3D and 4D Printing in the Fight against Breast Cancer
Abstract
:1. Introduction
2. Principles of Therapy for Breast Cancer: Current Treatments and Limitations
2.1. Surgery and Radiotherapy
Breast Reconstruction
2.2. Systemic Therapy
2.2.1. Chemotherapy
2.2.2. Endocrine Therapy
3. Personalized Medicine and Additive Manufacturing
4. Three-Dimensional and Four-Dimensional Printing
4.1. Advantages and Disadvantages
4.2. Materials for 3DP and 4DP
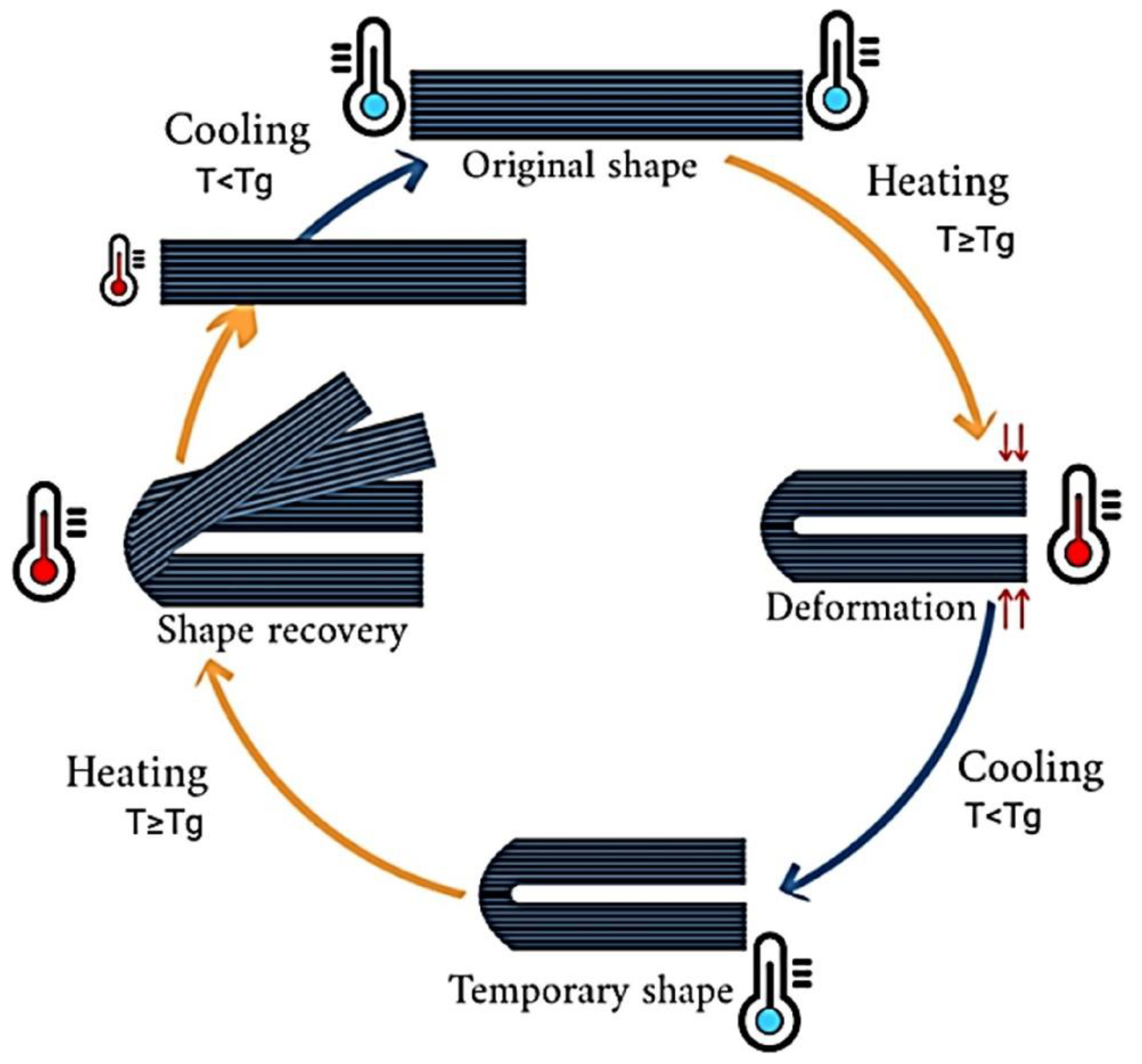
4.2.1. Shape Memory Materials
Shape Memory Alloys
Shape Memory Polymers (SMPs)
4.2.2. Smart Hydrogels
5. Three-Dimensional Printing in the Fight against Breast Cancer
5.1. Three-Dimensional-Printed Prototypes
5.2. Three-Dimensional Printing Application in the Treatment of Breast Cancer
Drug-Loaded Implants
5.3. Three-Dimensional Printing Application in Breast Reconstruction
5.3.1. Scaffold-Guided Reconstruction
5.3.2. External Prostheses
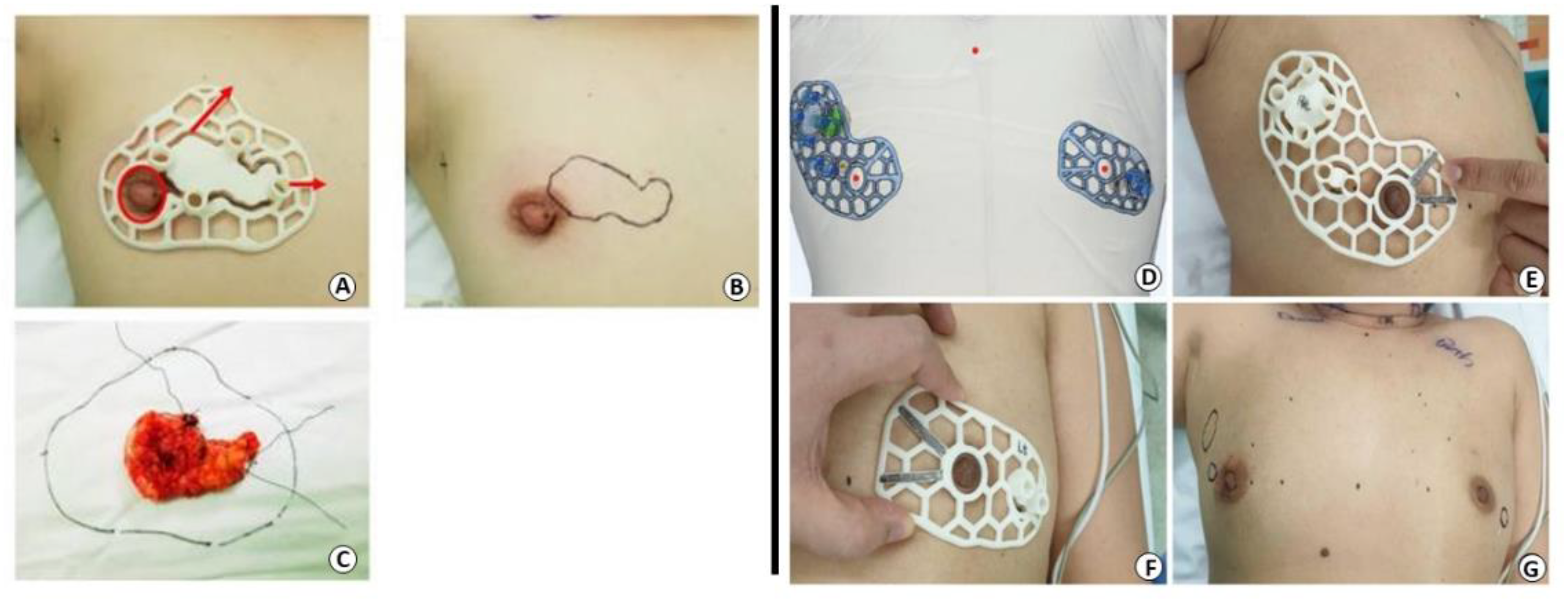
5.3.3. Nipple–Areola Complex Reconstruction
6. Four-Dimensional Printing Applications in Breast Cancer
7. Regulatory Considerations
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- NIH. Breast Cancer. Available online: https://www.cancer.gov/types/breast/hp/breast-prevention-pdq (accessed on 16 June 2022).
- World Health Organization. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 15 June 2022).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, R.E.; Blackburn, H.L.; Shriver, C.D.; Soon-Shiong, P.; Ellsworth, D.L. Molecular Heterogeneity in Breast Cancer: State of the Science and Implications for Patient Care. Semin. Cell Dev. Biol. 2017, 64, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Place, A.E.; Huh, S.J.; Polyak, K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espina, V.; Liotta, L.A. What