Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range
Abstract
:1. Introduction
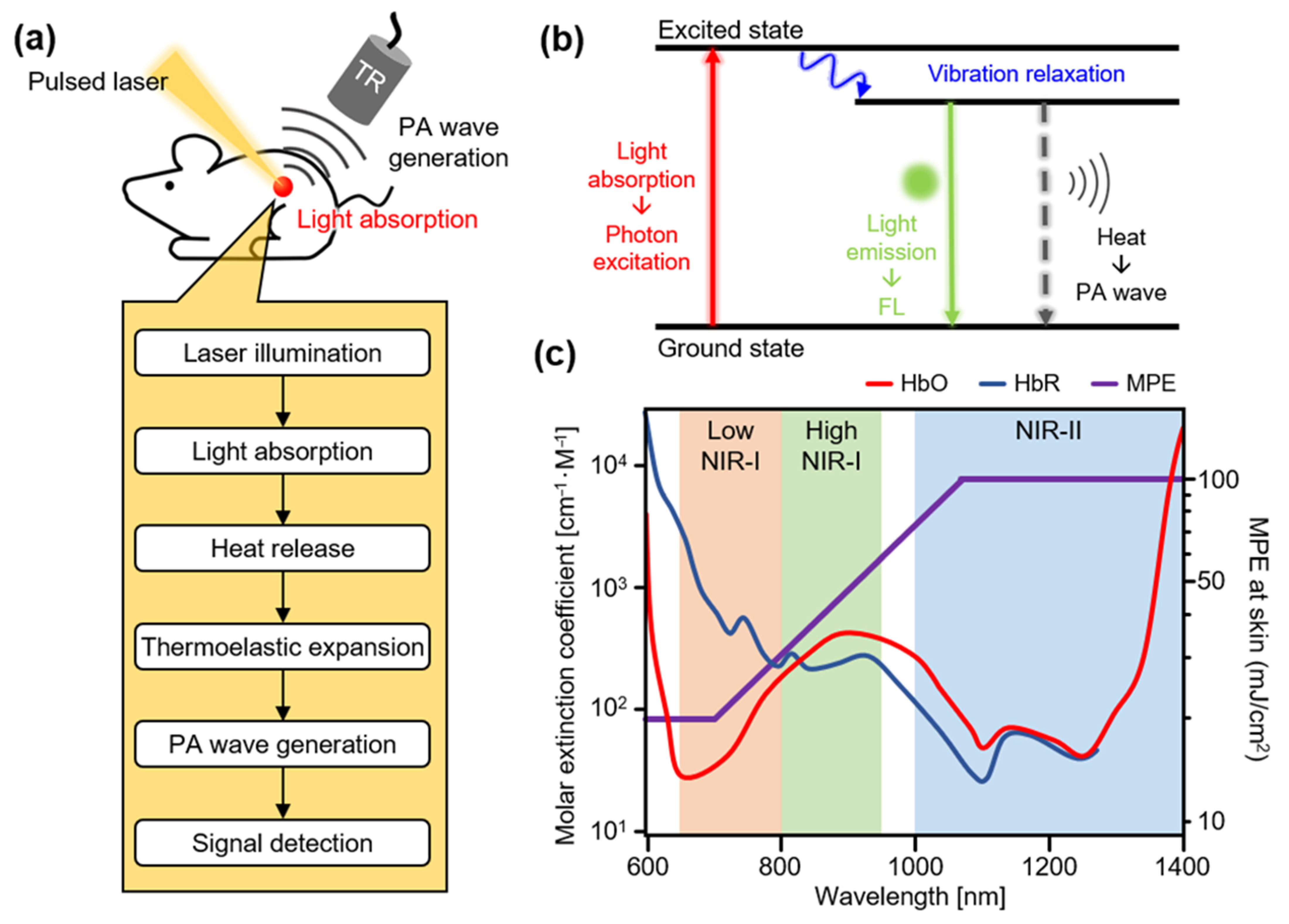
2. Principles of Photoacoustic Imaging
3. Contrast-Enhanced Photoacoustic Imaging
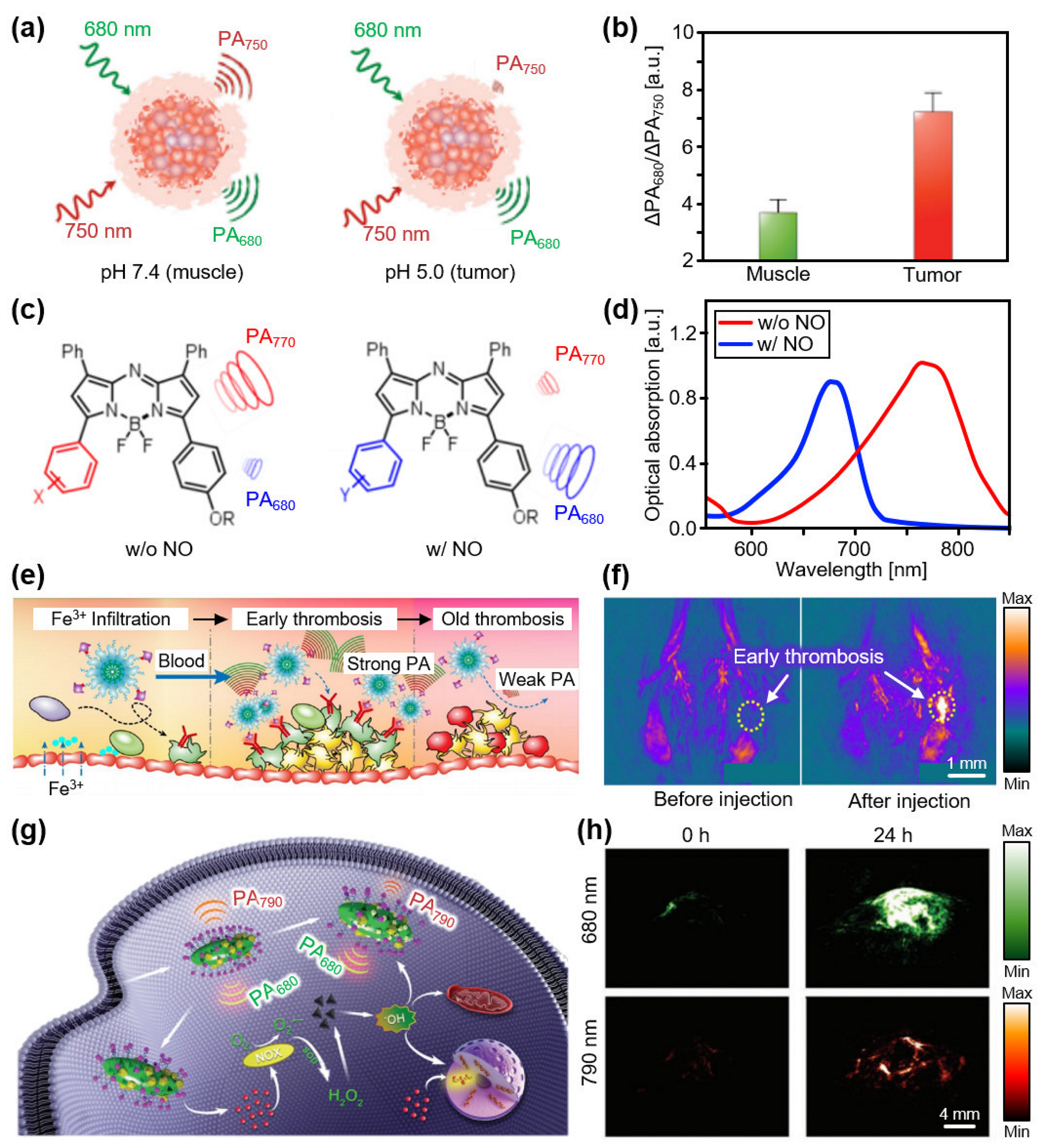
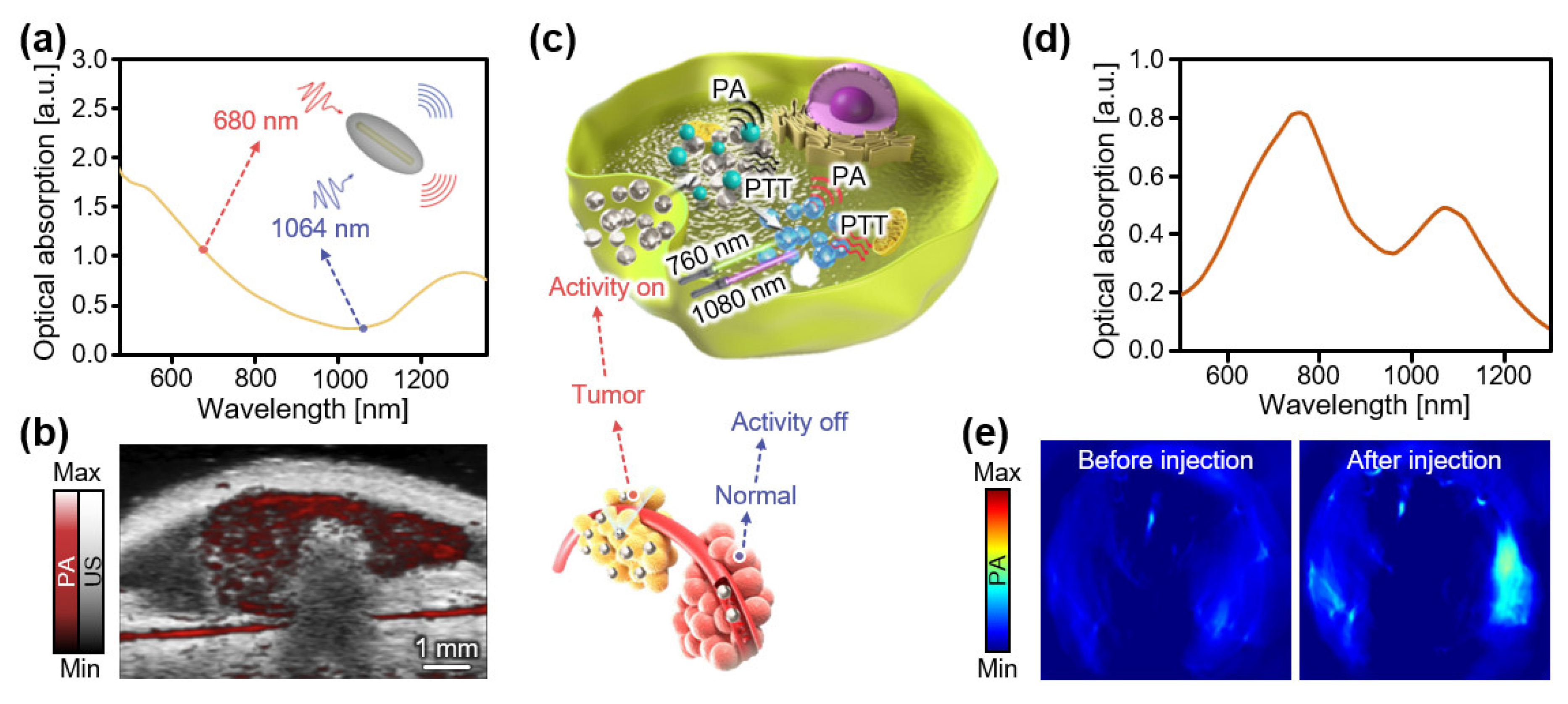
3.1. Contrast Agents at Low NIR-I (650–800 nm)
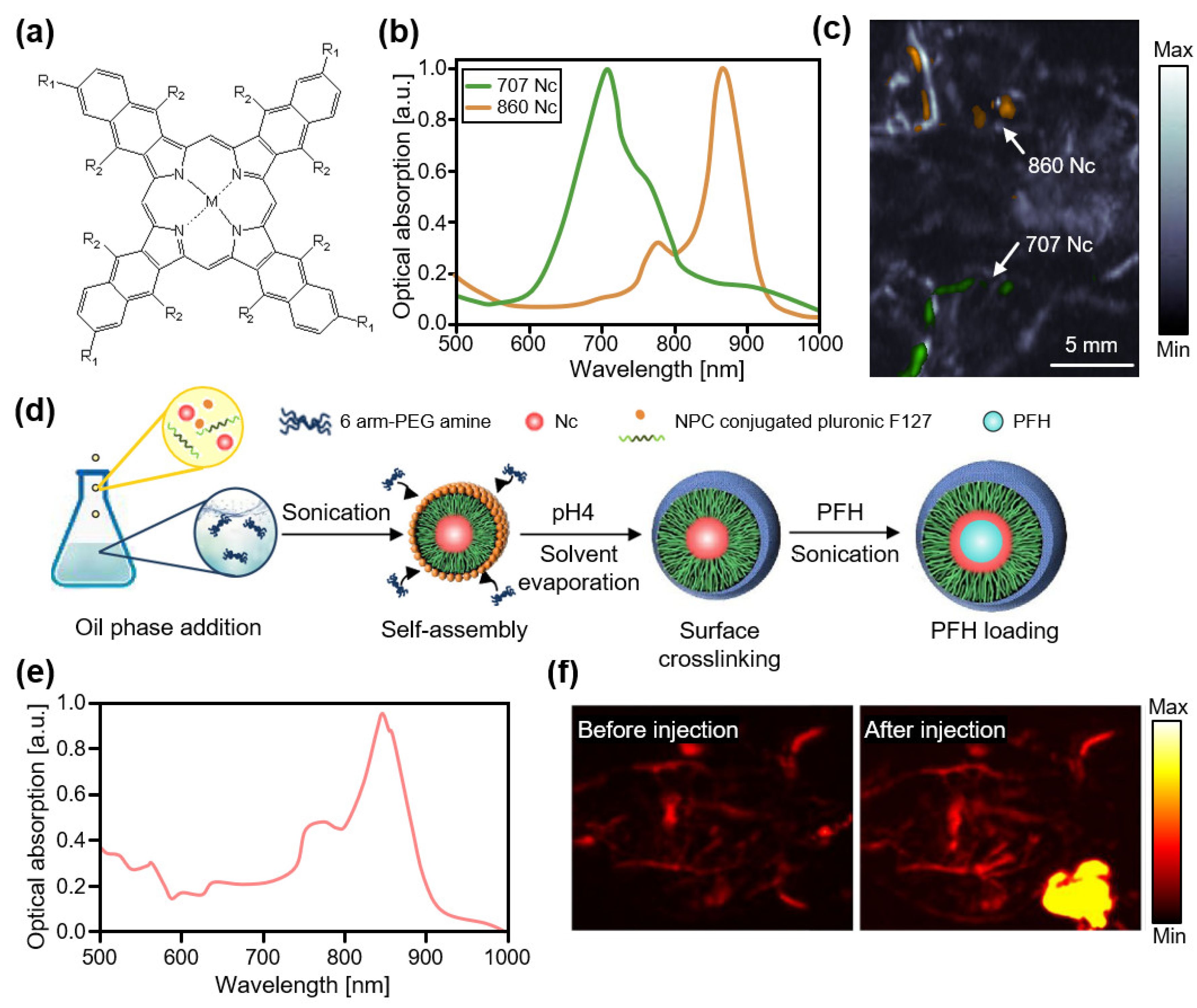
3.2. Contrast Agents at High NIR-I (800–950 nm)
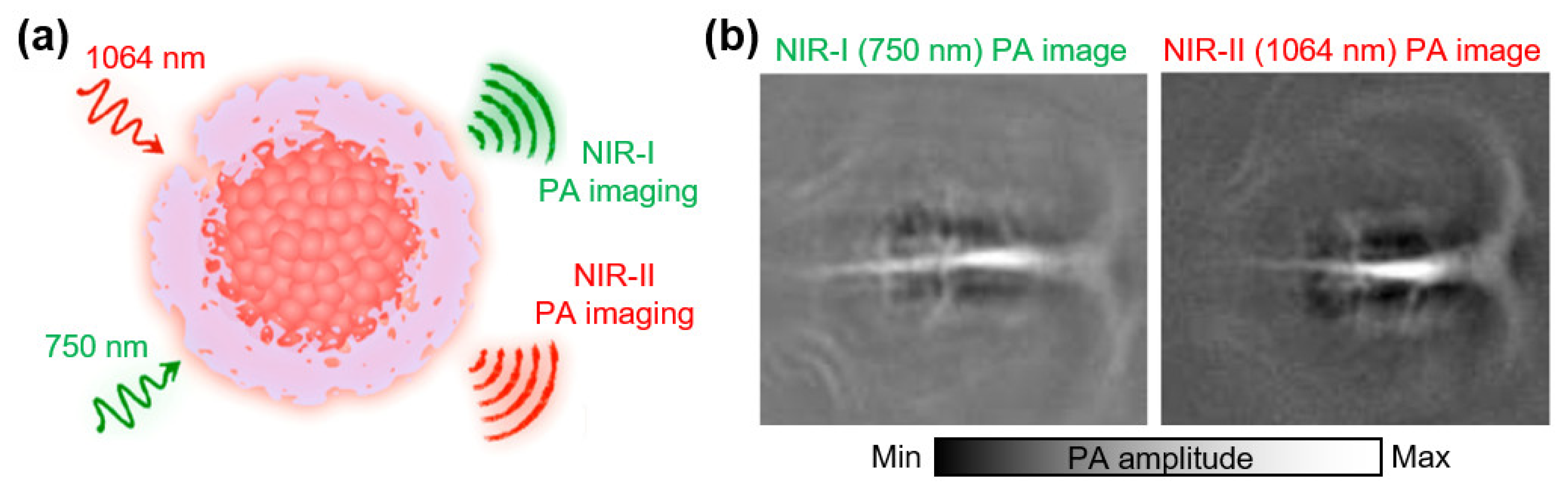
3.3. Contrast Agents at NIR-II (>1000 nm)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kagadis, G.C.; Loudos, G.; Katsanos, K.; Langer, S.G.; Nikiforidis, G.C. In Vivo Small Animal Imaging: Current Status and Future Prospects. Med. Phys. 2010, 37, 6421–6442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maramraju, S.H.; Smith, S.D.; Junnarkar, S.S.; Schulz, D.; Stoll, S.; Ravindranath, B.; Purschke, M.L.; Rescia, S.; Southekal, S.; Pratte, J.-F. Small Animal Simultaneous PET/MRI: Initial Experiences in a 9.4 T microMRI. Photoacoustics 2011, 56, 2459. [Google Scholar]
- Jung, K.O.; Kim, T.J.; Yu, J.H.; Rhee, S.; Zhao, W.; Ha, B.; Red-Horse, K.; Gambhir, S.S.; Pratx, G. Whole-Body Tracking of Single Cells via Positron Emission Tomography. Nat. Biomed. Eng. 2020, 4, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, D.W.; Thornton, M.M. Micro-CT in Small Animal and Specimen Imaging. Trends Biotechnol. 2002, 20, S34–S39. [Google Scholar] [CrossRef]
- Schambach, S.J.; Bag, S.; Schilling, L.; Groden, C.; Brockmann, M.A. Application of Micro-CT in Small Animal Imaging. Methods 2010, 50, 2–13. [Google Scholar] [CrossRef]
- Ritman, E.L. Current Status of Developments and Applications of Micro-CT. Annu. Rev. Biomed. Eng. 2011, 13, 531–552. [Google Scholar] [CrossRef]
- Foster, F.S.; Hossack, J.; Adamson, S.L. Micro-Ultrasound for Preclinical Imaging. Interface Focus 2011, 1, 576–601. [Google Scholar] [CrossRef]
- Needles, A.; Arditi, M.; Rognin, N.; Mehi, J.; Coulthard, T.; Bilan-Tracey, C.; Gaud, E.; Frinking, P.; Hirson, D.; Foster, F. Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med. Biol. 2010, 36, 2097–2106. [Google Scholar] [CrossRef]
- Greco, A.; Mancini, M.; Gargiulo, S.; Gramanzini, M.; Claudio, P.; Brunetti, A.; Salvatore, M. Ultrasound biomicroscopy in small animal research: Applications in molecular and preclinical imaging. J. Biomed. Biotechnol. 2012, 2012, 519238. [Google Scholar] [CrossRef]
