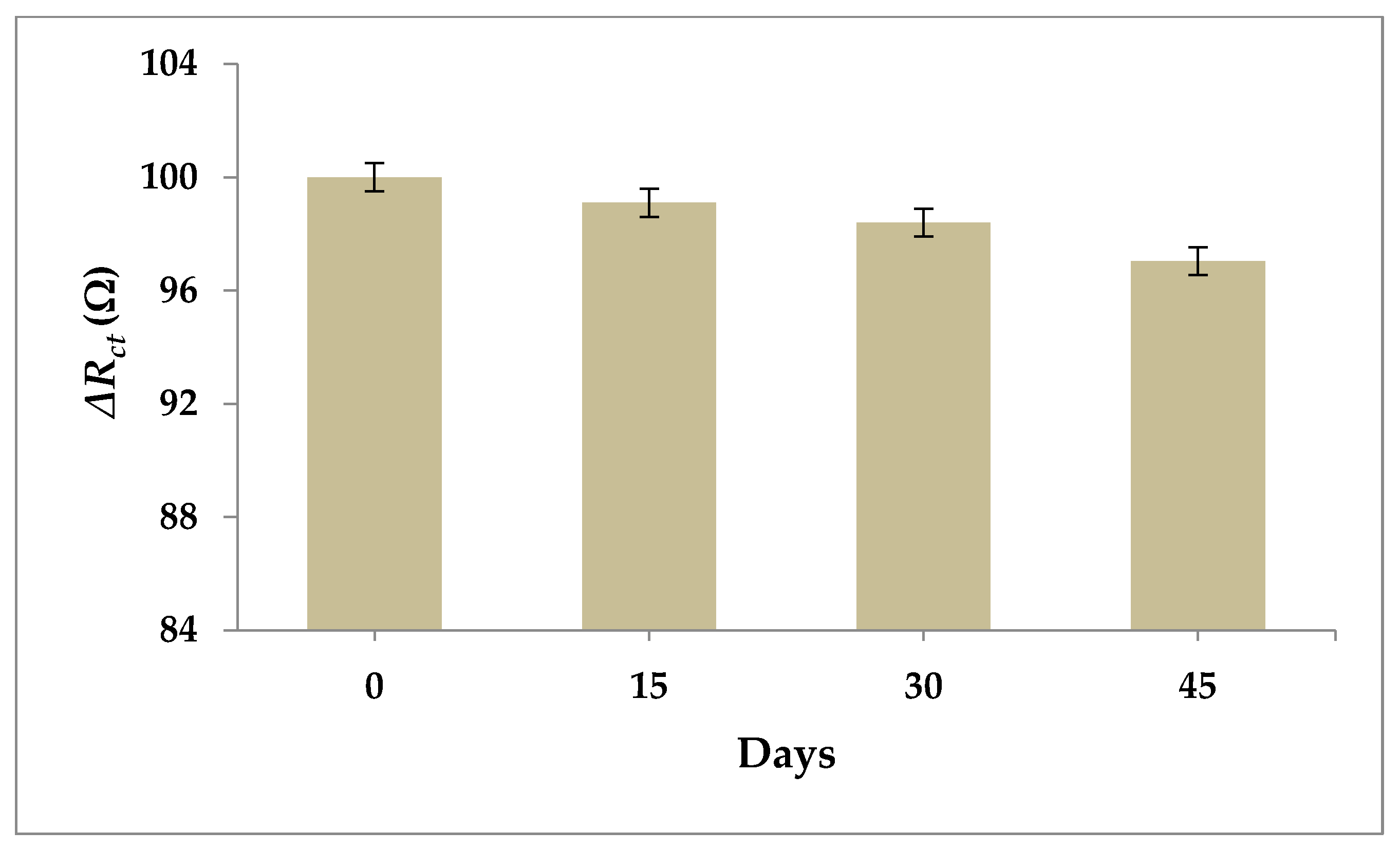
3.7. Selectivity and Stability of the Aptasensor
3.8. Performance of Aptasensor in Stimulated Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freudenmann, R.W.; Öxler, F.; Bernschneider-Reif, S. The origin of MDMA (ecstasy) revisited: The true story reconstructed from the original documents. Addiction 2006, 101, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.L.; Pintao, E.R.; Corrigliano, C.M.C.; Negrini Neto, O. Determination of 3,4-methylenedioxymethamphetamine (MDMA) in Ecstasy tablets by high performance liquid chromatography with fluorescence detection (HPLC-FD). Química Nova 2009, 32, 965–969. [Google Scholar]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Newcombe, D. Methylenedioxymethamphetamine (MDMA) in Psychiatry. J. Clin. Psychopharmacol. 2018, 38, 632–638. [Google Scholar] [CrossRef]
- Tadini, M.C.; Balbino, M.A.; Eleoterio, I.C.; de Oliveira, L.S.; Dias, L.G.; Jean-François Demets, G.; de Oliveira, M.F. Developing electrodes chemically modified with cucurbit[6]uril to detect 3,4-methylenedioxymethamphetamine (MDMA) by voltammetry. Electrochim. Acta 2014, 121, 188–193. [Google Scholar] [CrossRef]
- Mauri, M.C.; Paletta, S.; Di Pace, C. Hallucinations in the Substance-Induced Psychosis. In Hallucinations in Psychoses and Affective Disorders; Brambilla, P., Mauri, M.C., Altamura, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–83. [Google Scholar]
- Duarte, L.O.; Ferreira, B.; Silva, G.R.; Ipólito, A.J.; de Oliveira, M.F. Validated green phenyl reversed-phase LC method using ethanol to determine MDMA in seized ecstasy tablets. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 761–769. [Google Scholar] [CrossRef]
- Naqi, H.A.; Husbands, S.M.; Blagbrough, I.S. 1 H quantitative NMR and UHPLC-MS analysis of seized MDMA/NPS mixtures and tablets from night-club venues. Anal. Methods 2019, 11, 4795–4807. [Google Scholar] [CrossRef]
- Jain, U.; Soni, S.; Balhara, Y.P.S.; Khanuja, M.; Chauhan, N. Dual-Layered Nanomaterial-Based Molecular Pattering on Polymer Surface Biomimetic Impedimetric Sensing of a Bliss Molecule, Anandamide Neurotransmitter. ACS Omega 2020, 5, 10750–10758. [Google Scholar] [CrossRef]
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Chauhan, N.; Soni, S.; Jain, U. Recent advances in nanosensors development for biomarker alpha-synuclein protein detection. Process Biochem. 2021, 111, 105–113. [Google Scholar] [CrossRef]
- Soni, S.; Jain, U.; Burke, D.H.; Chauhan, N. Recent trends and emerging strategies for aptasensing technologies for illicit drugs detection. J. Electroanal. Chem. 2022, 116128. [Google Scholar] [CrossRef]
- Röthlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20, 11959–11980. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Savory, N.; Abe, K.; Ikebukuro, K. Methods for Improving Aptamer Binding Affinity. Molecules 2016, 21, 421. [Google Scholar] [CrossRef] [PubMed]
- Balayan, S.; Chauhan, N.; Chandra, R.; Jain, U. Electrochemical Based C-Reactive Protein (CRP) Sensing Through Molecularly Imprinted Polymer (MIP) Pore Structure Coupled with Bi-Metallic Tuned Screen-Printed Electrode. Biointerface Res. Appl. Chem. 2021, 12, 7697–7714. [Google Scholar]
- Singh, A.P.; Balayan, S.; Hooda, V.; Sarin, R.K.; Chauhan, N. Nano-interface driven electrochemical sensor for pesticides detection based on the acetylcholinesterase enzyme inhibition. Int. J. Biol. Macromol. 2020, 164, 3943–3952. [Google Scholar] [CrossRef]
- Jain, U.; Singh, A.; Kuchhal, N.K.; Chauhan, N. Glycated hemoglobin biosensing integration formed on Au nanoparticle-dotted tubular TiO2 nanoarray. Anal. Chim. Acta 2016, 945, 67–74. [Google Scholar] [CrossRef]
- Wu, Q.; Tan, R.; Mi, X.; Tu, Y. Electrochemiluminescent aptamer-sensor for alpha synuclein oligomer based on a metal–organic framework. Analyst 2020, 145, 2159–2167. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Zhang, F.-T.; Cui, Y.-R.; Deng, Q.-P.; Krause, S.; Zhou, Y.-L.; Zhang, X.-X. A label-free aptasensor for the sensitive and specific detection of cocaine using supramolecular aptamer fragments/target complex by electrochemical impedance spectroscopy. Talanta 2012, 92, 65–71. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Noorbakhsh, A. Sensitive Electrochemical Prostate Specific Antigen Aptasensor: Effect of Carboxylic Acid Functionalized Carbon Nanotube and Glutaraldehyde Linker. Electroanalysis 2016, 28, 1134–1145. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Gupta, S.; Avasthi, D.K.; Adelung, R.; Mishra, Y.K.; Jain, U. Zinc Oxide Tetrapods Based Biohybrid Interface for Voltammetric Sensing of Helicobacter pylori. ACS Appl. Mater. Interfaces 2018, 10, 30631–30639. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Narang, J.; Pundir, C.S. Immobilization of rat brain acetylcholinesterase on ZnS and poly(indole-5-carboxylic acid) modified Au electrode for detection of organophosphorus insecticides. Biosens. Bioelectron. 2011, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.; Chauhan, N.; Tomar, M.; Gupta, V. A contrivance based on electrochemical integration of graphene oxide nanoparticles/nickel nanoparticles for bilirubin biosensing. Biochem. Eng. J. 2017, 125, 238–245. [Google Scholar] [CrossRef]