is the malignant nature of human ductal carcinoma in situ? Nat. Rev. Cancer 2011, 11, 68–75. Available online: https://www.nature.com/articles/nrc2950 (accessed on 27 June 2022). [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer What Is Breast Cancer? Am. Cancer Soc. Cancer Facts Figure Atlanta Ga Am. Cancer Soc. 2022, 1–19. [Google Scholar]
- Viale, G.; Regan, M.M.; Maiorano, E.; Mastropasqua, M.G.; Dell’Orto, P.; Rasmussen, B.B.; Raffoul, J.; Neven, P.; Orosz, Z.; Braye, S.; et al. Prognostic and Predictive Value of Centrally Reviewed Expression of Estrogen and Progesterone Receptors in a Randomized Trial Comparing Letrozole and Tamoxifen Adjuvant Therapy for Postmenopausal Early Breast Cancer: BIG 1. J. Clin. Oncol. 2022, 25, 3846–3852. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Johnston, S.R. Endocrine Therapy–Current Benefits and Limitations. Breast Cancer Res. Treat. 2005, 93, S3–S10. [Google Scholar] [CrossRef]
- Huang, M.; Wu, J.; Ling, R.; Li, N. Quadruple Negative Breast Cancer. Breast Cancer 2020, 27, 527–533. [Google Scholar] [CrossRef]
- Disparity, R.; Determinants, S. Racial Disparity and Triple-Negative Breast Cancer in African-American women: A multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers 2018, 10, 514. [Google Scholar] [CrossRef] [Green Version]
- Minami, C.A.; King, T.A.; Mittendorf, E.A. Patient Preferences for Locoregional Therapy in Early-Stage Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Breast, E.; Trialists, C.; Group, C. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10 801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Surgery for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer.html (accessed on 15 June 2022).
