A Neural Sensor with a Nanocomposite Interface for the Study of Spike Characteristics of Hippocampal Neurons under Learning Training
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
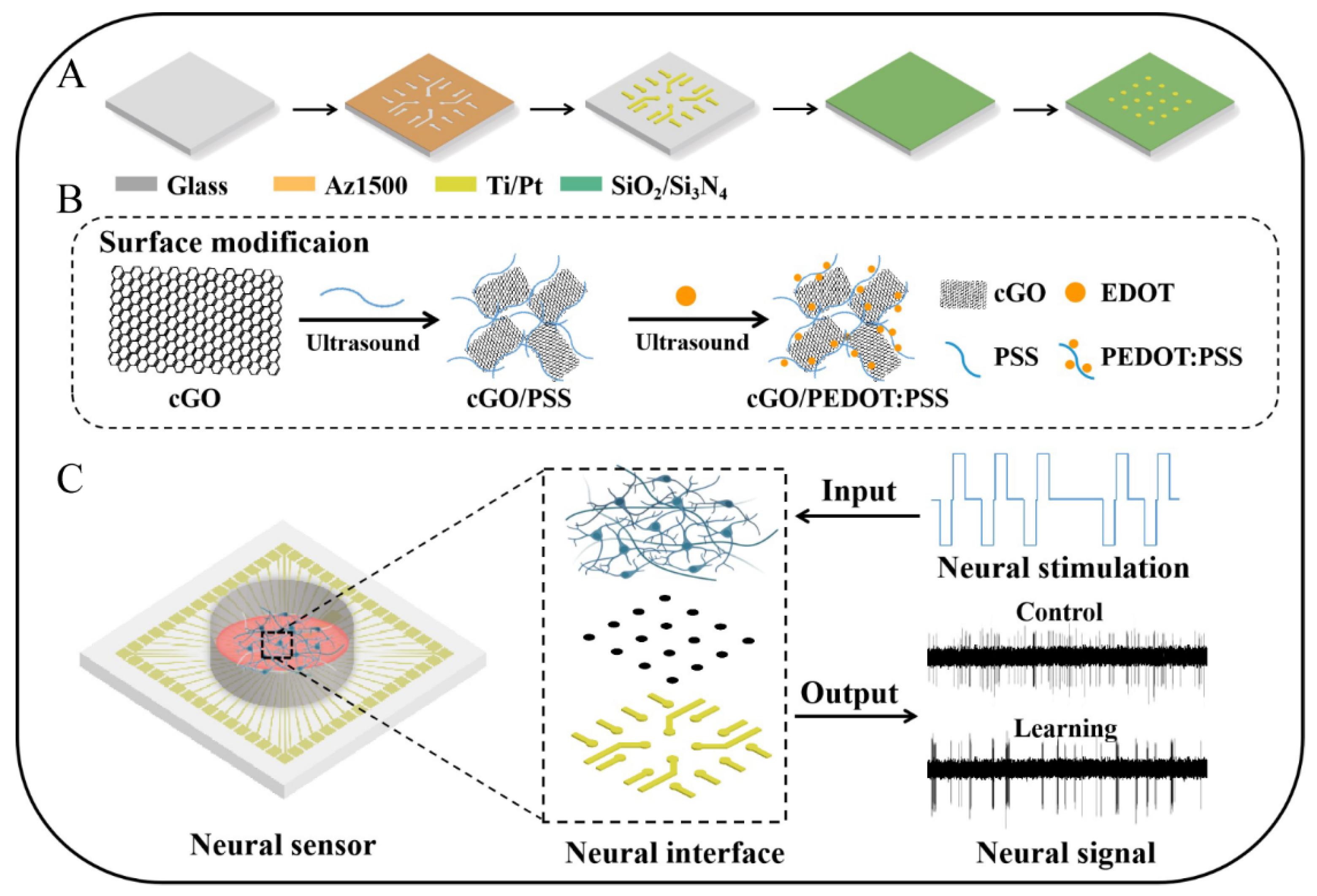
2.2. Fabrication of Neural Sensor and Cell Culture
2.3. Modification of Neural Interface
2.4. Protocol for Learning Training Hippocampal Neurons
2.5. Data Processing and Analysis
3. Results and Discussion
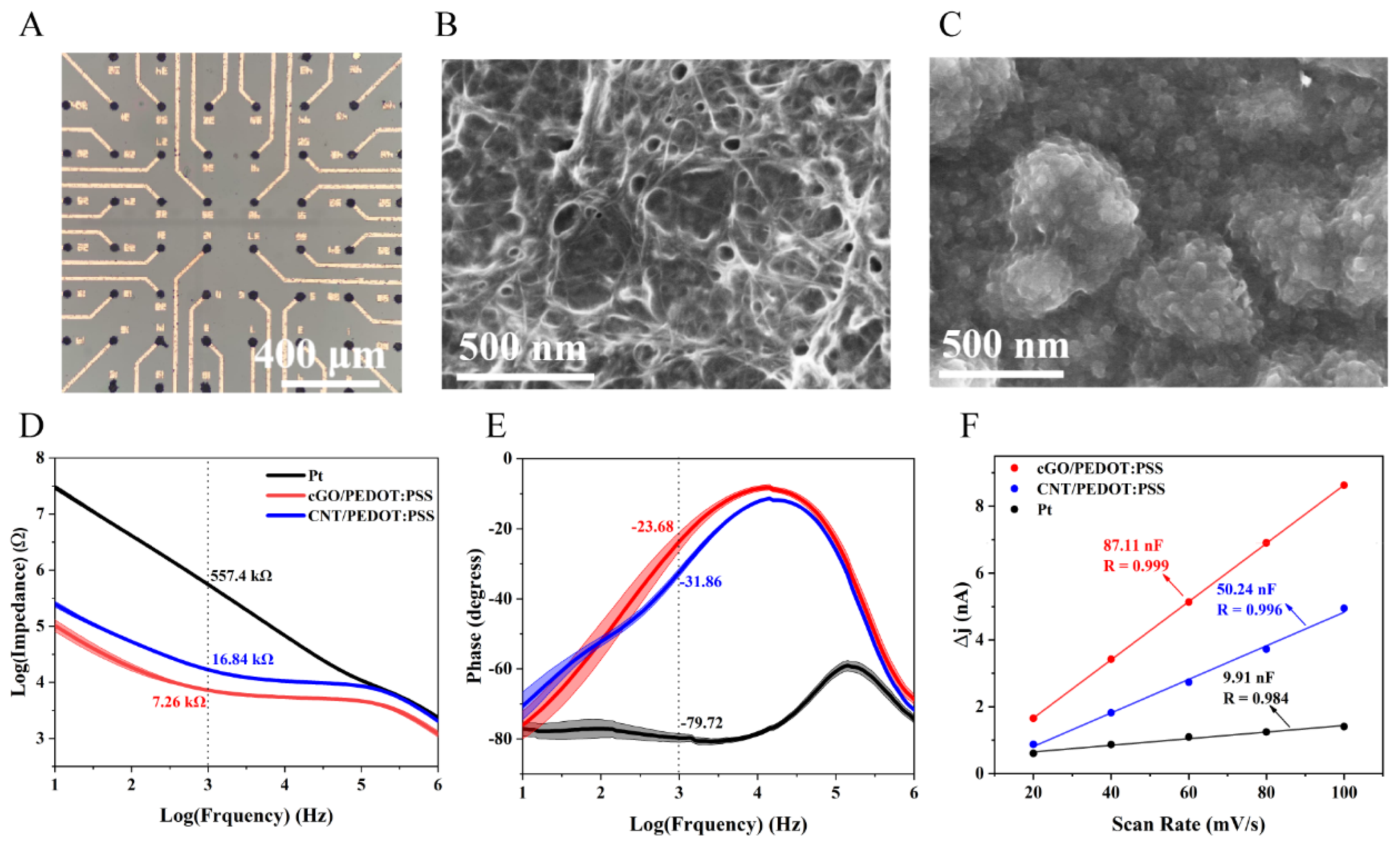
3.1. Morphology and Recording Characteristics of Neural Sensor
3.2. Stimulation Characteristics of Neural Sensor
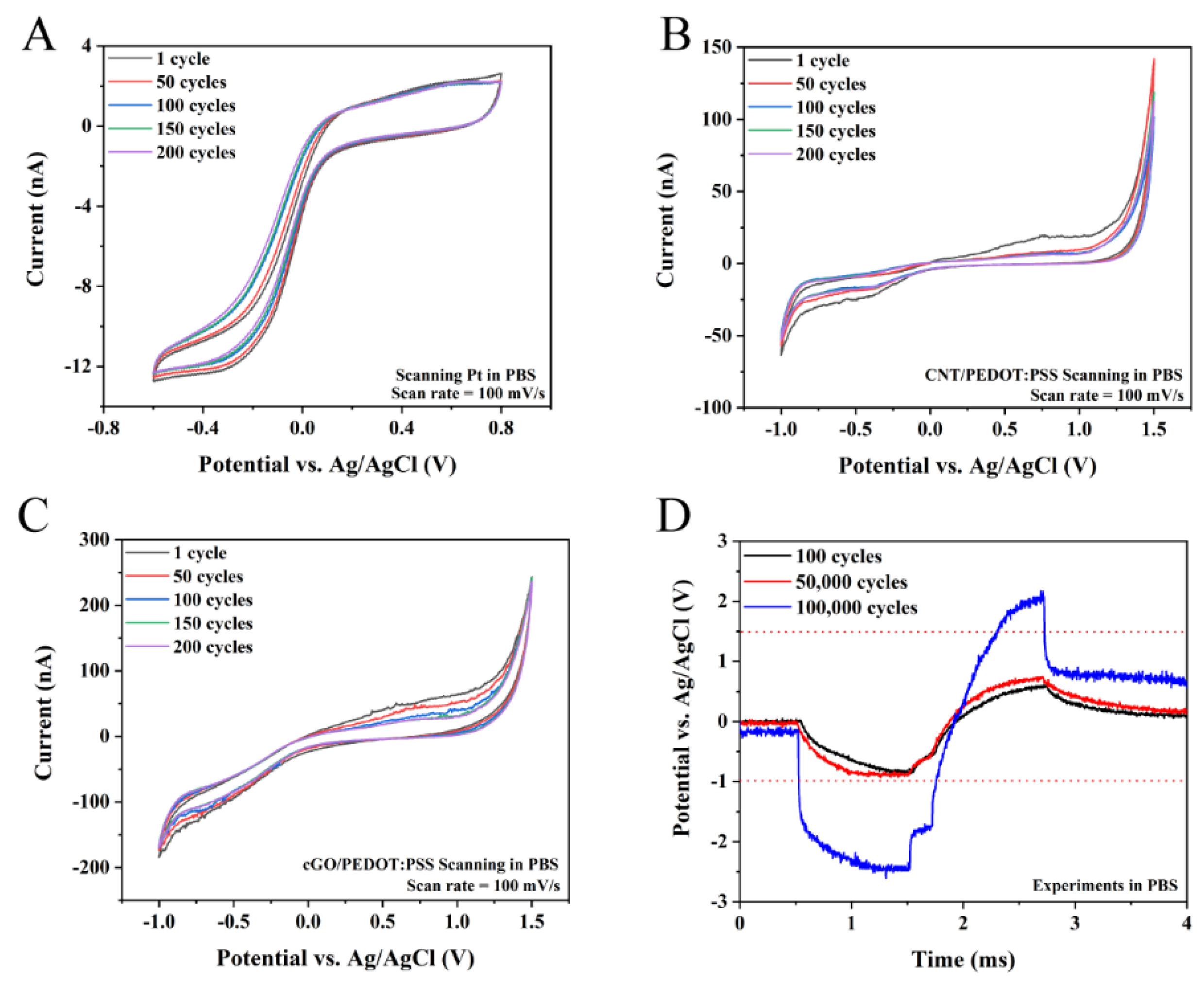
3.3. Stability of Neural Interface
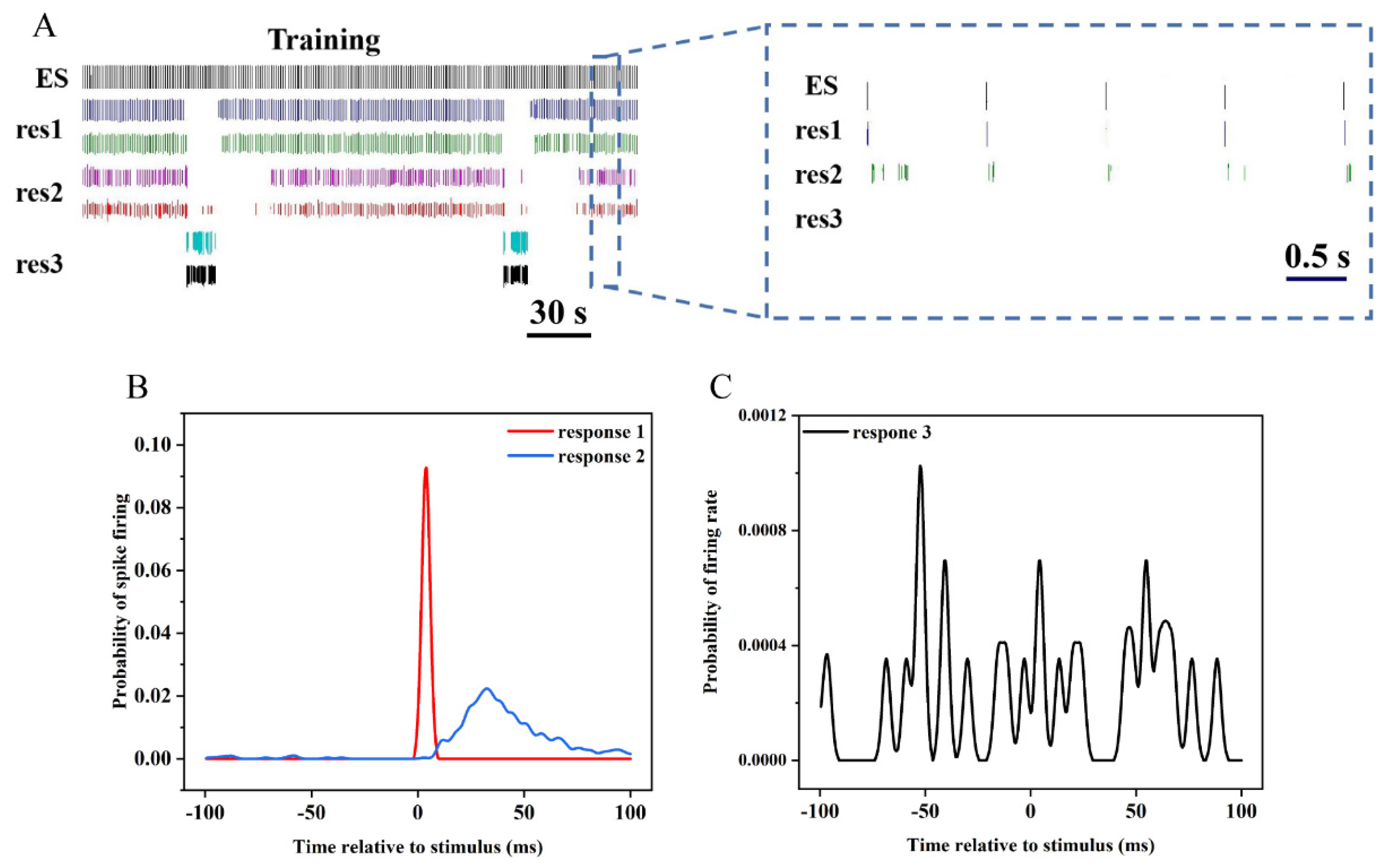
3.4. Response of Hippocampal Neurons to Electrical Stimulation
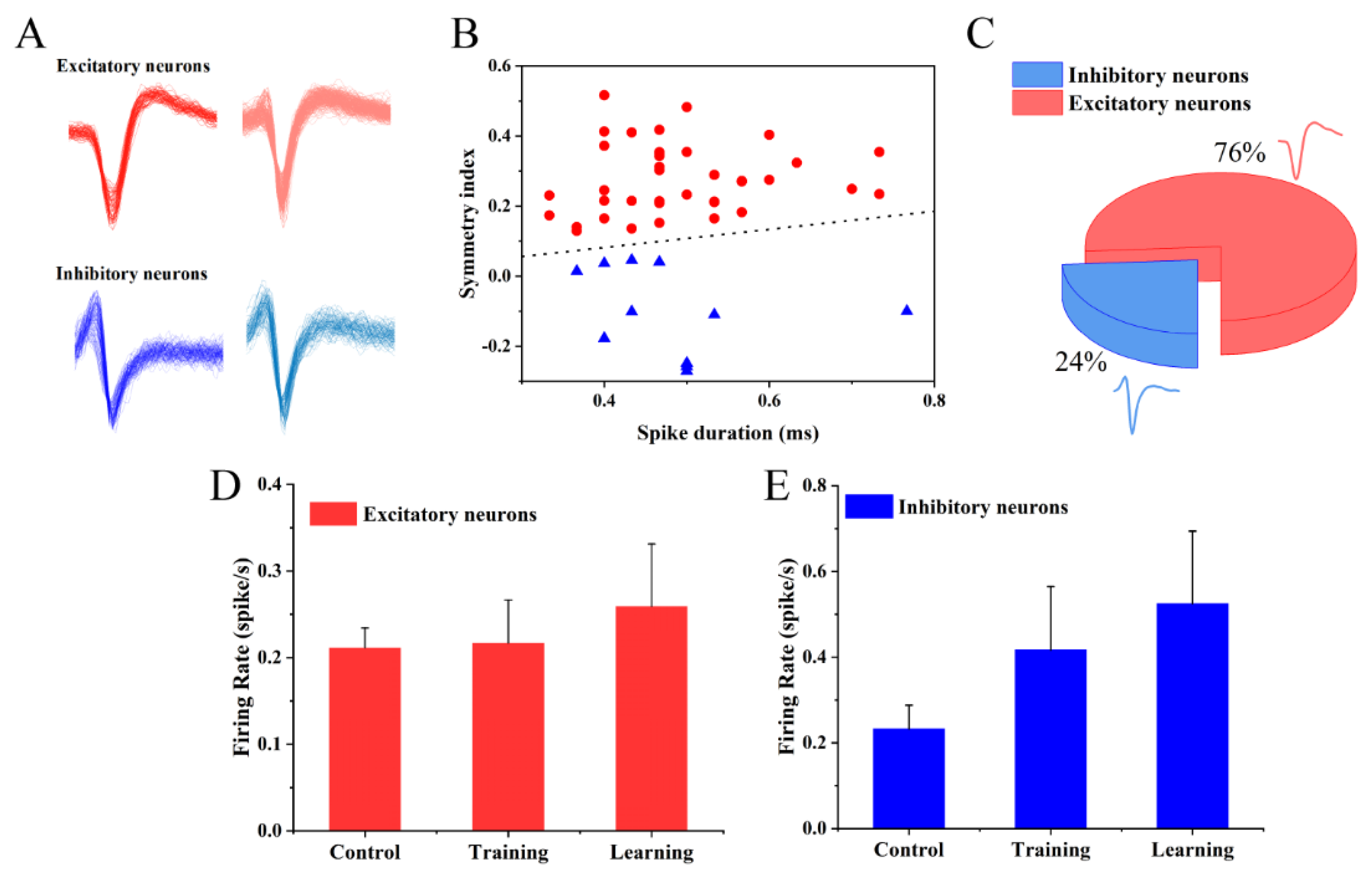
3.5. Effects of Electrical Stimulation on Different Types of Neurons
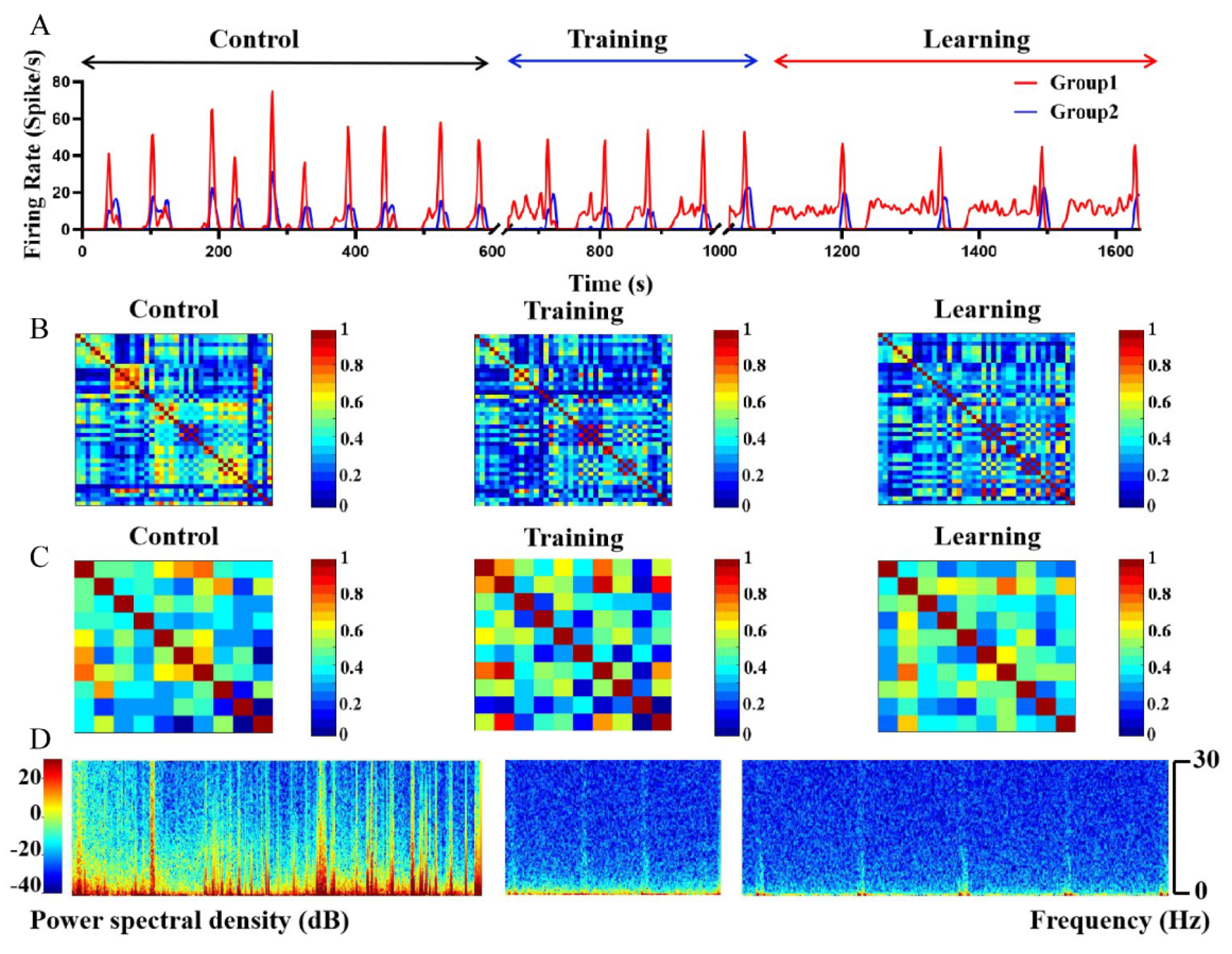
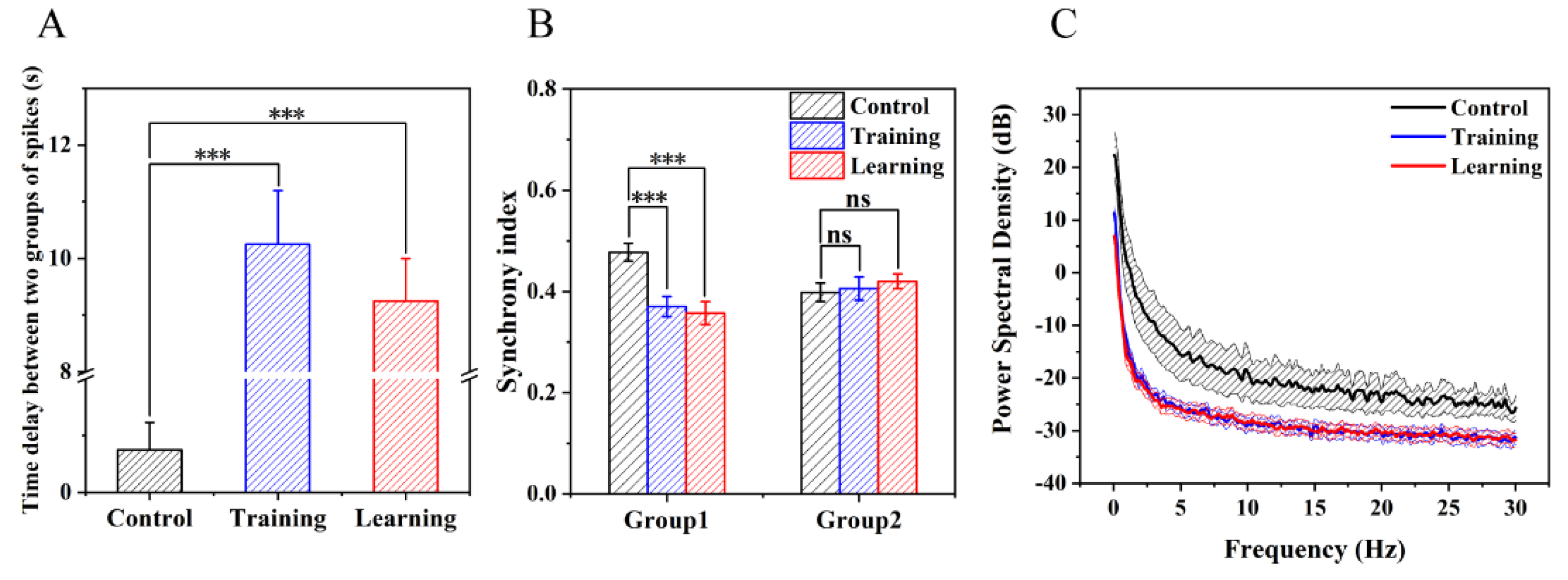
3.6. Electrophysiological Characteristics at Population Level
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachidhanandam, S.; Sreenivasan, V.; Kyriakatos, A.; Kremer, Y.; Petersen, C.C. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat. Neurosci. 2013, 16, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.E.; Yang, H.; Minamisawa, G.; O’Connor, D.H. Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat. Neurosci. 2016, 19, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.; Ohstrom, K.R.P.; Lindenberger, U. Interactive brains, social minds: Neural and physiological mechanisms of interpersonal action coordination. Neurosci. Biobehav. Rev. 2021, 128, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Reinert, S.; Hubener, M.; Bonhoeffer, T.; Goltstein, P.M. Mouse prefrontal cortex represents learned rules for categorization. Nature 2021, 593, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Wischnewski, M.; Ghanavati, E.; Mosayebi-Samani, M.; Kuo, M.-F.; Nitsche, M.A. Cognitive functions and underlying parameters of human brain physiology are associated with chronotype. Nat. Commun. 2021, 12, 4672. [Google Scholar] [CrossRef]
- Li, C.; Li, Y. A Review on Synergistic Learning. IEEE Access 2016, 4, 119–134. [Google Scholar] [CrossRef]
- le Feber, J.; Stegenga, J.; Rutten, W.L. The effect of slow electrical stimuli to achieve learning in cultured networks of rat cortical neurons. PLoS ONE 2010, 5, e8871. [Google Scholar] [CrossRef]
- Stegenga, J.; Le Feber, J.; Marani, E.; Rutten, W.L. The effect of learning on bursting. IEEE Trans. Biomed. Eng. 2009, 56, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Di Credico, A.; Gaggi, G.; Izzicupo, P.; Ferri, L.; Bonanni, L.; Iannetti, G.; Di Baldassarre, A.; Ghinassi, B. Real-Time Monitoring of Levetiracetam Effect on the Electrophysiology of an Heterogenous Human iPSC-Derived Neuronal Cell Culture Using Microelectrode Array Technology. Biosensors 2021, 11, 450. [Google Scholar] [CrossRef]
- Kussauer, S.; David, R.; Lemcke, H. hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells 2019, 8, 1331. [Google Scholar] [CrossRef] [Green Version]