- Singh, A.P.; Balayan, S.; Gupta, S.; Jain, U.; Sarin, R.K.; Chauhan, N. Detection of pesticide residues utilizing enzyme-electrode interface via nano-patterning of TiO2 nanoparticles and molybdenum disulfide (MoS2) nanosheets. Process Biochem. 2021, 108, 185–193. [Google Scholar] [CrossRef]
- Li, B.; Mei, T.; Li, D.; Du, S. Ultrasonic-assisted electrodeposition of Ni-Cu/TiN composite coating from sulphate-citrate bath: Structural and electrochemical properties. Ultrason. Sonochem. 2019, 58, 104680. [Google Scholar] [CrossRef]
- Abutbul, R.E.; Segev, E.; Samuha, S.; Zeiri, L.; Ezersky, V.; Makov, G.; Golan, Y. A new nanocrystalline binary phase: Synthesis and properties of cubic tin monoselenide. CrystEngComm 2016, 18, 1918–1923. [Google Scholar] [CrossRef]
- Riedel, O.; Düttmann, A.; Dühnen, S.; Kolny-Olesiak, J.; Gutsche, C.; Parisi, J.; Winter, M.; Knipper, M.; Placke, T. Surface-Modified Tin Nanoparticles and Their Electrochemical Performance in Lithium Ion Battery Cells. ACS Appl. Nano Mater. 2019, 2, 3577–3589. [Google Scholar] [CrossRef]
- Saleem, M.; Mehboob, G.; Ahmed, M.S.; Khisro, S.N.; Ansar, M.Z.; Mehmood, K.; Rafiqa-Tul-Rasool; Alamgir, M.K.; Ejaz, A.; Ghazanfar, M.; et al. Electrochemical Properties of Tin Sulfide Nano-Sheets as Cathode Material for Lithium-Sulfur Batteries. Front. Chem. 2020, 8, 254. [Google Scholar] [CrossRef]
- Khatoon, Z.; Hassanein, A.S.; Fouad, H.; Ansari, Z.A.; Alothman, O.Y.; Alnbaheen, M.S.; Ansari, S.G. Fabrication and Characterization of Electrochemical Organophosphate Sensor Device Based on Doped Tin Oxide Nanoparticles. J. Nanoelectron. Optoelectron. 2018, 13, 1082–1089. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review—Recent Advances in Tin Oxide Nanomaterials as Electrochemical/Chemiresistive Sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Soni, S.; Jain, U.; Burke, D.H.; Chauhan, N. A label free, signal off electrochemical aptasensor for amphetamine detection. Surf. Interfaces 2022, 31, 102023. [Google Scholar] [CrossRef]
- Kumar, R.; Kushwaha, N.; Mittal, J. Ammonia Gas Sensing Activity of Sn Nanoparticles Film. Sens. Lett. 2016, 14, 300–303. [Google Scholar] [CrossRef]
- Shi, Q.; Shi, Y.; Pan, Y.; Yue, Z.; Zhang, H.; Yi, C. Colorimetric and bare eye determination of urinary methylamphetamine based on the use of aptamers and the salt-induced aggregation of unmodified gold nanoparticles. Microchim. Acta 2015, 182, 505–511. [Google Scholar] [CrossRef]
- Randles, J.E.B. A cathode ray polarograph. Part II.—The current-voltage curves. Trans. Faraday Soc. 1948, 44, 322–327. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattarai, J.K.; Alla, A.J.; Demchenko, A.V.; Stine, K.J. Electrochemical annealing of nanoporous gold by application of cyclic potential sweeps. Nanotechnology 2015, 26, 085602. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Chauhan, N.; Balayan, S.; Jain, U. Sensitive biosensing of neurotransmitter: 2D material wrapped nanotubes and MnO2 composites for the detection of acetylcholine. Synth. Met. 2020, 263, 116354. [Google Scholar] [CrossRef]
- Zaikovskii, A.V.; Iurchenkova, A.A.; Kozlachkov, D.V.; Fedorovskaya, E.O. Effects of Tin on the Morphological and Electrochemical Properties of Arc-Discharge Nanomaterials. JOM 2021, 73, 847–855. [Google Scholar] [CrossRef]
- Rosy, R.; Goyal, R.N.; Shim, Y.-B. Glutaraldehyde sandwiched amino functionalized polymer based aptasensor for the determination and quantification of chloramphenicol. RSC Adv. 2015, 5, 69356–69364. [Google Scholar] [CrossRef]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.D.; Reddy, S.P.; Deepthi, A.; Prashanthi, J.; Rao, G.N. Synthesis, Characterization of Tin Oxide (SnO) Nanoparticles via Autoclave synthesis protocol for H2 sensing. Int. J. Nanotechnol. Appl. 2017, 11, 265–276. [Google Scholar]
- Lee, K.-M.; Lee, D.-J.; Ahn, H. XRD and TEM studies on tin oxide (II) nanoparticles prepared by inert gas condensation. Mater. Lett. 2004, 58, 3122–3125. [Google Scholar] [CrossRef]
- Arora, N.; Jagirdar, B.R. From (Au5Sn + AuSn) physical mixture to phase pure AuSn and Au5Sn intermetallic nanocrystals with tailored morphology: Digestive ripening assisted approach. Phys. Chem. Chem. Phys. 2014, 16, 11381–11389. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ji, X.; Yang, Y.; Deng, W.; Banks, C.E. Room temperature ionic liquid assisted well-dispersed core-shell tin nanoparticles through cathodic corrosion. RSC Adv. 2013, 3, 18791. [Google Scholar] [CrossRef]
- Dhillon, S.; Kant, R. Theory for electrochemical impedance spectroscopy of heterogeneous electrode with distributed capacitance and charge transfer resistance. J. Chem. Sci. 2017, 129, 1277–1292. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J. Electroanal. Chem. 2010, 646, 43–51. [Google Scholar] [CrossRef]
- Mehennaoui, S.; Poorahong, S.; Jimenez, G.C.; Siaj, M. Selection of high affinity aptamer-ligand for dexamethasone and its electrochemical biosensor. Sci. Rep. 2019, 9, 6600. [Google Scholar] [CrossRef]
- Pérez de Carvasal, K.; Riccardi, C.; Russo Krauss, I.; Cavasso, D.; Vasseur, J.-J.; Smietana, M.; Morvan, F.; Montesarchio, D. Charge-Transfer Interactions Stabilize G-Quadruplex-Forming Thrombin Binding Aptamers and Can Improve Their Anticoagulant Activity. Int. J. Mol. Sci. 2021, 22, 9510. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kelly, R.S. Analytical Electrochemistry: The Basic Concepts. Available online: https://amser.org/index.php?P=AMSER--ResourceFrame&resourceId=17150 (accessed on 2 June 2022).