- American Cancer Society. Radiation for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/radiation-for-breast-cancer.html (accessed on 22 June 2022).
- Lee, G.K.; Sheckter, C.C. Breast Reconstruction Following Breast Cancer Treatment-2018. JAMA J. Am. Med. Assoc. 2018, 320, 1277–1278. [Google Scholar] [CrossRef]
- Gladfelter, J. Breast Augmentation 101. Plast. Surg. Nurs. 2007, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.P.; Gabriel, A. Breast Implant Design. Gland Surg. 2017, 6, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shridharani, S.M.; Bellamy, J.L.; Mofid, M.M.; Singh, N.K. Interesting Case Series Breast Augmentation. Eplasty 2013, 13. Available online: https://www.yumpu.com/en/document/view/28977958/interesting-case-series-breast-augmentation-eplasty (accessed on 22 June 2022).
- Spear, S.L.; Mesbahi, A.N. Implant-Based Reconstruction. Clin. Plast. Surgery 2007, 34, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Manyam, B.V.; Shah, C.; Woody, N.M.; Reddy, C.A.; Weller, M.A.; Juloori, A.; Naik, M.; Valente, S.; Grobmyer, S.; Durand, P.; et al. Long-Term Complications and Reconstruction Failures in Previously Radiated Breast Cancer Patients Receiving Salvage Mastectomy with Autologous Reconstruction or Tissue Expander/Implant-Based Reconstruction. Breast J. 2019, 25, 1071–1078. [Google Scholar] [CrossRef]
- Visscher, L.E.; Cheng, M.; Chhaya, M.; Hintz, M.L.; Schantz, J.T.; Tran, P.; Ung, O.; Wong, C.; Hutmacher, D.W. Breast Augmentation and Reconstruction from a Regenerative Medicine Point of View: State of the Art and Future Perspectives. Tissue Eng. Part B Rev. 2017, 23, 281–293. [Google Scholar] [CrossRef]
- Glaus, S.W.; Carlson, G.W. Long-Term Role of External Breast Prostheses After Total Mastectomy. Breast J. 2009, 15, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sisti, A.; Grimaldi, L.; Tassinari, J.; Cuomo, R.; Fortezza, L. Nipple-Areola Complex Reconstruction Techniques: A Literature Review. Eur. J. Surg. Oncol. 2016, 42, 441–465. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Chemotherapy for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/chemotherapy-for-breast-cancer.html (accessed on 22 June 2022).
- American Cancer Society. Hormone Therapy for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/hormone-therapy-for-breast-cancer.html (accessed on 22 June 2022).
- Vogenberg, F.R.; Barash, C.I.; Pursel, M. Personalized Medicine—Part 1: Evolution and Development into Theranostics. Pharm. Ther. 2010, 35, 560. [Google Scholar]
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Bhuskute, H.; Shende, P.; Prabhakar, B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2022, 23, 8. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards Precision Medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From Oral Formulations to Drug-Eluting Implants: Using 3D and 4D Printing to Develop Drug Delivery Systems and Personalized Medicine. Bio-Design Manuf. 2022, 5, 85–106. [Google Scholar] [CrossRef]
- Prakash, K.S.; Nancharaih, T.; Rao, V.V.S. Additive Manufacturing Techniques in Manufacturing—An Overview. Mater. Today Proc. 2018, 5, 3873–3882. [Google Scholar] [CrossRef]
- ISO/ASTM. Additive Manufacturing—General Principles Terminology (ASTM52900). Rapid Manuf. Assoc. 2013, 10–12. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, II. Johns. Matthey Technol. Rev. 2015, 59, 193–198. [Google Scholar]
- Zhang, W.; Liu, H.; Zhang, X.; Li, X.; Zhang, G.; Cao, P. 3D Printed Micro-Electrochemical Energy Storage Devices: From Design to Integration. Adv. Funct. Mater. 2021, 31, 2104909. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A New Chapter in Pharmaceutical Manufacturing: 3D-Printed Drug Products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Steward, B. Compliance, Adherence and Concordance: A Review of Engaging Patients in Their Treatment. Br. J. Hand Ther. 2004, 9, 88–95. [Google Scholar] [CrossRef]
- Tibbits, S. The Emergence of 4D Printing. Available online: https://www.ted.com/talks/skylar_tibbits_the_emergence_of_4d_printing (accessed on 24 June 2022).