- Luker, G.D.; Luker, K.E. Optical Imaging: Current Applications and Future Directions. J. Nucl. Med. 2008, 49, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Graves, E.E.; Ripoll, J.; Weissleder, R.; Ntziachristos, V. A Submillimeter Resolution Fluorescence Molecular Imaging System for Small Animal Imaging. Med. Phys. 2003, 30, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-Photon Small-Molecule Fluorescence-Based Agents for Sensing, Imaging, and Therapy within Biological Systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging From Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, J.; Wang, T.; Chen, X.; Huang, P. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics 2016, 6, 2394. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.V.; Yao, J. A Practical Guide to Photoacoustic Tomography in the Life Sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Taruttis, A.; Ntziachristos, V. Advances in Real-Time Multispectral Optoacoustic Imaging and Its Applications. Nat. Photonics 2015, 9, 219–227. [Google Scholar] [CrossRef]
- Dima, A.; Burton, N.C.; Ntziachristos, V. Multispectral Optoacoustic Tomography at 64, 128, and 256 Channels. J. Biomed. Opt. 2014, 19, 036021. [Google Scholar] [CrossRef] [Green Version]
- Razansky, D. Multispectral Optoacoustic Tomography—Volumetric Color Hearing in Real Time. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1234–1243. [Google Scholar] [CrossRef]
- Kroenke, M.; Karlas, A.; Fasoula, N.; Markwardt, N.; Scheidhauer, K.; Eiber, M.; Weber, W.; Ntziachristos, V. Multispectral Optoacoustic Tomography: A Novel Label-Free Imaging Technique for the Assessment of Hyperthyroid Diseases. J. Nucl. Med. 2019, 60, 525. [Google Scholar]
- Bi, R.; Dinish, U.; Goh, C.C.; Imai, T.; Moothanchery, M.; Li, X.; Kim, J.Y.; Jeon, S.; Pu, Y.; Kim, C.; et al. In Vivo Label-Free Functional Photoacoustic Monitoring of Ischemic Reperfusion. J. Biophotonics 2019, 12, e201800454. [Google Scholar] [CrossRef]
- Hu, S.; Maslov, K.; Tsytsarev, V.; Wang, L.V. Functional Transcranial Brain Imaging by Optical-Resolution Photoacoustic Microscopy. J. Biomed. Opt. 2009, 14, 040503. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, H.; Xi, L. Wearable Optical Resolution Photoacoustic Microscopy. J. Biophotonics 2019, 12, e201900066. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gong, H.; Jiang, B.; Wang, H.; Xu, G.; Deng, Y. Assessing the Effects of Norepinephrine on Single Cerebral Microvessels using Optical-Resolution Photoacoustic Microscope. J. Biomed. Opt. 2013, 18, 076007. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Jin, T.; Guo, H.; Xi, L. Large-Field-of-View Optical Resolution Photoacoustic Microscopy. Opt. Express 2018, 26, 4271–4278. [Google Scholar] [CrossRef]
- Liu, W.; Schultz, K.M.; Zhang, K.; Sasman, A.; Gao, F.; Kume, T.; Zhang, H.F. In Vivo Corneal Neovascularization Imaging by Optical-Resolution Photoacoustic Microscopy. Photoacoustics 2014, 2, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhang, H.F. Photoacoustic Imaging of the Eye: A Mini Review. Photoacoustics 2016, 4, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Silverman, R.H.; Kong, F.; Chen, Y.; Lloyd, H.O.; Kim, H.H.; Cannata, J.M.; Shung, K.K.; Coleman, D.J. High-Resolution Photoacoustic Imaging of Ocular Tissues. Ultrasound Med. Biol. 2010, 36, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Song, K.H.; Wang, L.V. Deep Reflection-Mode Photoacoustic Imaging of Biological Tissue. J. Biomed. Opt. 2007, 12, 060503. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Wang, L.V. Noninvasive Photoacoustic Imaging of the Thoracic Cavity and the Kidney in Small and Large Animals. Med. Phys. 2008, 35, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Kim, J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Imaging of Small Animals In Vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Aguirre, A.; Gamelin, J.; Maurudis, A.; Zhu, Q.; Wang, L.V. Real-Time Photoacoustic Tomography of Cortical Hemodynamics in Small Animals. J. Biomed. Opt. 2010, 15, 010509. [Google Scholar] [CrossRef]
- Brecht, H.-P.; Su, R.; Fronheiser, M.; Ermilov, S.A.; Conjusteau, A.; Oraevsky, A.A. Whole-Body Three-Dimensional Optoacoustic Tomography System for Small Animals. J. Biomed. Opt. 2009, 14, 064007. [Google Scholar] [CrossRef]
- Xia, J.; Chatni, M.R.; Maslov, K.; Guo, Z.; Wang, K.; Anastasio, M.; Wang, L.V. Whole-Body Ring-Shaped Confocal Photoacoustic Computed Tomography of Small Animals In Vivo. J. Biomed. Opt. 2012, 17, 050506. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J. Single-Impulse Panoramic Photoacoustic Computed Tomography of Small-Animal Whole-Body Dynamics at High Spatiotemporal Resolution. Nat. Biomed. Eng. 2017, 1, 0071. [Google Scholar] [CrossRef]