- Chen, Z.; Li, L.; Zhao, H.; Guo, L.; Mu, X. Electrochemical impedance spectroscopy detection of lysozyme based on electrodeposited gold nanoparticles. Talanta 2011, 83, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Cowen, T.; Piletsky, S.; De Wael, K. Electrochemical sensing of cocaine in real samples based on electrodeposited biomimetic affinity ligands. Analyst 2019, 144, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Shahdost-fard, F. Impedimetric detection of cocaine by using an aptamer attached to a screen printed electrode modified with a dendrimer/silver nanoparticle nanocomposite. Microchim. Acta 2018, 185, 214. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, U.; Murti, B.T.; Putri, A.D.; Tiwari, A.; Chauhan, N. Nanohybrid-based immunosensor prepared for Helicobacter pylori BabA antigen detection through immobilized antibody assembly with @Pdnano/rGO/PEDOT sensing platform. Sci. Rep. 2020, 10, 21217. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.A.; Teck, H.B.; Adnan, R. Surfactant-controlled aqueous synthesis of SnO2 nanoparticles via the hydrothermal and conventional heating methods. Turk. J. Chem. 2010, 34, 537–550. [Google Scholar]
- Akram, M.; Saleh, A.T.; Ibrahim, W.A.W.; Awan, A.S.; Hussain, R. Continuous microwave flow synthesis (CMFS) of nano-sized tin oxide: Effect of precursor concentration. Ceram. Int. 2016, 42, 8613–8619. [Google Scholar] [CrossRef]