- Gramowski, A.; Flossdorf, J.; Bhattacharya, K.; Jonas, L.; Lantow, M.; Rahman, Q.; Schiffmann, D.; Weiss, D.G.; Dopp, E. Nanoparticles induce changes of the electrical activity of neuronal networks on microelectrode array neurochips. Environ. Health Perspect. 2010, 118, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Xu, G.; Guan, M.; Zhang, Q.; Wang, Z.; Dong, Z.; Chen, W.; Yang, X.; Qiao, A.; et al. A multiplexed electrochemical quantitative polymerase chain reaction platform for single-base mutation analysis. Biosens. Bioelectron. 2022, 214, 114496. [Google Scholar] [CrossRef]
- He, E.; Xu, S.; Xiao, G.; Dai, Y.; Li, X.; Song, Y.; Gao, F.; Zhang, Y.; Xu, S.; Cai, X. MWCNTs/PEDOT:PSS nanocomposites-modified microelectrode array for spatial dynamics recording of epileptic discharges in multi-subregion of hippocampal slice. Sens. Actuators B Chem. 2021, 329, 129190. [Google Scholar] [CrossRef]
- Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Xu, S.; Lu, Z.; Wang, M.; Cai, X. Cellular-Scale Microelectrode Arrays to Monitor Movement-Related Neuron Activities in the Epileptic Hippocampus of Awake Mice. IEEE Trans. Biomed. Eng. 2021, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, A.; Mzezewa, R.; Sukki, L.; Ryynanen, T.; Kreutzer, J.; Hyvarinen, T.; Vinogradov, A.; Aarnos, L.; Lekkala, J.; Kallio, P.; et al. A modular brain-on-a-chip for modelling epileptic seizures with functionally connected human neuronal networks. Biosens. Bioelectron. 2020, 168, 112553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Li, X.; Zeng, S.; Liu, M.; Luo, Q. Characterization of synchronized bursts in cultured hippocampal neuronal networks with learning training on microelectrode arrays. Biosens. Bioelectron. 2007, 22, 2976–2982. [Google Scholar] [CrossRef]