- Momeni, F.; Mehdi, M.; Hassani, N.S.; Liu, X.; Ni, J. A Review of 4D Printing. Mater. Des. 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Zafar, M.Q.; Zhao, H. 4D Printing: Future Insight in Additive Manufacturing. Met. Mater. Int. 2020, 26, 564–585. [Google Scholar] [CrossRef]
- Siang, Y.; Ting, W.; Poh, L.; Wu, Y.; Lai, Y.; Li, H. Acta Biomaterialia 4D Printing and Stimuli-Responsive Materials in Biomedical Applications. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef]
- Saad, Y.; Nafea, M.; Sultan, M.; Ali, M.; Haider, A.; Almurib, F. Review on Recent Advances in 4D Printing of Shape Memory Polymers. Eur. Polym. J. 2021, 159, 110708. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. 4D Printing Applications in Medical Field: A Brief Review. Clin. Epidemiol. Glob. Heal. 2019, 7, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lu, B.; Li, H.; Pan, Z.; Jiang, J.; Qian, S. A Comparative Study on Environmental Performance of 3D Printing and Conventional Casting of Concrete Products with Industrial Wastes. Chemosphere 2022, 298, 134310. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future Perspectives and Recent Advances in Stimuli-Responsive Materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Fu, P.; Li, H.; Gong, J.; Fan, Z.; Smith, A.T.; Shen, K.; Khalfalla, T.O.; Huang, H.; Qian, X.; McCutcheon, J.R.; et al. 4D Printing of Polymers: Techniques, Materials, and Prospects. Prog. Polym. Sci. 2022, 126, 101506. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W. 4D Printing: Technological and Manufacturing Renaissance. Macromol. Mater. Eng. 2022, 2200003, 1–22. [Google Scholar] [CrossRef]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Demei, K.; Zhang, M.; Phuhongsung, P.; Mujumdar, A.S. 3D Food Printing: Controlling Characteristics and Improving Technological Effect during Food Processing. Food Res. Int. 2022, 156, 111120. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape Memory Polymers: Past, Present and Future Developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Akbar, I.; El, M.; El, M.; Lagoudas, D. Toward Enabling Manufacturing Paradigm of 4D Printing of Shape Memory Materials: Open Literature Review. Eur. Polym. J. 2022, 168, 111106. [Google Scholar] [CrossRef]
- Imrie, P.; Jin, J. Polymer 4D Printing: Advanced Shape-Change and Beyond. J. Polym. Sci. 2022, 60, 149–174. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-Responsive Shape Memory Materials: A Review. Mater. Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Sabahi, N.; Chen, W.; Wang, C.; Kruzic, J.J.; Li, X. Advanced Manufacturing for Biomaterials and Biological Materials A Review on Additive Manufacturing of Shape-Memory Materials for Biomedical Applications. JOM 2020, 72, 1229–1253. [Google Scholar] [CrossRef]
- Naresh, C.; Bose, P.S.C.; Rao, C.S.P. Shape Memory Alloys: A State of Art Review Shape Memory Alloys: A State of Art Review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012054. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape Memory Materials and 4D Printing in Pharmaceutics. Adv. Drug Deliv. Rev. 2021, 173, 216–237. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Wu, W.; Wang, P.; Wang, B.; Li, G.; Zhang, S. Radial Compressive Property and the Proof-of-Concept Study for Realizing Self-Expansion of 3D Printing Polylactic Acid Vascular Stents with Negative Poisson’s Ratio Structure. Materials 2018, 11, 1357. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Shen, P.; Cheng, Z.; Chu, C.; Xue, F.; Bai, J. Preparation of 4D Printed Peripheral Vascular Stent and Its Degradation Behavior under Fluid Shear Stress after Deployment. Biomater. Sci. 2022, 10, 2302–2314. [Google Scholar] [CrossRef]