- Babar, A.R.; Shinde, S.S.; Moholkar, A.V.; Rajpure, K.Y. Electrical and dielectric properties of co-precipitated nanocrystalline tin oxide. J. Alloys Compd. 2010, 505, 743–749. [Google Scholar] [CrossRef]
- Yang, S.; Song, Q.; Gao, K.; Wang, H. EuF3 nanotubes fabricated via Eu(NO3)3/cysteamine as precursor and their derived thermosensitive nanogels. Solid State Sci. 2014, 34, 17–23. [Google Scholar] [CrossRef]
- Singh, K.; Srivastava, G.; Talat, M.; Srivastava, O.N.; Kayastha, A.M. α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: Its characterization, kinetics and potential applications in starch based industries. Biochem. Biophys. Rep. 2015, 3, 18–25. [Google Scholar] [CrossRef]
- Lović, J. Electrochemical glucose biosensor with the characterization of surface morphology and content of glucose oxidase- glutaraldehyde-cysteine layers on gold electrode. Int. J. Electrochem. Sci. 2018, 13, 12340–12348. [Google Scholar] [CrossRef]
- Etesami, M.; Salehi Karoonian, F.; Mohamed, N. Electrochemical Deposition of Gold Nanoparticles on Pencil Graphite by Fast Scan Cyclic Voltammetry. J. Chin. Chem. Soc. 2011, 58, 688–693. [Google Scholar] [CrossRef]
- Ustarroz, J.; Hubin, A.; Terryn, H. Supported Nanoparticle Synthesis by Electrochemical Deposition. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 603–631. [Google Scholar]