- Park, E.-Y.; Park, S.; Lee, H.; Kang, M.; Kim, C.; Kim, J. Simultaneous Dual-Modal Multispectral Photoacoustic and Ultrasound Macroscopy for Three-Dimensional Whole-Body Imaging of Small Animals. Photonics 2021, 8, 13. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.H.; Lee, C.; Han, J.H.; Choi, W.; Kim, J.; Park, G.S.; Rhie, J.W.; Lee, J.H.; Kim, C. 3D Wide-Field Multispectral Photoacoustic Imaging of Human Melanomas In Vivo: A Pilot Study. J. Eur. Acad. Dermatol. 2020, 35, 669–676. [Google Scholar] [CrossRef]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer. Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [Green Version]
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A. Multispectral Optoacoustic Tomography for Assessment of Crohn’s Disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294. [Google Scholar] [CrossRef]
- Roll, W.; Markwardt, N.A.; Masthoff, M.; Helfen, A.; Claussen, J.; Eisenblätter, M.; Hasenbach, A.; Hermann, S.; Karlas, A.; Wildgruber, M. Multispectral Optoacoustic Tomography of Benign and Malignant Thyroid Disorders: A Pilot Study. J. Nucl. Med. 2019, 60, 1461–1466. [Google Scholar] [CrossRef]
- Becker, A.; Masthoff, M.; Claussen, J.; Ford, S.J.; Roll, W.; Burg, M.; Barth, P.J.; Heindel, W.; Schaefers, M.; Eisenblaetter, M. Multispectral Optoacoustic Tomography of the Human Breast: Characterisation of Healthy Tissue and Malignant Lesions using a Hybrid Ultrasound-Optoacoustic Approach. Eur. Radiol. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Fadhel, M.N.; Hysi, E.; Assi, H.; Kolios, M.C. Fluence-Matching Technique using Photoacoustic Radiofrequency Spectra for Improving Estimates of Oxygen Saturation. Photoacoustics 2020, 19, 100182. [Google Scholar] [CrossRef]
- Merčep, E.; Deán-Ben, X.L.; Razansky, D. Imaging of Blood Flow and Oxygen State with a Multi-Segment Optoacoustic Ultrasound Array. Photoacoustics 2018, 10, 48–53. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In Vitro Photoacoustic Measurement of Hemoglobin Oxygen Saturation using a Single Pulsed Broadband Supercontinuum Laser Source. Appl. Optics 2014, 53, 3884–3889. [Google Scholar] [CrossRef]