- Langford, T.; Mohammed, A.; Essa, K.; Elshaer, A.; Hassanin, H. 4D Printing of Origami Structures for Minimally Invasive Surgeries Using Functional Scaffold. Appl. Sci. 2021, 11, 332. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Z.; Liu, L.; Wang, W.; Leng, J.; Liu, Y. Porous Bone Tissue Scaffold Concept Based on Shape Memory PLA/Fe3O4. Compos. Sci. Technol. 2021, 203, 108563. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Ren, L.; Liu, Q.; Ren, L.; Yang, X.; Zhou, X. Advances in 4D Printed Shape Memory Polymers: From 3D Printing, Smart Excitation, and Response to Applications. Adv. Mater. Technol. 2022, 2101568, 1–18. [Google Scholar] [CrossRef]
- Muthe, L.P.; Pickering, K.; Gauss, C. A Review of 3D/4D Printing of Poly-Lactic Acid Composites with Bio-Derived Reinforcements. Compos. Part C Open Access 2022, 8, 100271. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Aminabad, N.S.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and Biomimetic 3d and 4d Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio. 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alshehri, S. Four-Dimensional Printing for Hydrogel: Theoretical Concept, 4D Materials, Shape-Morphing Way, and Future Perspectives. Polymers 2021, 13, 3858. [Google Scholar] [CrossRef]
- Zu, S.; Wang, Z.; Zhang, S.; Guo, Y.; Chen, C.; Zhang, Q.; Wang, Z.; Liu, T.; Liu, Q.; Zhang, Z. A Bioinspired 4D Printed Hydrogel Capsule for Smart Controlled Drug Release. Mater. Today Chem. 2022, 24, 100789. [Google Scholar] [CrossRef]
- Varnamkhasti, Z.K.; Konh, B. Design, Fabrication, and Testing of a Flexible Three-Dimensional Printed Percutaneous Needle with Embedded Actuators. J. Med. Devices, Trans. ASME 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, M.; Tang, D.; Han, Y. A 3D-Printed Electrical Impedance Flow Cytometer Array for Parallel Detection of Cellular Biomarkers. Proc. IEEE Int. Conf. Micro Electro Mech. Syst. 2021, 2021, 490–493. [Google Scholar] [CrossRef]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.K. Electrochemiluminescence Detection of Human Breast Cancer Cells Using Aptamer Modified Bipolar Electrode Mounted into 3D Printed Microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef]
- Bliznakova, K. The Advent of Anthropomorphic Three-Dimensional Breast Phantoms for X-Ray Imaging. Phys. Medica 2020, 79, 145–161. [Google Scholar] [CrossRef]
- Boita, J.; Mackenzie, A.; Engen, R.E. Van. Validation of a Mammographic Image Quality Modification Algorithm Using 3D-Printed Breast Phantoms. J. Med. Imaging 2021, 8, 033502. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, I.J.; Park, K.; Park, K.R.; Cho, Y.; Kim, J.W.; Lee, H. Patient-Speci Fi c Quality Assurance Using a 3D-Printed Chest Phantom for Intraoperative Radiotherapy in Breast Cancer. Front. Oncol. 2021, 11, 629927. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.A.; Deptula, P.L.; Inchauste, S.M.; Zelones, J.T.; Walters, S.; Gifford, K.; Lecastillo, C.; Napel, S.; Fleischmann, D.; Nguyen, D.H. The Utility of Three-Dimensional Models in Complex Microsurgical Reconstruction Original Article. Arch. Plast. Surg. 2020, 47, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Kim, H.J.; Lee, J.; Chung, I.Y.; Kim, J.; Lee, S.; Son, B.H.; Ahn, S.H.; Kim, H.H.; Seo, J.B.; et al. Breast—Conserving Surgery with 3D—Printed Surgical Guide: A Single—Center, Prospective Clinical Study. Sci. Rep. 2021, 11, 2252. [Google Scholar] [CrossRef]
- Wu, Z.; Lee, Y.J.; Shin, Y.; Choi, S.; Baek, S.Y.; Chun, J.W.; Albinsaad, L.S. Usefulness of 3-Dimensional-Printed Breast Surgical Guides for Undetectable Ductal Carcinoma In Situ on Ultrasonography: A Report of 2 Cases. J. Breast Cancer 2021, 24, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.P.; Hutmacher, D.W.; Tran, P.A. The Use of 3D Printed Microporous-Strut Polycaprolactone Scaffolds for Targeted Local Delivery of Chemotherapeutic Agent for Breast Cancer Application. IFMBE Proc. 2020, 69, 153–157. [Google Scholar] [CrossRef]