- Liu, Y.; Tuleouva, N.; Ramanculov, E.; Revzin, A. Aptamer-Based Electrochemical Biosensor for Interferon Gamma Detection. Anal. Chem. 2010, 82, 8131–8136. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Shi, L.; Zheng, W.; Jing, X. Electroactive Cu2O nanoparticles and Ag nanoparticles driven ratiometric electrochemical aptasensor for prostate specific antigen detection. Sens. Actuators B Chem. 2020, 315, 128155. [Google Scholar] [CrossRef]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Ardakani, Y.H.; Madrakian, T. Fabrication of a novel aptasensor based on three-dimensional reduced graphene oxide/polyaniline/gold nanoparticle composite as a novel platform for high sensitive and specific cocaine detection. Anal. Chim. Acta 2017, 996, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cumba, L.R.; Smith, J.P.; Zuway, K.Y.; Sutcliffe, O.B.; Do Carmo, D.R.; Banks, C.E. Forensic electrochemistry: Simultaneous voltammetric detection of MDMA and its fatal counterpart “dr Death” (PMA). Anal. Methods 2016, 8, 142–152. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Chang, J.-S.; Lin, S.; Chao, S.D.; Liu, C.-H. 3,4-Methylenedioxymethylamphetamine detection using a microcantilever-based biosensor. Sens. Actuators A Phys. 2012, 182, 163–167. [Google Scholar] [CrossRef]