- Wilson, D.F.; Vinogradov, S.A.; Grosul, P.; Vaccarezza, M.N.; Kuroki, A.; Bennett, J. Oxygen Distribution and Vascular Injury in the Mouse Eye Measured by Phosphorescence-Lifetime Imaging. Appl. Optics 2005, 44, 5239–5248. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- James, S.; Neuhaus, K.; Murphy, M.; Leahy, M. Contrast Agents for Photoacoustic Imaging: A Review of Stem Cell Tracking. Stem Cell Res. Ther. 2021, 12, 511. [Google Scholar] [CrossRef]
- Li, W.; Brown, P.K.; Wang, L.V.; Xia, Y. Gold Nanocages as Contrast Agents for Photoacoustic Imaging. Contrast Media Mol. Imaging 2011, 6, 370–377. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef]
- Park, J.; Jeon, S.; Meng, J.; Song, L.; Lee, J.S.; Kim, C. Delay-Multiply-and-Sum-Based Synthetic Aperture Focusing in Photoacoustic Microscopy. J. Biomed. Opt. 2016, 21, 036010. [Google Scholar] [CrossRef]
- Jeon, S.; Park, E.-Y.; Choi, W.; Managuli, R.; Jong Lee, K.; Kim, C. Real-Time Delay-Multiply-and-Sum Beamforming with Coherence Factor for In Vivo Clinical Photoacoustic Imaging of Humans. Photoacoustics 2019, 15, 100136. [Google Scholar] [CrossRef]
- Lutzweiler, C.; Razansky, D. Optoacoustic Imaging and Tomography: Reconstruction Approaches and Outstanding Challenges in Image Performance and Quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef] [Green Version]
- Köstli, K.P.; Frenz, M.; Weber, H.P.; Paltauf, G.; Schmidt-Kloiber, H. Optoacoustic Tomography: Time-Gated Measurement of Pressure Distributions and Image Reconstruction. Appl. Optics 2001, 40, 3800–3809. [Google Scholar] [CrossRef]
- Kruger, R.A.; Liu, P.; Appledorn, C.R. Photoacoustic Ultrasound (PAUS)—Reconstruction Tomography. Med. Phys. 1995, 22, 1605–1609. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.V.; Ambartsoumian, G.; Kuchment, P. Reconstructions in Limited-View Thermoacoustic Tomography. Med. Phys. 2004, 31, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, I.; Kim, J.; Schneider, M.K.; Hyun, D.; Zlitni, A.; Hopper, S.M.; Klap, T.; Sonn, G.A.; Dahl, J.J.; Kim, C. Superiorized Photo-Acoustic Non-NEgative Reconstruction (SPANNER) for Clinical Photoacoustic Imaging. IEEE T. Med. Imaging 2021, 40, 1888–1897. [Google Scholar] [CrossRef]
- Koman, V.B.; Bakh, N.A.; Jin, X.; Nguyen, F.T.; Son, M.; Kozawa, D.; Lee, M.A.; Bisker, G.; Dong, J.; Strano, M.S. A Wavelength-Induced Frequency Filtering Method for Fluorescent Nanosensors In Vivo. Nat. Nanotechnol. 2022, 17, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Jung, U.; Kim, C. Photoacoustic Imaging Platforms for Multimodal Imaging. Ultrasonography 2015, 34, 88. [Google Scholar] [CrossRef] [PubMed]
