Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Reagents
2.2. Sensor Preparation
2.3. Electrochemical Measurements for MC-LR Determination
3. Results and Discussion
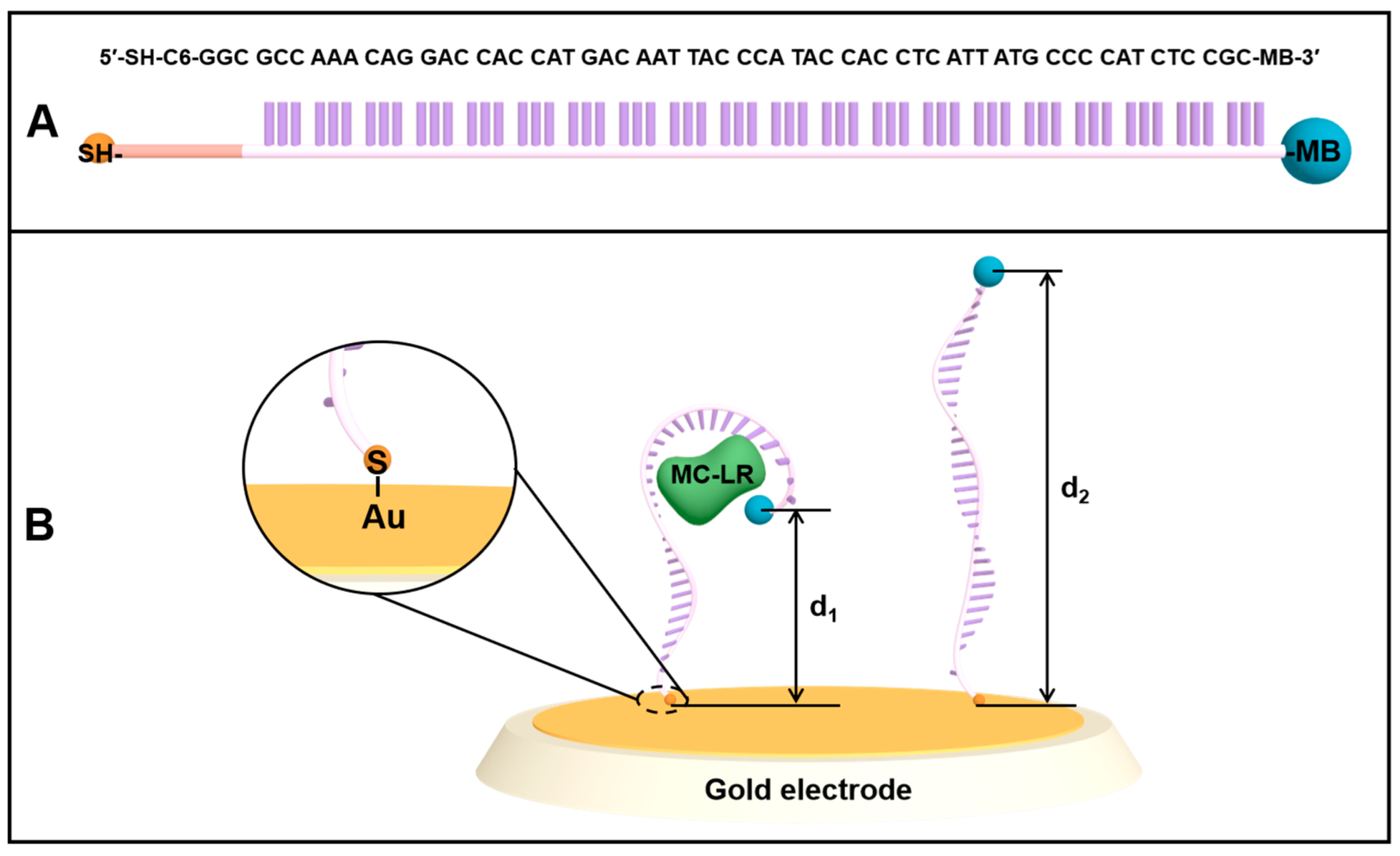
3.1. E-AB Platform Design and Sensing Mechanism
3.2. Sensor Characterization Using CV and SWV
3.3. Optimization of the Aptasensor Reaction Conditions
3.4. Sensor Sensitivity
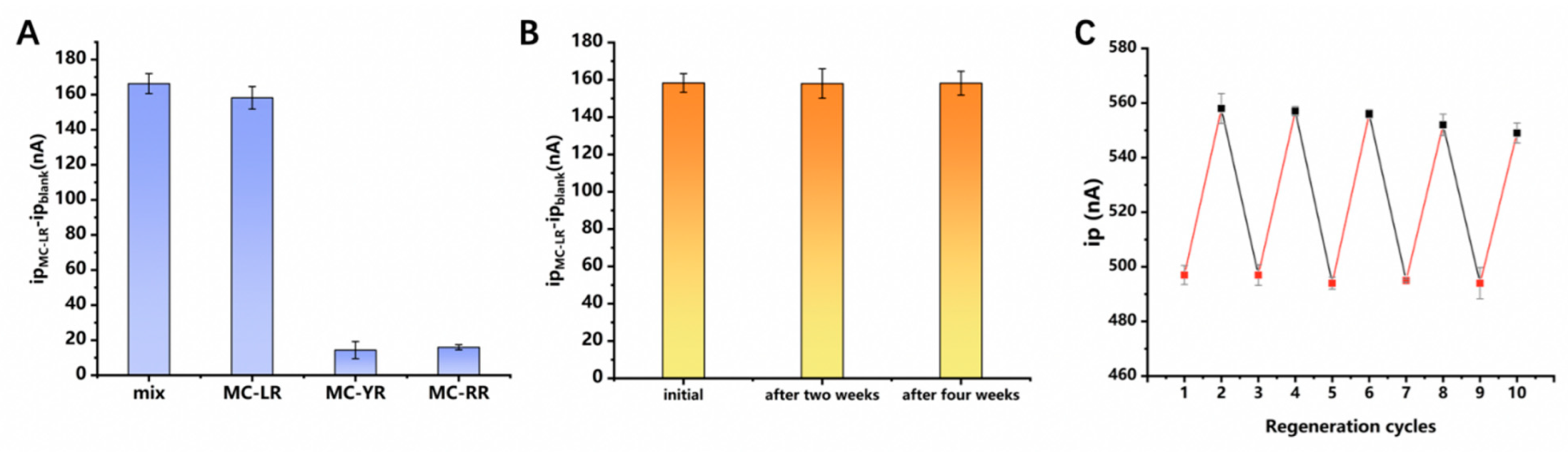
3.5. Sensor Selectivity, Stability, and Reusability
3.6. Real Sample Detection in Simulated Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lotierzo, M.; Abuknesha, R.; Davis, F.; Tothill, I.E. A Membrane-Based ELISA Assay and Electrochemical Immunosensor for Microcystin-LR in Water Samples. Environ. Sci. Technol. 2012, 46, 5504–5510. [Google Scholar] [CrossRef] [PubMed]
- Plaas, H.E.; Paerl, H.W. Toxic Cyanobacteria: A Growing Threat to Water and Air Quality. Environ. Sci. Technol. 2020, 55, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Schreidah, C.M.; Ratnayake, K.; Senarath, K.; Karunarathne, A. Microcystins: Biogenesis, Toxicity, Analysis, and Control. Chem. Res. Toxicol. 2020, 33, 2225–2246. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Zourob, M. Label-Free Voltammetric Aptasensor for the Sensitive Detection of Microcystin-LR Using Graphene-Modified Electrodes. Anal. Chem. 2014, 86, 7551–7557. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, S.; Ahn, C.-Y.; Oh, H.-M.; Asthana, R.K. Monitoring Approaches for a Toxic Cyanobacterial Bloom. Environ. Sci. Technol. 2013, 47, 8999–9013. [Google Scholar] [CrossRef]
- Long, F.; He, M.; Zhu, A.N.; Shi, H.C. Portable optical immunosensor for highly sensitive detection of microcystin-LR in water samples. Biosens. Bioelectron. 2009, 24, 2346–2351. [Google Scholar] [CrossRef]
- Campàs, M.; Marty, J.-L. Highly sensitive amperometric immunosensors for microcystin detection in algae. Biosens. Bioelectron. 2007, 22, 1034–1040. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Du, B.; Wu, D.; Cai, Y.; Mao, K.; Li, H.; Xu, C. Nanoporous PtRu Alloy Enhanced Nonenzymatic Immunosensor for Ultrasensitive Detection of Microcystin-LR. Adv. Funct. Mater. 2011, 21, 4193–4198. [Google Scholar] [CrossRef]
- Tong, P.; Tang, S.; He, Y.; Shao, Y.; Zhang, L.; Chen, G. Label-free immunosensing of microcystin-LR using a gold electrode modified with gold nanoparticles. Mikrochim. Acta 2011, 173, 299–305. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Pan, R.; Leng, C.; Hu, Z.; Ju, H. In situassembly of gold nanoparticles on nitrogen-doped carbon nanotubes for sensitive immunosensing of microcystin-LR. Chem. Commun. 2011, 47, 668–670. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Xu, D.; Shim, B.S.; Zhu, Y.; Sun, F.; Liu, L.; Peng, C.; Jin, Z.; Xu, C.; et al. Simple, Rapid, Sensitive, and Versatile SWNT−Paper Sensor for Environmental Toxin Detection Competitive with ELISA. Nano Lett. 2009, 9, 4147–4152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J.; et al. A Multiwalled-Carbon-Nanotube-Based Biosensor for Monitoring Microcystin-LR in Sources of Drinking Water Supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]
- Zhang, W.; Han, C.; Jia, B.; Saint, C.; Nadagouda, M.; Falaras, P.; Sygellou, L.; Vogiazi, V.; Dionysiou, D.D. A 3D graphene-based biosensor as an early microcystin-LR screening tool in sources of drinking water supply. Electrochim. Acta 2017, 236, 319–327. [Google Scholar] [CrossRef]
- Tan, F.; Saucedo, N.M.; Ramnani, P.; Mulchandani, A. Label-Free Electrical Immunosensor for Highly Sensitive and Specific Detection of Microcystin-LR in Water Samples. Environ. Sci. Technol. 2015, 49, 9256–9263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Dong, H.; Zhang, X.; Wang, W.; Chen, Z. An electrochemical non-enzymatic immunosensor for ultrasensitive detection of microcystin-LR using carbon nanofibers as the matrix. Sens. Actuators B Chem. 2016, 233, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Ruiyi, L.; Qianfang, X.; Zaijun, L.; Xiulan, S.; Junkang, L. Electrochemical immunosensor for ultrasensitive detection of microcystin-LR based on graphene–gold nanocomposite/functional conducting polymer/gold nanoparticle/ionic liquid composite film with electrodeposition. Biosens. Bioelectron. 2013, 44, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, J.; Quan, X. A graphene and multienzyme functionalized carbon nanosphere-based electrochemical immunosensor for microcystin-LR detection. Colloids Surf. B Biointerfaces 2013, 103, 38–44. [Google Scholar] [CrossRef]
- Gan, C.; Ling, L.; He, Z.; Lei, H.; Liu, Y. In-situ assembly of biocompatible core–shell hierarchical nanostructures sensitized immunosensor for microcystin-LR detection. Biosens. Bioelectron. 2016, 78, 381–389. [Google Scholar] [CrossRef]
- Deng, Q.; Watson, C.J.; Kennedy, R.T. Aptamer affinity chromatography for rapid assay of adenosine in microdialysis samples collected in vivo. J. Chromatogr. A 2003, 1005, 123–130. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef]