- Nevescanin, M.; Avramov-Ivic, M.; Petrovic, S.; Mijin, D.; Banovic-Stevic, S.; Jovanovic, V. The use of a gold electrode for the determination of amphetamine derivatives and application to their analysis in human urine. J. Serb. Chem. Soc. 2013, 78, 1373–1385. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Moreira Gonçalves, L.; et al. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Cohen, I.V.; Makunts, T.; Abagyan, R.; Thomas, K. Concomitant drugs associated with increased mortality for MDMA users reported in a drug safety surveillance database. Sci. Rep. 2021, 11, 5997. [Google Scholar] [CrossRef]
- Papaseit, E.; Torrens, M.; Pérez-Mañá, C.; Muga, R.; Farré, M. Key interindividual determinants in MDMA pharmacodynamics. Expert Opin. Drug Metab. Toxicol. 2018, 14, 183–195. [Google Scholar] [CrossRef]
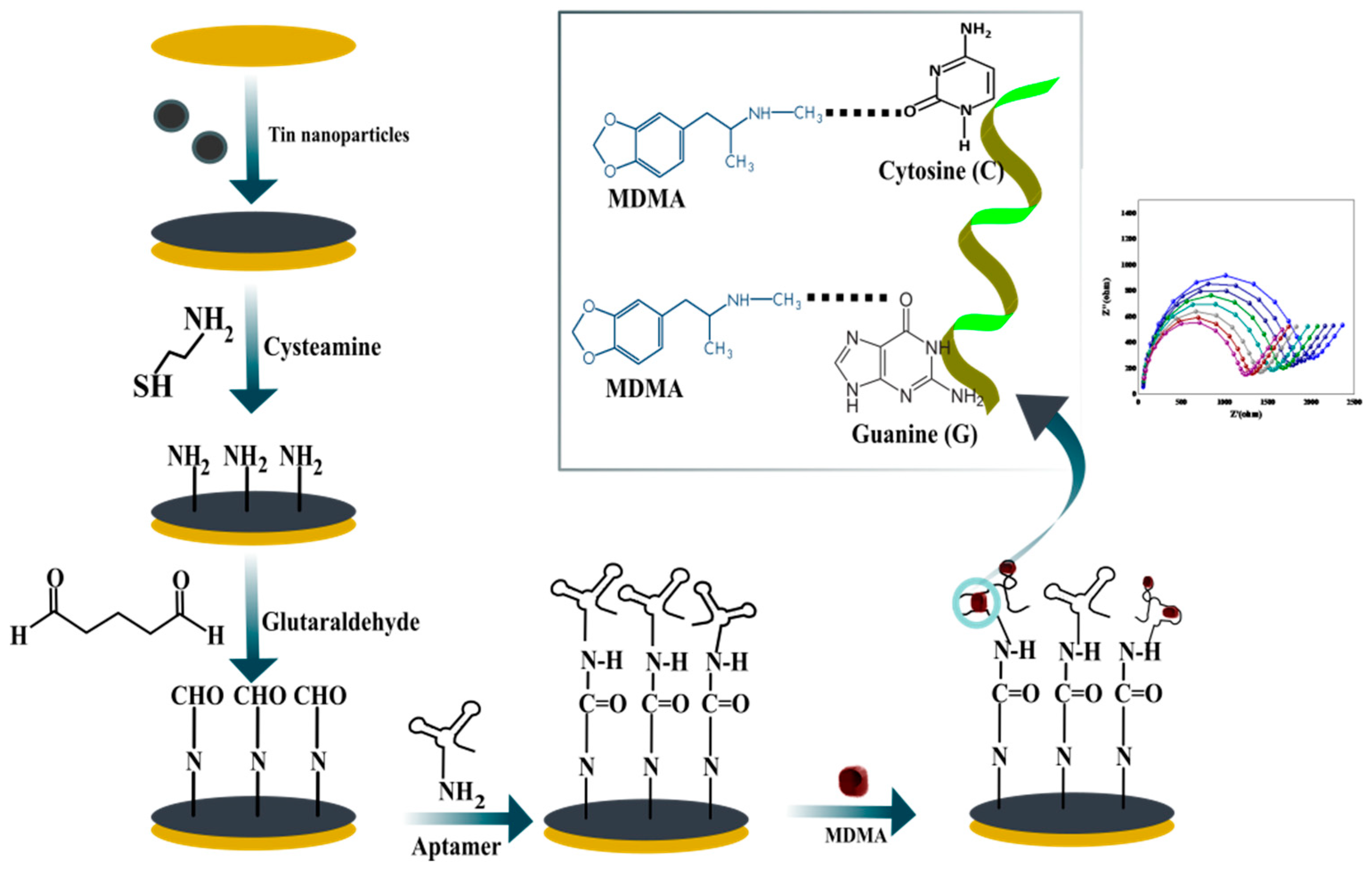

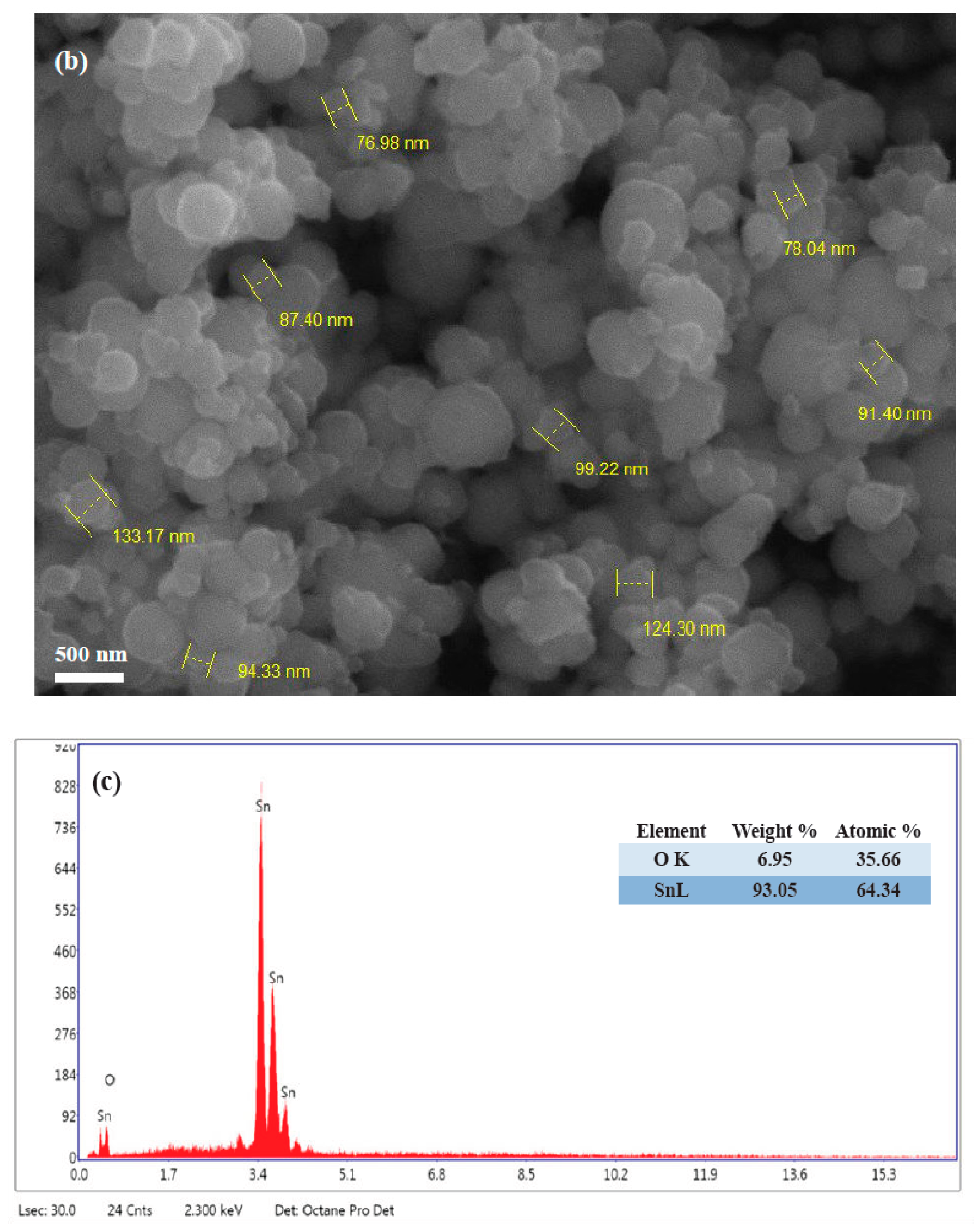
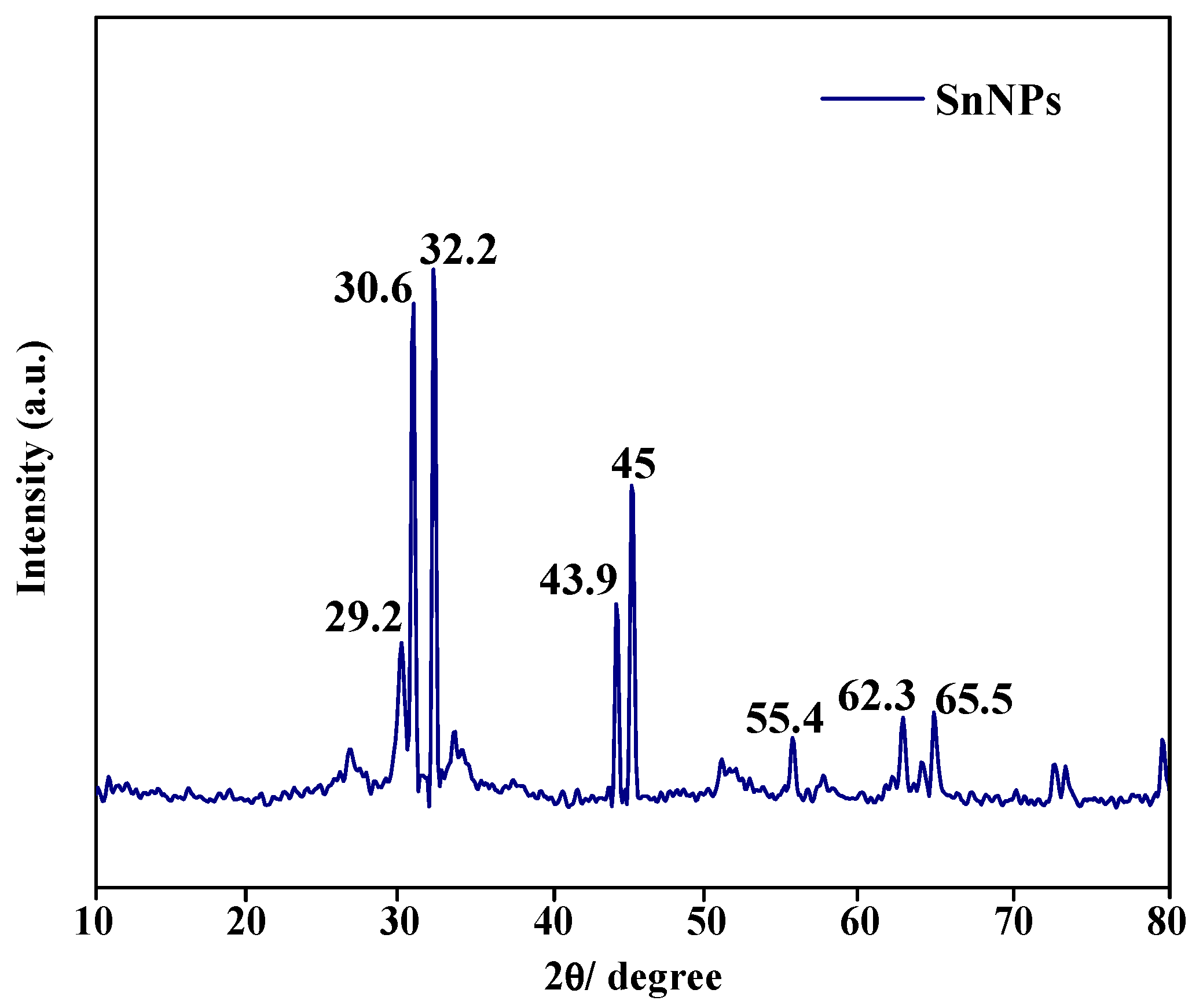
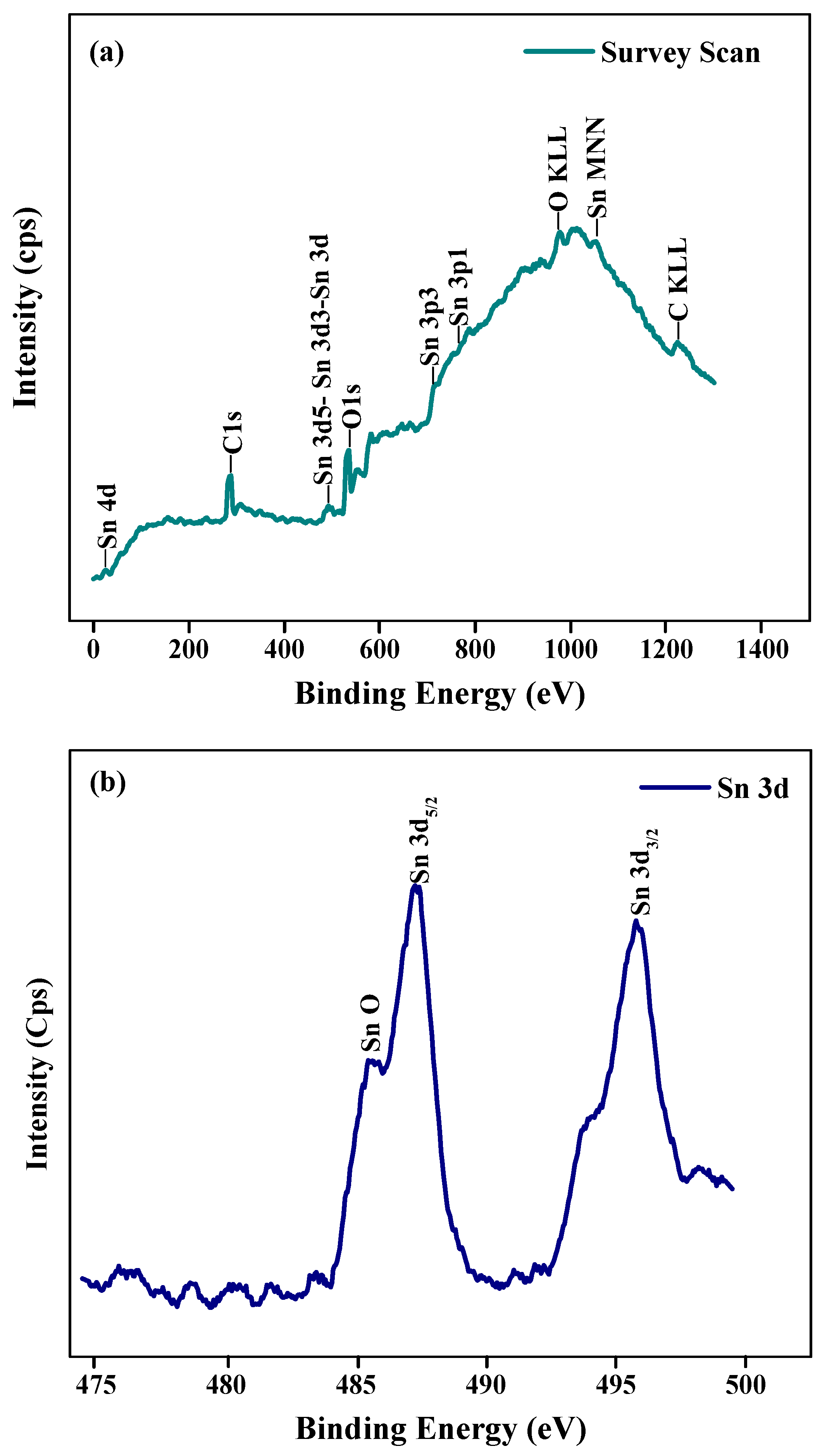

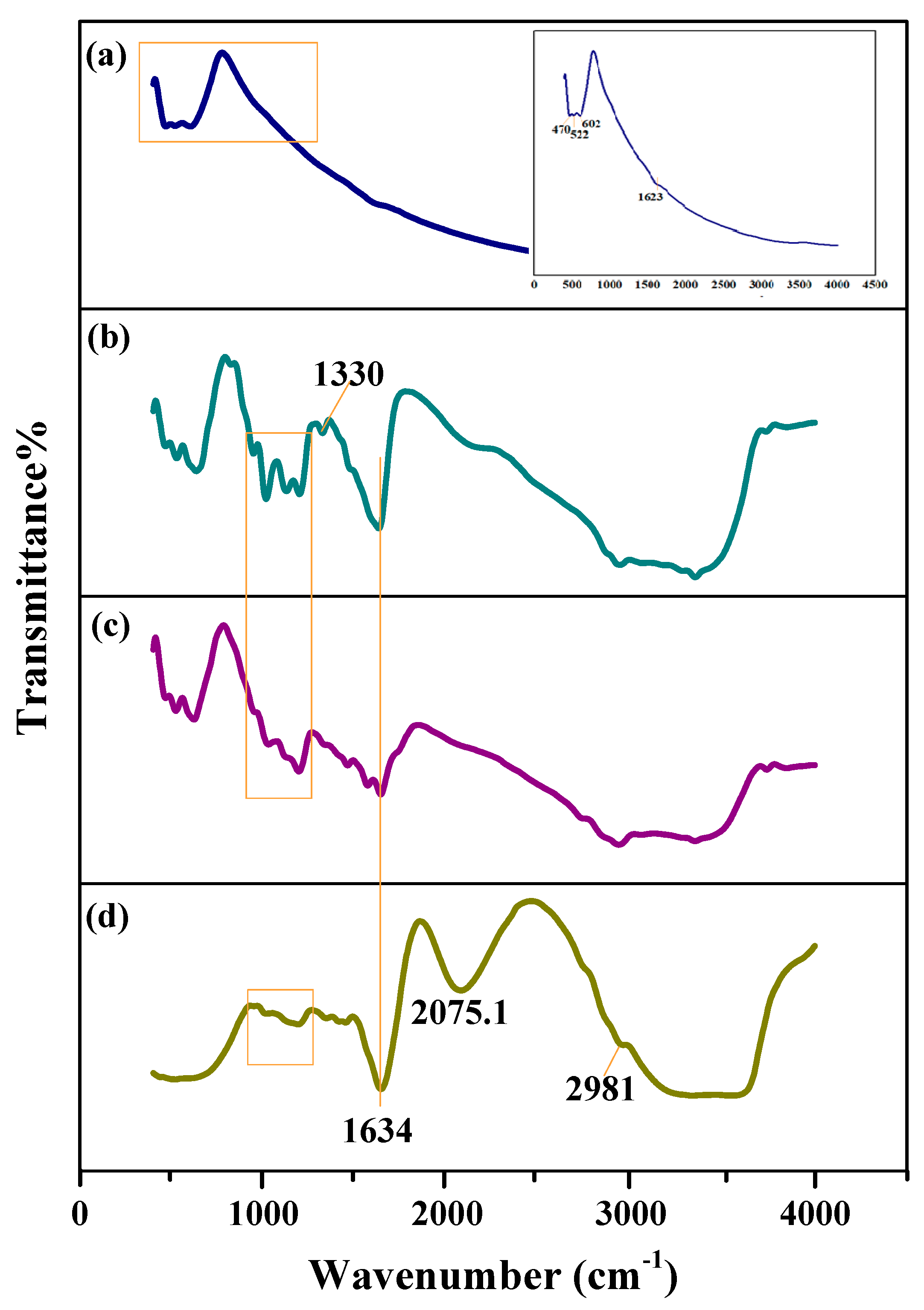
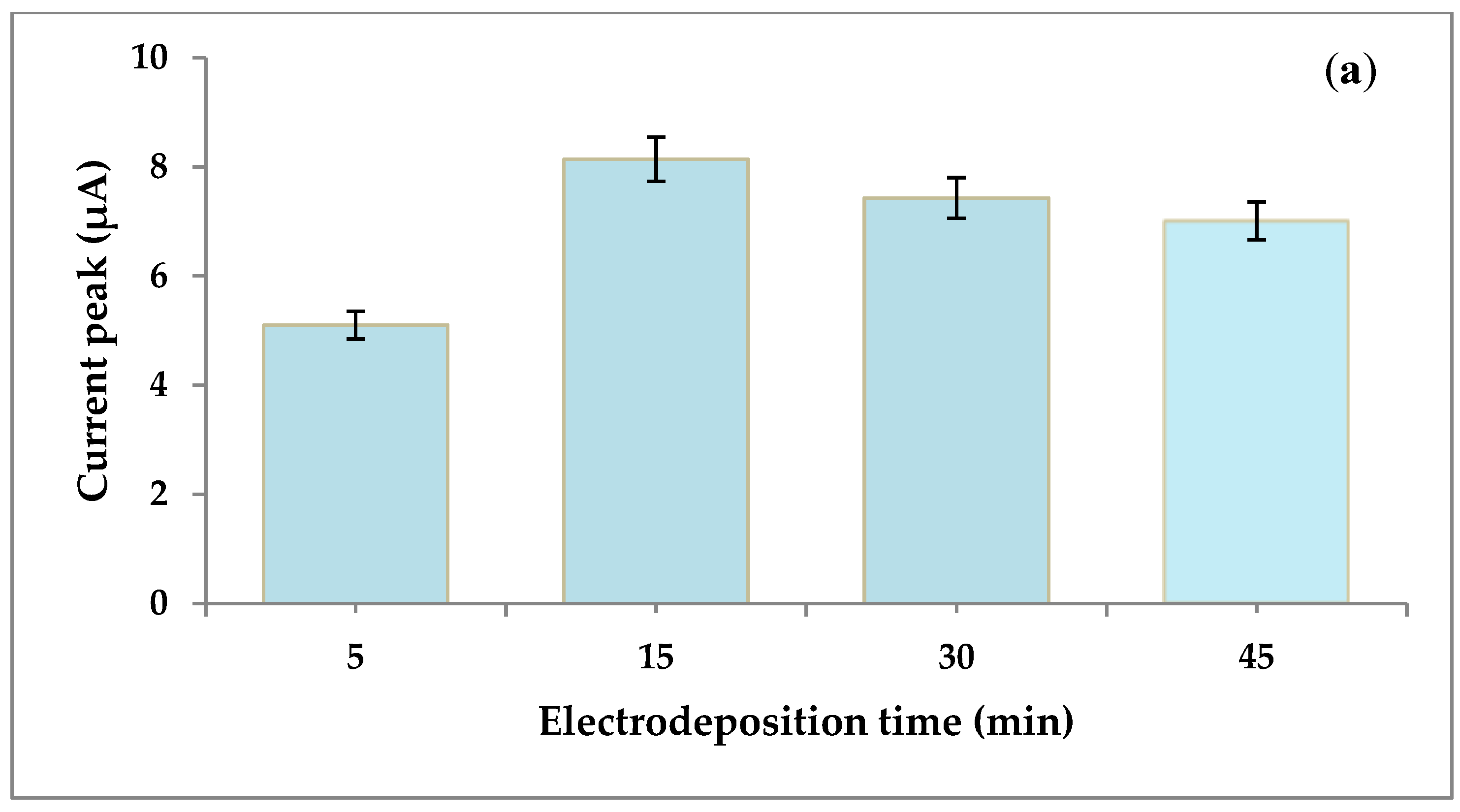

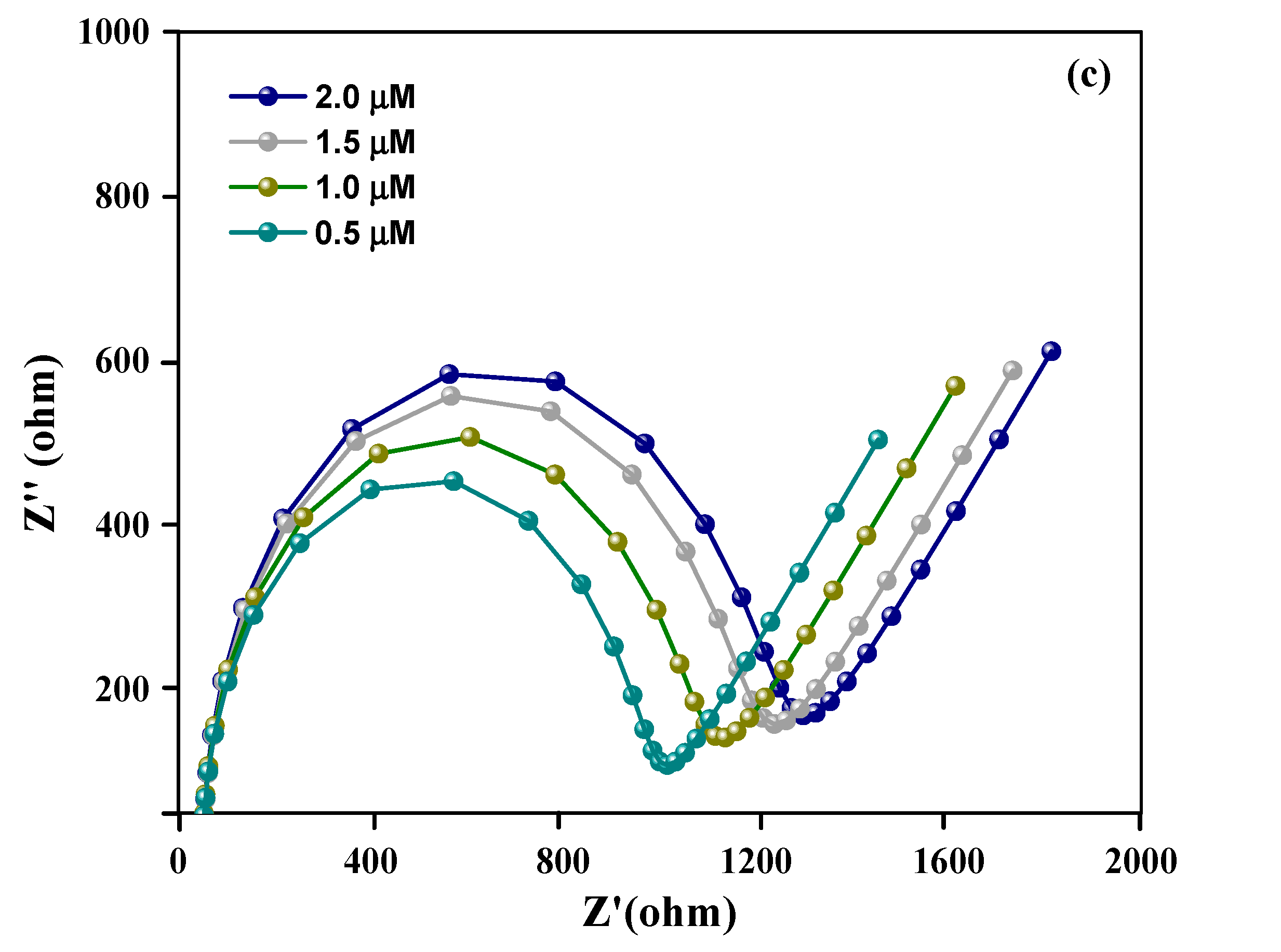
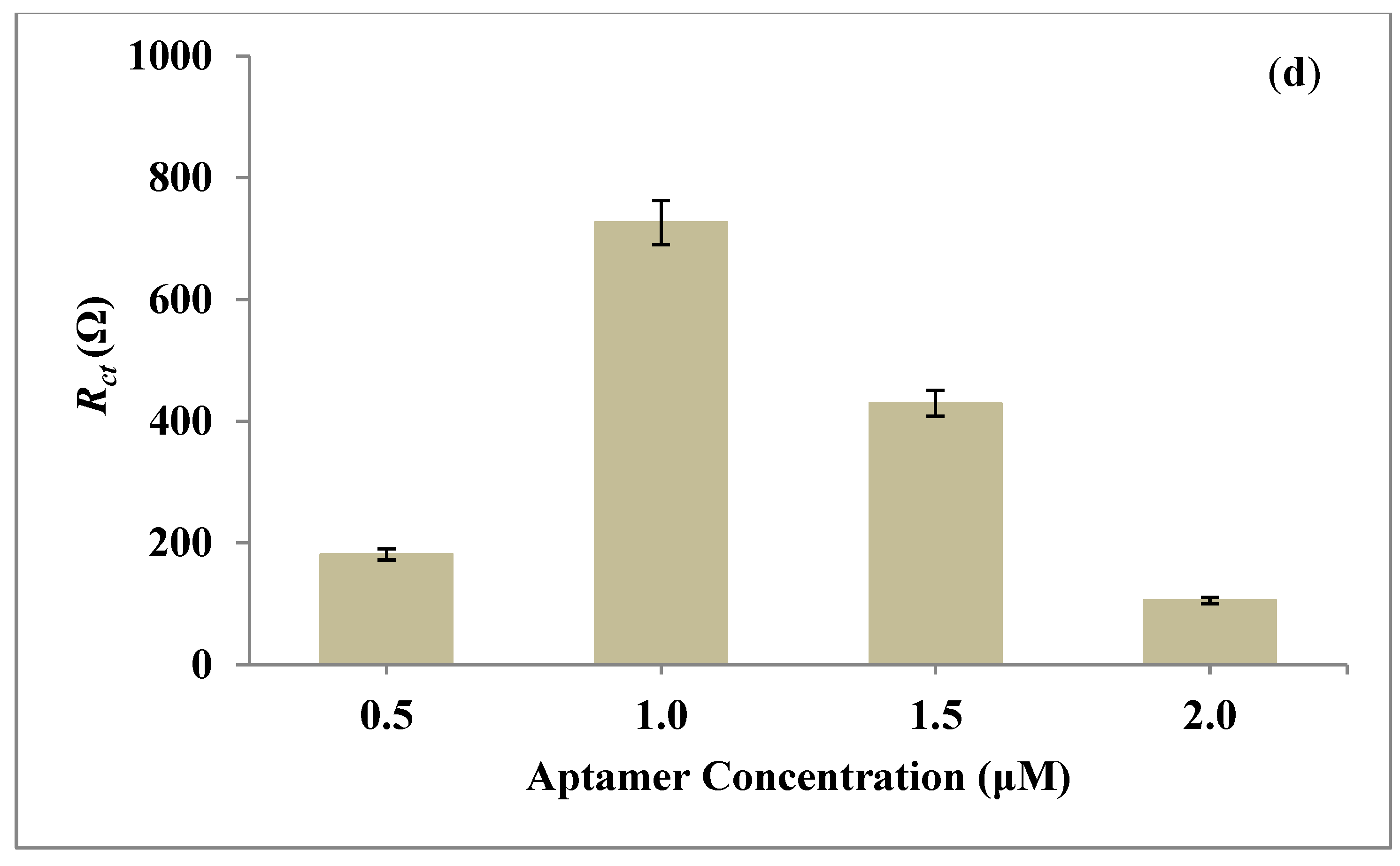

| SN | 2θ (Degree) | d (A°) | FWHM (Degree) | Crystalline Size (nm) | Dislocation Density (δ) | Microstrain (ε) |
|---|---|---|---|---|---|---|
| 1. | 29.9 | 1.98 | 0.5538 | 25.2 | 0.157 | 0.037 |
| 2. | 30.6 | 2.03 | 0.263 | 53.0 | 0.035 | 0.179 |
| 3. | 32.2 | 2.12 | 0.371 | 37.5 | 0.071 | 0.026 |
| 4. | 43.9 | 2.87 | 0.403 | 33.3 | 0.090 | 0.040 |