- Massobrio, P.; Tessadori, J.; Chiappalone, M.; Ghirardi, M. In vitro studies of neuronal networks and synaptic plasticity in invertebrates and in mammals using multielectrode arrays. Neural Plast. 2015, 2015, 196195. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-S.; Whang, C.-N.; Park, J.-C.; Lee, D.-H.; Seo, W.-S. Biocompatibility and charge injection property of iridium film formed by ion beam assisted deposition. Biomaterials 2003, 24, 2225–2231. [Google Scholar] [CrossRef]
- Heim, M.; Yvert, B.; Kuhn, A. Nanostructuration strategies to enhance microelectrode array (MEA) performance for neuronal recording and stimulation. J. Physiol. Paris 2012, 106, 137–145. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Song, Y.; Luo, J.; Wei, W.; Xu, S.; Cai, X. Highly sensitive detection of quantal dopamine secretion from pheochromocytoma cells using neural microelectrode array electrodeposited with polypyrrole graphene. ACS Appl. Mater. Interfaces 2015, 7, 7619–7626. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Xu, S.; Dai, Y.; Wang, Y.; Xiao, G.; Xie, J.; Xu, S.; Fan, P.; Mo, F.; Wang, M.; et al. SWCNTs/PEDOT:PSS-Modified Microelectrode Arrays for Dual-Mode Detection of Electrophysiological Signals and Dopamine Concentration in the Striatum under Isoflurane Anesthesia. ACS Sens. 2021, 6, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, N.; Kim, Y.; Baik, M.-S.; Yoo, M.; Kim, D.; Lee, W.-J.; Kang, D.-H.; Kim, S.; Lee, K.; et al. High-performance, polymer-based direct cellular interfaces for electrical stimulation and recording. NPG Asia Mater. 2018, 10, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Susloparova, A.; Halliez, S.; Begard, S.; Colin, M.; Buee, L.; Pecqueur, S.; Alibart, F.; Thomy, V.; Arscott, S.; Pallecchi, E.; et al. Low impedance and highly transparent microelectrode arrays (MEA) for in vitro neuron electrical activity probing. Sens. Actuators B Chem. 2021, 327, 128895. [Google Scholar] [CrossRef]