- Fan, L.; Mei, Y.; Gao, C.; Zhu, P. Three-Dimensional Printed 5-Fluorouracil /UHMWPE Scaffolds for the Treatment of Breast Cancer. Bioprinting 2021, 24, e00174. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, Y.; Huang, R.; Shi, X.; Chen, H.; Wang, J.; Chen, Y.; Tan, Y.; Tan, Z. E-Jet 3D-Printed Scaffolds as Sustained Multi-Drug Delivery Vehicles in Breast Cancer Therapy. Pharm. Res. 2019, 36, 182. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-Jet 3D Printed Drug Delivery Implants to Inhibit Growth and Metastasis of Orthotopic Breast Cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Y.; Wang, J.; Chen, H.; Wang, X.; Li, X.; Tan, W.; Tan, Z. 3D Printed Intelligent Scaffold Prevents Recurrence and Distal Metastasis of Breast Cancer. Theranostics 2020, 10, 10652–10664. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. Acta Biomaterialia 3D Printing of Hydrogel Scaffolds for Future Application in Photothermal Therapy of Breast Cancer and Tissue Repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- He, C.; Yu, L.; Yao, H.; Chen, Y.; Hao, Y. Combinatorial Photothermal 3D-Printing Scaffold and Checkpoint Blockade Inhibits Growth/Metastasis of Breast Cancer to Bone and Accelerates Osteogenesis. Adv. Funct. Mater. 2021, 31, 2006214. [Google Scholar] [CrossRef]
- Wolberg, W.H.; Romsaas, E.P.; Tanner, M.A.; Malec, J.F. Psychosexual Adaptation to Breast Cancer Surgery. Cancer 1989, 63, 1645–1655. [Google Scholar] [CrossRef]
- Cornell, C.E. Clinical Topics Psychiatric Problems in the First Year after Mastectomy. Br. Med. J. 1978, 1, 963–965. [Google Scholar]
- Mu, X.; Zhang, J.; Jiang, Y. 3D Printing in Breast Reconstruction: From Bench to Bed. Front. Surg. 2021, 8, 641370. [Google Scholar] [CrossRef] [PubMed]
- Rocco, N.; Gloria, A.; De Santis, R.; Catanuto, G.; Bruno, M.; Accurso, A. Improving Outcomes in Breast Reconstruction: From Implant-Based Techniques Towards Tissue Regeneration. Procedia CIRP 2016, 49, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.P.; Hunter-Smith, D.J.; Murphy, S.V.; Findlay, M.W. 3D Bioprinting Adipose Tissue for Breast Reconstruction; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Chhaya, M.P.; Balmayor, E.R.; Hutmacher, D.W.; Schantz, J.T. Transformation of Breast Reconstruction via Additive Biomanufacturing. Sci. Rep. 2016, 6, 28030. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, M.; Bas, O.; Castro, N.J.; Schmutz, B.; Hutmacher, D.W. Additive Biomanufacturing of Scaffolds for Breast Reconstruction. Addit. Manuf. 2019, 30, 100845. [Google Scholar] [CrossRef]
- Bao, W.; Cao, L.; Wei, H.; Zhu, D.; Zhou, G.; Wang, J. Materials Science & Engineering C Effect of 3D Printed Polycaprolactone Scaffold with a Bionic Structure on the Early Stage of Fat Grafting. Mater. Sci. Eng. C 2021, 123, 111973. [Google Scholar]
- Zhou, M.; Hou, J.; Zhang, G.; Luo, C.; Zeng, Y.; Mou, S.; Xiao, P.; Zhong, A.; Yuan, Q.; Yang, J.; et al. Tuning the Mechanics of 3D-Printed Scaffolds by Crystal Lattice-like Structural Design for Breast Tissue Engineering. Biofabrication 2020, 12, 015023. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Van Damme, L.; Arevalo, M.d.P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.P.; Shabab, T.; Sha, A.; Peiffer, Q.C.; Fox, K.; Tran, N. 3D Printed Dual Macro-, Microscale Porous Network as a Tissue Engineering Scaffold with Drug Delivering Function. Biofabrication 2019, 11, 035014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattice Medical. Available online: https://www.lattice-medical.com/ (accessed on 22 June 2022).
- BellaSeno-SenellaBreast. Available online: https://www.bellaseno.com/products/senella-breast/ (accessed on 22 June 2022).
- Gallagher, P.; Buckmaster, A.; Kiernan, G. Experiences in the Provision, Fitting and Supply of External Breast Prostheses: Findings from a National Survey. Eur. J. Center Care 2009, 18, 556–568. [Google Scholar] [CrossRef] [PubMed]