| 5. | 45.0 | 2.95 | 0.307 | 43.2 | 0.053 | 0.032 |
| 6. | 55.4 | 3.57 | 0.407 | 31.4 | 0.101 | 0.534 |
| 7. | 62.3 | 3.98 | 0.369 | 33.5 | 0.089 | 0.055 |
| 8. | 65.5 | 4.16 | 0.465 | 26.1 | 0.146 | 0.074 |
| SN | Electrochemical Sensor Type | Sensing Mechanism | Detection Limit | Linear Range | Incubation Time | Samples | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Cucurbit[6]uril-based sensor | CV | 3.5 and 2.7 μM | 4.2 × 10−3–4.8 × 10−2 μM | NR | NR | [5] |
| 2. | Graphite-based sensor | DPV | 40 μM | 500–4980 μM | NR | PBS buffer | [68] |
| 3. | Microcantilever-based immunosensor | Frequency shift | 5.0 × 103μM | 5.0 × 103–50 × 103μM | NR | NR | [69] |
| 4. | Gold electrode-based sensor | SWV | NR | 110.9–258.9 μM | NR | Urine | [70] |
| 5. | MIP-based sensor | SWV | 0.7 μM | 2.5–200 μM | 10 min | Serum and urine | [71] |
| 6. | Tin nanoparticle-based aptasensor | PEIS | 0.33 nM | 0.01–1.0 nM | 30 min | Diluted blood, urine, and water | Present study |
| Mean ΔRct | Standard Deviation | Coefficient of Variance (CV) | |
|---|---|---|---|
| Intra-batch | 541.97 | 9.28 | 1.71% |
| Inter-batch | 546.98 | 13.2 | 2.42% |
| Added MDMA Conc. (nM) | Observed MDMA Conc. (nM) | Recovery % | RSD % (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Urine | Blood | Water | Urine | Blood | Water | Urine | Blood | Water | |
| 0.0 | ND | ND | ND | 0.0 | 0.1 | 0.0 | 0.0 | - | |