- Bilibana, M.P.; Williams, A.R.; Rassie, C.; Sunday, C.E.; Makelane, H.; Wilson, L.; Ntshongontshi, N.; Jijana, A.N.; Masikini, M.; Baker, P.G.L.; et al. Electrochemical Aptatoxisensor Responses on Nanocomposites Containing Electro-Deposited Silver Nanoparticles on Poly(Propyleneimine) Dendrimer for the Detection of Microcystin-LR in Freshwater. Sensors 2016, 16, 1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, A.; Chinnappan, R.; Eissa, S.; Liu, H.; Tlili, C.; Zourob, M. Selection, Characterization, and Biosensing Application of High Affinity Congener-Specific Microcystin-Targeting Aptamers. Environ. Sci. Technol. 2012, 46, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Z.; Teng, X.; Lai, Y.; Pu, S.; Pang, P.; Wang, H.; Yang, C.; Barrow, C.; Yang, W. Enzyme-free fluorescent detection of microcystin-LR using hairpin DNA-templated copper nanoclusters as signal indicator. Talanta 2019, 202, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Baker, S.L.; Lai, R.Y. Effects of DNA Probe Length on the Performance of a Dynamics-based Electrochemical Hg(II) Sensor. Electroanalysis 2017, 29, 2239–2245. [Google Scholar] [CrossRef]
- Wu, Y.; Lai, R.Y. Tunable Signal-Off and Signal-On Electrochemical Cisplatin Sensor. Anal. Chem. 2017, 89, 9984–9989. [Google Scholar] [CrossRef]
- Yu, Z.-G.; Sutlief, A.L.; Lai, R.Y. Towards the development of a sensitive and selective electrochemical aptamer-based ampicillin sensor. Sens. Actuators B Chem. 2018, 258, 722–729. [Google Scholar] [CrossRef]
- Kang, D.; Zuo, X.; Yang, R.; Xia, F.; Plaxco, K.W.; White, R.J. Comparing the Properties of Electrochemical-Based DNA Sensors Employing Different Redox Tags. Anal. Chem. 2009, 81, 9109–9113. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.D.; Lai, R.Y. Effects of redox label location on the performance of an electrochemical aptamer-based tumor necrosis factor-alpha sensor. Talanta 2018, 189, 585–591. [Google Scholar] [CrossRef]
- Lubin, A.A.; Hunt, B.V.S.; White, R.J.; Plaxco, K.W. Effects of Probe Length, Probe Geometry, and Redox-Tag Placement on the Performance of the Electrochemical E-DNA Sensor. Anal. Chem. 2009, 81, 2150–2158. [Google Scholar] [CrossRef]
- Schoukroun-Barnes, L.R.; Wagan, S.; White, R.J. Enhancing the Analytical Performance of Electrochemical RNA Aptamer-Based Sensors for Sensitive Detection of Aminoglycoside Antibiotics. Anal. Chem. 2013, 86, 1131–1137. [Google Scholar] [CrossRef]
- Uzawa, T.; Cheng, R.R.; White, R.J.; Makarov, D.E.; Plaxco, K.W. A Mechanistic Study of Electron Transfer from the Distal Termini of Electrode-Bound, Single-Stranded DNAs. J. Am. Chem. Soc. 2010, 132, 16120–16126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Ding, Y.; Zhang, L.; Guo, Y.; Li, M.; Chen, Z.; Wu, X. An ultrasensitive competitive immunosensor for impedimetric detection of microcystin-LR via antibody-conjugated enzymatic biocatalytic precipitation. Sens. Actuators B Chem. 2016, 233, 63–70. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Xu, Y.; Gao, X.; Qiu, B.; Chen, X.; Chen, G. Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta 2013, 103, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Lotfi Zadeh Zhad, H.R.; Rodríguez Torres, Y.M.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of Cd(II). J. Electroanal. Chem. 2017, 803, 89–94. [Google Scholar] [CrossRef]



| Electrode | Signal Labels | Linear Range | Detection Range (LOD) | Recovery (%) | Detection Techniques | Ref |
|---|---|---|---|---|---|---|
| Gold-GNPs | mAb | 0.05–15 µg/L | 20 ng/L | 95.6−105 | DPV | [9] |
| SWCNT/SiO2/Go ld | mAb | 1−1000 ng/L | 0.6 ng/L | 84.7–124.2 | Electrical resistance | [14] |
| AuNPs/GCE | HRP-mAb | 0.01−100 µg/L | 4 ng/L | 94.1−98.1 | EIS | [32] |
| Au (Non-labeled) | Ab, Au NPs | 0.05−15.00 μg/L | 20 ng/L | 95.6−105 | DPV | [9] |
| AuNp-polyDPB- G-AuNP/GCE | Polyclonal Ab | 0.1−8 pg/L | 0.037 pg/L | 96.3–105.8 | DPV | [16] |
| GSs-CS/GCE | HRP-CNS-Ab | 0.05−15 µg/L | 16 ng/L | 88–107.8 | DPV | [17] |
| CNT@Co silicate | Multi-HRP- (Fe3O4@PDA-Au)- Ab | 0.005−50 µg/L | 4 ng/L | 91.6–110.7 | CV | [18] |
| GS/GCE | Ab1, PtRu-Ab2 | 0.01−28 ng/mL | 9.63 pg/mL | 99.5–102 | Chronoamper ometry | [8] |
| CNFs/PEG/GCE | Ab1, Au-Ab2 | 0.0025−5 µg/L | 1.68 ng/L | 98−99.2 | DPV | [15] |
| GCE modified | aptamer | 0.1−1.1 µg/L | 40 pg/mL | 94.3−115 | CV | [21] |
| GSPE | Ferrocene, aptamer | 0.1−1000 ng/L | 1.9 ng/L | 91.7 | SWV | [4] |
| Au (Non-labeled) | aptamer | 1.0 × 10−7−5.0 × 10−11 mol/L | 1.8 × 10−11 mol/L | 91.2−113.7 | EIS | [33] |
| Au (Labeled) | aptamer | 0.001−0.75 ng/L | 0.53 pg/mL | 96.11−108.43 | SWV | this method |
| Sample | [MC-LR] Added (ng/L) | [MC-LR] Detected (ng/L) | Recovery % | RDS % |
|---|---|---|---|---|
| Tap water | 20 | 19.18 | 96.11 | 1.3 |
| 150 | 147.75 | 102.36 | 2.7 | |
| 500 | 494.45 | 98.89 | 3.3 | |
| The pond water | 5 | 5.14 | 102.87 | 1.03 |
| 20 | 21.12 | 105.60 | 1.56 | |
| 150 | 150.49 | 100.33 | 0.33 | |
| 200 | 197.33 | 98.66 | 0.99 | |
| 500 | 497.19 | 99.44 | 1.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, S.; Zhan, Y.; Kai, T.; Ding, P. Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer. Biosensors 2022, 12, 556. https://doi.org/10.3390/bios12080556
Wei X, Wang S, Zhan Y, Kai T, Ding P. Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer. Biosensors. 2022; 12(8):556. https://doi.org/10.3390/bios12080556
Chicago/Turabian StyleWei, Xiaoqian, Shanlin Wang, Yujuan Zhan, Tianhan Kai, and Ping Ding. 2022. "Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer" Biosensors 12, no. 8: 556. https://doi.org/10.3390/bios12080556