- White, V.M.; Roberts, S.B.; Pritchard, E.; Hill, D.J. Women’s Satisfaction with Their Breast Prosthesis What Determines a Quality Prosthesis? Eval. Rev. 2005, 29, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Rostkowska, E.; Bąk, M.; Samborski, W. Body Posture in Women after Mastectomy and Its Changes as a Result of Rehabilitation. Adv. Med. Sci. 2006, 51, 287–297. [Google Scholar]
- Powell, S.K.; Cruz, R.L.J.; Ross, M.T.; Woodruff, M.A. Past, Present, and Future of Soft-Tissue Prosthetics: Advanced Polymers and Advanced Manufacturing. Adv. Mater. 2020, 32, 2001122. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.L.J.; Ross, M.T.; Powell, S.K.; Woodruff, M.A. Advancements in Soft-Tissue Prosthetics Part A: The Art of Imitating Life. Front. Bioeng. Biotechnol. 2020, 8, 121. [Google Scholar] [CrossRef]
- Maillo, B.M.; Blaya, A.; Pedro, S.; Bobo, P.A.; Juanes, J.A. Methodology of Custom Design and Manufacturing of 3D External Breast Prostheses ∗. In Proceedings of the Eighth International Conference on Technological Ecosystems for Enhancing Multiculturality, Salamanca, Spain, 21–23 October 2020. [Google Scholar] [CrossRef]
- Eggbeer, D.; Evans, P. Computer-Aided Methods in Bespoke Breast Prosthesis Design and Fabrication. Proc. Inst. Mech. Eng. H. 2010, 225, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Hummelink, S.; Verhulst, A.C.; Maal, T.J.J.; Ulrich, D.J.O. Applications and Limitations of Using Patient-Specific 3D Printed Molds in Autologous Breast Reconstruction. Eur. J. Plast. Surg. 2018, 41, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Feng, C.J.; Ma, H.; Hsiao, F.Y.; Tseng, L.M.; Tsai, Y.F.; Lin, Y.S.; Huang, L.Y.; Yu, W.C.; Perng, C.K. Preoperative Breast Volume Evaluation of One-Stage Immediate Breast Reconstruction Using Three-Dimensional Surface Imaging and a Printed Mold. J. Chinese Med. Assoc. 2019, 82, 732–739. [Google Scholar] [CrossRef]
- Hao, W.; Zheng, Z.; Zhu, L.; Pang, L.; Ma, J. Prosthesis to Prevent Breast Cancer Recurrence Post-Conserving Surgery. Asian J. Pharm. Sci. 2021, 16, 86–96. [Google Scholar] [CrossRef]
- Van Belleghem, S.; Mahadik, B.; Snodderly, K.; Mote, Z.; Jiang, B.; Yu, J.R.; Mcloughlin, S.; He, X.; Nam, A.J.; Fisher, J.P. Dual Extrusion Patterning Drives Tissue Development Aesthetics and Shape Retention in 3D Printed Nipple-Areola Constructs. Adv. Healthc. Mater. 2021, 10, e2101249. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J. 3D Bioprinting as a Solution for Engineering the Nipple Areola Complex for Breast Cancer Reconstruction. Int. J. Surg. 2017, 41, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Premaratne, I.D.; Wright, M.A.; Bernstein, J.L.; Lara, D.O.; Kim, J.; Zhao, R.; Bonassar, L.J.; Spector, J.A. Nipple Engineering: Maintaining Nipple Geometry with Externally Scaffolded Processed Autologous Costal Cartilage. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Premaratne, I.D.; Sariibrahimoglu, K.; Limem, S.; Scott, J.; Gadjiko, M.; Berri, N.; Ginter, P.; Spector, J.A. 3D-Printed Poly-4-Hydroxybutyrate Bioabsorbable Scaffolds for Nipple Reconstruction. Acta Biomater. 2022, 143, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Healshape. Available online: https://healshape.com/regenerative-products-for-breast-reconstructions/ (accessed on 22 June 2022).
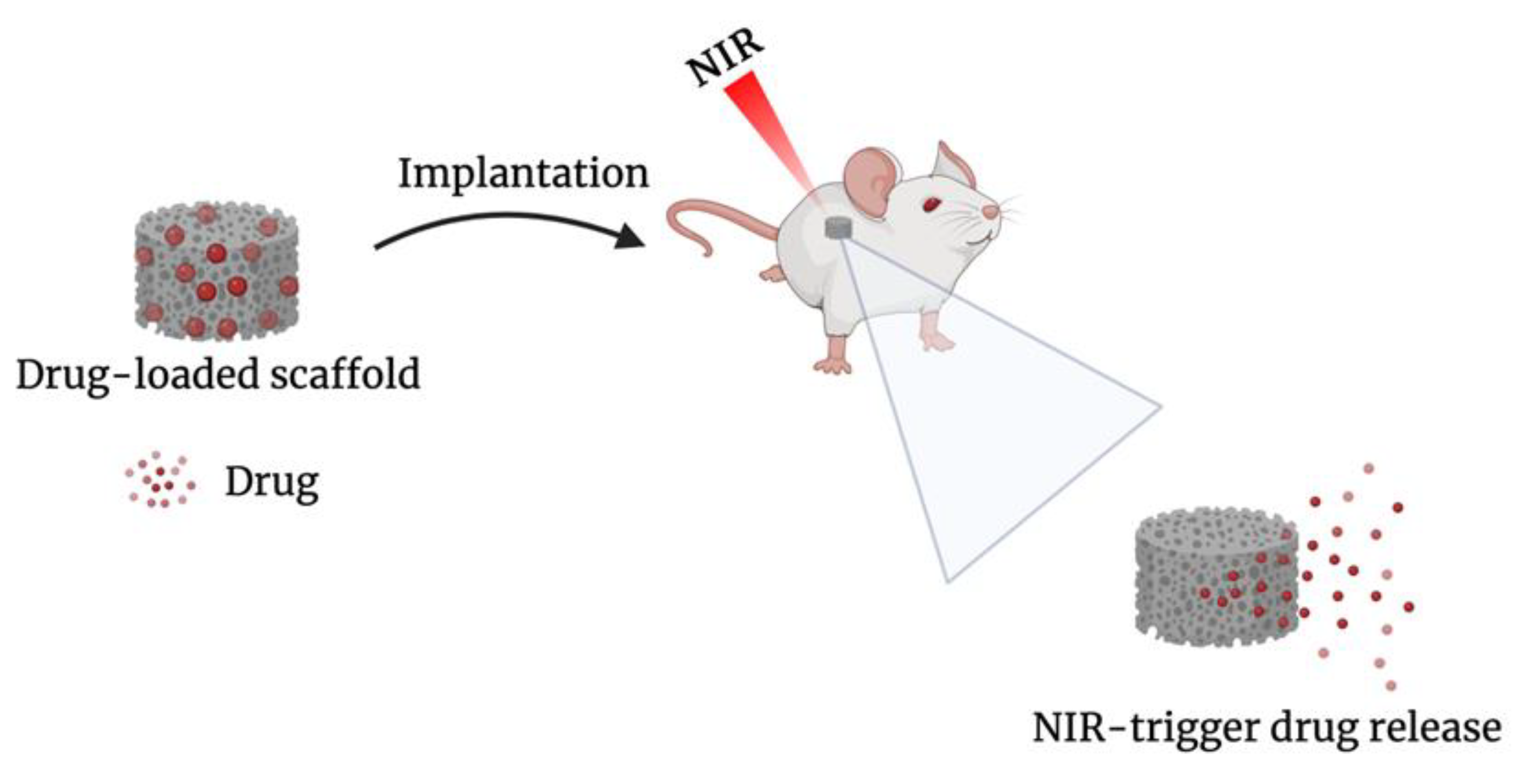
- Wei, X.; Liu, C.; Wang, Z.; Luo, Y. 3D Printed Core-Shell Hydrogel Fiber Scaffolds with NIR-Triggered Drug Release for Localized Therapy of Breast Cancer. Int. J. Pharm. 2020, 580, 119219. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D Printed Hydrogel/PCL Core/Shell Fiber Scaffolds with NIR-Triggered Drug Release for Cancer Therapy and Wound Healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef] [PubMed]
- FDA. Classify Your Medical Device. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device (accessed on 14 June 2022).