- Xu, S.; Deng, Y.; Luo, J.; He, E.; Liu, Y.; Zhang, K.; Yang, Y.; Xu, S.; Sha, L.; Song, Y.; et al. High-Throughput PEDOT:PSS/PtNPs-Modified Microelectrode Array for Simultaneous Recording and Stimulation of Hippocampal Neuronal Networks in Gradual Learning Process. ACS Appl. Mater. Interfaces 2022, 14, 15736–15746. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly stable carbon nanotube doped poly(3,4-ethylenedioxythiophene) for chronic neural stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Zhao, H.; Xie, J.; Xu, S.; Gao, F.; Wang, M.; Xing, G.; et al. Microelectrode Arrays Modified with Nanocomposites for Monitoring Dopamine and Spike Firings under Deep Brain Stimulation in Rat Models of Parkinson’s Disease. ACS Sens. 2019, 4, 1992–2000. [Google Scholar] [CrossRef]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Yu, X.; Wang, S.; Qiu, J.; Tang, W.; Li, L.; Liu, H.; Wang, Z.L. Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers. ACS Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Kuo, C.-W.; Chen, P. Multifunctional Graphene-PEDOT Microelectrodes for On-Chip Manipulation of Human Mesenchymal Stem Cells. Adv. Funct. Mater. 2013, 23, 4649–4656. [Google Scholar] [CrossRef]
- He, E.; Zhou, Y.; Luo, J.; Xu, S.; Zhang, K.; Song, Y.; Wang, M.; Xu, S.; Dai, Y.; Yang, G.; et al. Sensitive detection of electrophysiology and dopamine vesicular exocytosis of hESC-derived dopaminergic neurons using multifunctional microelectrode array. Biosens. Bioelectron. 2022, 209, 114263. [Google Scholar] [CrossRef] [PubMed]