| 0.1 | 0.094 | 0.091 | 0.087 | 94% | 91% | 87% | 1.79% | 1.71% | - |
| 0.4 | 0.384 | 0.380 | 0.354 | 96.7% | 95% | 88.5% | 2.04% | 1.99% | - |
| 0.7 | 0.67 | 0.651 | 0.612 | 95.7% | 93% | 87.4% | 2.18% | 2.12% | - |
| 1.0 | 0.92 | 0.923 | 0.901 | 92% | 100.3 | 90.1% | 1.14% | 1.37% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, S.; Jain, U.; Burke, D.H.; Chauhan, N. Development of Nanomaterial-Modified Impedimetric Aptasensor—A Single-Step Strategy for 3,4-Methylenedioxymethylamphetamine Detection. Biosensors 2022, 12, 538. https://doi.org/10.3390/bios12070538
Soni S, Jain U, Burke DH, Chauhan N. Development of Nanomaterial-Modified Impedimetric Aptasensor—A Single-Step Strategy for 3,4-Methylenedioxymethylamphetamine Detection. Biosensors. 2022; 12(7):538. https://doi.org/10.3390/bios12070538
Chicago/Turabian StyleSoni, Shringika, Utkarsh Jain, Donald H. Burke, and Nidhi Chauhan. 2022. "Development of Nanomaterial-Modified Impedimetric Aptasensor—A Single-Step Strategy for 3,4-Methylenedioxymethylamphetamine Detection" Biosensors 12, no. 7: 538. https://doi.org/10.3390/bios12070538
APA StyleSoni, S., Jain, U., Burke, D. H., & Chauhan, N. (2022). Development of Nanomaterial-Modified Impedimetric Aptasensor—A Single-Step Strategy for 3,4-Methylenedioxymethylamphetamine Detection. Biosensors, 12(7), 538. https://doi.org/10.3390/bios12070538