- Food and Drug Administration. Technical Considerations for Additive Manufactured Medical Devices:Guidance for Industry and Food and Drug Administration Staff Document. Materialia 2017, 12, 100732. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing ): A Review of Materials, Methods, Applications and Challenges. Compos. Part B 2018, 143, 172–196. [Google Scholar] [CrossRef]




| Treatment | Advantages | Disadvantages | |
| Surgery | Breast conserving | Not invasive, preserves the natural shape of the breast. | Possibility of recurrence. |
| Mastectomy | Lowers the chance of recurrence. | Invasive, poor cosmetic outcome, high emotional impact. | |
| Systemic Therapy | Chemotherapy | Before surgery facilitates the removal of the tumor and enables a less invasive procedure, lower chance of metastasis and recurrence. | Unpleasant side effects (e.g., nausea, fatigue, hair loss). |
| Endocrine Therapy | Milder side effects than chemotherapy, possibility to combine it with chemotherapy or radiotherapy to achieve better results. | Limited to hormone receptor-positive breast cancer, higher possibility of resistance to treatment, early menopause. |
| Category | Technique | Brief Description | Strengths | Weaknesses |
|---|---|---|---|---|
| Material Extrusion | Fused deposition modeling (FDM); direct ink writing (DIW) | Thermoplastic materials or semi-solid inks are extruded through a nozzle. | Good variety of materials, low costs. | Low resolution and time consuming. |
| Binder Jetting | The build material, in the form of powder, and the binder material, generally liquid, are alternatively deposited into the printing bed. | Scalability, high speed. | Poor accuracy, post processing. | |
| Photopolymerization | Stereolithography (SLA); digital light processing (DLP) | The final object is obtained through a chemical reaction (photopolymerization) triggered by irradiation. | High accuracy, high speed. | Limited material availability (photo-resins), high costs, post processing. |
| Material Jetting | Material is deposited dropwise or continuously onto the printing bed through a printing head. | Low cost, high speed, scalability. | Limited material availability (polymers or waxes), support necessary. | |
| Powder Bed Fusion | Direct metal laser sintering (DMLS); selective laser melting (SLM); electron beam melting (EBM); selective heat sintering (SHS); selective laser sintering (SLS) | Laser or electron beams are applied as thermal source to melt powder particles and build the device. | Good resolution, wide range of materials, complexity of the design achieved. | Small product size, high cost, time consuming. |
| Sheet Lamination | Ultrasonic additive manufacturing (UAM); laminated object manufacturing (LOM) | The material, in the form of sheets, is cut by a laser according to the desired design. Each layer is bonded by pressure, temperature, or adhesive coating. | Low costs, robust. | Low resolution and poor accuracy, post processing. |
| Direct Energy Deposition | Direct light fabrication (DLF); laser engineered net shaping (LENS); direct metal deposition (DMD) | Powder or wire material and the substrate are simultaneously melted using an energy source (laser or electron beam). Firstly, the substrate will create the melt pool where the material will be deposited. | Production of dense part with microstructures, ability to control the structure. | Post-processing, time consuming, low material availability. |
| Material | Characteristics | Breast Cancer Application |
|---|---|---|
| Polycaprolactone (PCL) | Low melting temperature, slow degradation rate, good mechanical properties. | Scaffold to guide breast reconstruction and drug delivery. |
| Polylactic acid (PLA) | flexible, slow degradation rate. | Nipple–areola complex scaffold. |
| Poly(lactic-co-glycolic acid) (PLGA) | High degradation rate. High mechanical strength, good processability, high melting temperature. | Scaffold for drug delivery. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. https://doi.org/10.3390/bios12080568
Moroni S, Casettari L, Lamprou DA. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors. 2022; 12(8):568. https://doi.org/10.3390/bios12080568
Chicago/Turabian StyleMoroni, Sofia, Luca Casettari, and Dimitrios A. Lamprou. 2022. "3D and 4D Printing in the Fight against Breast Cancer" Biosensors 12, no. 8: 568. https://doi.org/10.3390/bios12080568