- Aqrawe, Z.; Montgomery, J.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers for neuronal microelectrode array recording and stimulation. Sens. Actuators B Chem. 2018, 257, 753–765. [Google Scholar] [CrossRef]
- Chen, S.; Pei, W.; Gui, Q.; Tang, R.; Chen, Y.; Zhao, S.; Wang, H.; Chen, H. PEDOT/MWCNT composite film coated microelectrode arrays for neural interface improvement. Sens. Actuators A Phys. 2013, 193, 141–148. [Google Scholar] [CrossRef]
- Saunier, V.; Flahaut, E.; Blatche, M.C.; Bergaud, C.; Maziz, A. Carbon nanofiber-PEDOT composite films as novel microelectrode for neural interfaces and biosensing. Biosens. Bioelectron. 2020, 165, 112413. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Schlabach, S.; Stieglitz, T.; Asplund, M. Long-Term Stable Adhesion for Conducting Polymers in Biomedical Applications: IrOx and Nanostructured Platinum Solve the Chronic Challenge. ACS Appl. Mater. Interfaces 2017, 9, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Gross, G.W.; Rhoades, B.K.; Azzazy, H.M.E.; Wu, M.C. The Use of Neuronal Networks on Multielectrode Arrays as Biosensors. Biosens. Bioelectron. 1995, 10, 553–567. [Google Scholar] [CrossRef]
- Simmons, D.V.; Higgs, M.H.; Lebby, S.; Wilson, C.J. Predicting responses to inhibitory synaptic input in substantia nigra pars reticulata neurons. J. Neurophysiol. 2018, 120, 2679–2693. [Google Scholar] [CrossRef]
- Dai, Y.C.; Song, Y.L.; Xie, J.Y.; Xiao, G.H.; Li, X.Y.; Li, Z.Y.; Gao, F.; Zhang, Y.; He, E.H.; Xu, S.W.; et al. CB1-Antibody Modified Liposomes for Targeted Modulation of Epileptiform Activities Synchronously Detected by Microelectrode Arrays. ACS Appl. Mater. Interfaces 2020, 12, 41148–41156. [Google Scholar] [CrossRef]
- Sukenik, N.; Vinogradov, O.; Weinreb, E.; Segal, M.; Levina, A.; Moses, E. Neuronal circuits overcome imbalance in excitation and inhibition by adjusting connection numbers. Proc. Natl. Acad. Sci. USA 2021, 118, e2018459118. [Google Scholar] [CrossRef]
- Goel, A.; Buonomano, D.V. Chronic electrical stimulation homeostatically decreases spontaneous activity, but paradoxically increases evoked network activity. J. Neurophysiol. 2013, 109, 1824–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, T.C.; Chen, C.M. Learning-induced synchronization and plasticity of a developing neural network. J. Comput. Neurosci. 2005, 19, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.; Tetzlaff, T.; Potjans, T.C.; Pettersen, K.H.; Grun, S.; Diesmann, M.; Einevoll, G.T. Modeling the Spatial Reach of the LFP. Neuron 2011, 72, 859–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.J.; Luo, X.L.; Weaver, C.L.; Cui, X.T. Poly(3,4-ethylenedioxythiophene)-ionic liquid coating improves neural recording and stimulation functionality of MEAs. J. Mater. Chem. C 2015, 3, 6515–6524. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J.; Humayun, M.S. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 1574–1579. [Google Scholar] [CrossRef]
- Abidian, M.R.; Corey, J.M.; Kipke, D.R.; Martin, D.C. Conducting-Polymer Nanotubes Improve Electrical Properties, Mechanical Adhesion, Neural Attachment, and Neurite Outgrowth of Neural Electrodes. Small 2010, 6, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fishman, H.A.; Dai, H.J.; Harris, J.S. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef]
- Park, S.; Song, Y.J.; Boo, H.; Chung, T.D. Nanoporous Pt Microelectrode for Neural Stimulation and Recording: In Vitro Characterization. J. Phys. Chem. C 2010, 114, 8721–8726. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Deng, Y.; Luo, J.; Liu, Y.; He, E.; Yang, Y.; Zhang, K.; Sha, L.; Dai, Y.; Ming, T.; et al. A Neural Sensor with a Nanocomposite Interface for the Study of Spike Characteristics of Hippocampal Neurons under Learning Training. Biosensors 2022, 12, 546. https://doi.org/10.3390/bios12070546
Xu S, Deng Y, Luo J, Liu Y, He E, Yang Y, Zhang K, Sha L, Dai Y, Ming T, et al. A Neural Sensor with a Nanocomposite Interface for the Study of Spike Characteristics of Hippocampal Neurons under Learning Training. Biosensors. 2022; 12(7):546. https://doi.org/10.3390/bios12070546
Chicago/Turabian StyleXu, Shihong, Yu Deng, Jinping Luo, Yaoyao Liu, Enhui He, Yan Yang, Kui Zhang, Longze Sha, Yuchun Dai, Tao Ming, and et al. 2022. "A Neural Sensor with a Nanocomposite Interface for the Study of Spike Characteristics of Hippocampal Neurons under Learning Training" Biosensors 12, no. 7: 546. https://doi.org/10.3390/bios12070546
APA StyleXu, S., Deng, Y., Luo, J., Liu, Y., He, E., Yang, Y., Zhang, K., Sha, L., Dai, Y., Ming, T., Song, Y., Jing, L., Zhuang, C., Xu, Q., & Cai, X. (2022). A Neural Sensor with a Nanocomposite Interface for the Study of Spike Characteristics of Hippocampal Neurons under Learning Training. Biosensors, 12(7), 546. https://doi.org/10.3390/bios12070546