- ANSI Z136.1-2014; American National Standard for the Safe Use of Lasers. American National Standards Institute: New York, NY, USA, 2014.
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S. Gold Nanoparticles as a Contrast Agent for In Vivo Tumor Imaging with Photoacoustic Tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbrich, A.; Heinmiller, A.; Zemp, R.J. Photoacoustic Imaging of Lymphatic Pumping. J. Biomed. Opt. 2017, 22, 106003. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliver. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O’Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic Tomography of a Nanoshell Contrast Agent in the In Vivo Rat Brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, J.; Pu, K. Second Near-Infrared Absorbing Agents for Photoacoustic Imaging and Photothermal Therapy. Small Methods 2019, 3, 1900553. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for In Vivo Imaging. Nat. Nanotechnol. 2009, 4, 710. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Fan, Y.; Ma, X.; Zhan, Y.; Lu, X.; Sun, Y. Recent Development of Near-Infrared Photoacoustic Probes based on Small-Molecule Organic Dye. RSC Chem. Biol. 2021, 2, 743–758. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Advanced Photoacoustic Imaging Applications of Near-Infrared Absorbing Organic Nanoparticles. Small 2017, 13, 1700710. [Google Scholar] [CrossRef]
- Erpelding, T.N.; Kim, C.; Pramanik, M.; Jankovic, L.; Maslov, K.; Guo, Z.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Sentinel Lymph Nodes in the Rat: Noninvasive Photoacoustic and US Imaging with a Clinical US System 1. Radiology 2010, 256, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Erpelding, T.N.; Jankovic, L.; Pashley, M.D.; Wang, L.V. Deeply Penetrating In Vivo Photoacoustic Imaging using a Clinical Ultrasound Array System. Biomed. Opt. Express 2010, 1, 278–284. [Google Scholar] [CrossRef]
- Song, K.H.; Margenthaler, J.A.; Wang, L.V.; Stein, E.W. Noninvasive Photoacoustic Identification of Sentinel Lymph Nodes Containing Methylene Blue In Vivo in a Rat Model. J. Biomed. Opt. 2008, 13, 054033–054036. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Kim, C.; Maslov, K.; Shung, K.K.; Wang, L.V. High-Speed Dynamic 3D Photoacoustic Imaging of Sentinel Lymph Node in a Murine Model using an Ultrasound Array. Med. Phys. 2009, 36, 3724–3729. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Song, W.; Huynh, E.; Kim, J.; Kim, J.; Helfield, B.L.; Leung, B.Y.; Goertz, D.E.; Zheng, G.; Oh, J. Methylene Blue Microbubbles as a Model Dual-Modality Contrast Agent for Ultrasound and Activatable Photoacoustic Imaging. J. Biomed. Opt. 2014, 19, 016005. [Google Scholar] [CrossRef]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel Lymph Nodes and Lymphatic Vessels: Noninvasive Dual-Modality In Vivo Mapping by using Indocyanine Green in Rats—Volumetric Spectroscopic Photoacoustic Imaging and Planar Fluorescence Imaging 1. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Jeon, M.; Song, J.; Kim, C. In Vivo Photoacoustic and Fluorescence Cystography Using Clinically Relevant Dual Modal Indocyanine Green. Sensors 2014, 14, 19660–19668. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Truini, C.; Ariyan, S. A Randomized Study Comparing the Effectiveness of Methylene Blue Dye with Lymphazurin Blue Dye in Sentinel Lymph Node Biopsy for the Treatment of Cutaneous Melanoma. Ann. Surg. Oncol. 2008, 15, 2412–2417. [Google Scholar] [CrossRef]
- Isak, S.J.; Eyring, E.M.; Spikes, J.D.; Meekins, P.A. Direct Blue Dye Solutions: Photo Properties. J. Photochem. Photobiol. A Chem. 2000, 134, 77–85. [Google Scholar] [CrossRef]
- Luetkemeier, M.J.; Fattor, J.A. Measurement of Indocyanine Green Dye is Improved by Use of Polyethylene Glycol to Reduce Plasma Turbidity. Clin. Chem. 2001, 47, 1843–1845. [Google Scholar] [CrossRef] [Green Version]
- Miao, Q.; Lyu, Y.; Ding, D.; Pu, K. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Adv. Mater. 2016, 28, 3662–3668. [Google Scholar] [CrossRef]
- Reinhardt, C.J.; Zhou, E.Y.; Jorgensen, M.D.; Partipilo, G.; Chan, J. A Ratiometric Acoustogenic Probe for In Vivo Imaging of Endogenous Nitric Oxide. J. Am. Chem. Soc. 2018, 140, 1011–1018. [Google Scholar] [CrossRef]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Yin, C.; Fan, Q.; Zhang, W.; Song, J.; Yu, G.; Tang, W.; Fan, W.; Yung, B.C. Activatable Semiconducting Theranostics: Simultaneous Generation and Ratiometric Photoacoustic Imaging of Reactive Oxygen Species In Vivo. Adv. Mater. 2018, 30, 1707509. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Cole, A.J.; Van de Sompel, D.; Gambhir, S.S. Gold Nanorods for Ovarian Cancer Detection with Photoacoustic Imaging and Resection Guidance via Raman Imaging in Living Mice. ACS Nano 2012, 6, 10366–10377. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, J.; Yang, K.; Yi, C.; Liu, Y.; Nie, L.; Khashab, N.M.; Chen, X.; Nie, Z. Folding up of Gold Nanoparticle Strings into Plasmonic Vesicles for Enhanced Photoacoustic Imaging. Angew. Chem. 2015, 54, 15809–15812. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Zhang, Q.; Li, J.; Zhang, L.; Jokerst, J.V. A Gold/Silver Hybrid Nanoparticle for Treatment and Photoacoustic Imaging of Bacterial Infection. ACS Nano 2018, 12, 5615–5625. [Google Scholar] [CrossRef]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O. Carbon Nanotubes as Photoacoustic Molecular Imaging Agents in Living Mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for In Vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Zeng, G.; You, Y.; Wang, H.; Gong, X.; Zheng, R.; Kim, J.; Kim, C.; Song, L. Indocyanine Green Loaded Reduced Graphene Oxide for In Vivo Photoacoustic/Fluorescence Dual-Modality Tumor Imaging. Nanoscale Res. Lett. 2016, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Liu, C.; Hu, D.; Wang, F.; Wu, H.; Gong, X.; Liu, X.; Song, L.; Sheng, Z.; Zheng, H. Single-Layer MoS2 Nanosheets with Amplified Photoacoustic Effect for Highly Sensitive Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Funct. Mater. 2016, 26, 8715–8725. [Google Scholar] [CrossRef]
- Park, E.-Y.; Oh, D.; Park, S.; Kim, W.; Kim, C. New Contrast Agents for Photoacoustic Imaging and Theranostics: Recent 5-year Overview on Phthalocyanine/Naphthalocyanine-Based Nanoparticles. APL Bioeng. 2021, 5, 031510. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexandridis, P.; Huizinga, J.D. Non-Invasive Multimodal Functional Imaging of the Intestine with Frozen Micellar Naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631–638. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-Color Photoacoustic Lymph Node Imaging using Nanoformulated Naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Sun, B.; Carter, K.; Qin, Y.; Wei, W.; Wang, D.; Jeon, M.; Geng, J.; Nickles, R.J. Surfactant-Stripped Naphthalocyanines for Multimodal Tumor Theranostics with Upconversion Guidance Cream. Nanoscale 2017, 9, 3391–3398. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.; Kim, J.; Kong, W.H.; Kim, K.S.; Kim, C.; Hahn, S.K. Multifunctional Nanodroplets Encapsulating Naphthalocyanine and Perfluorohexane for Bimodal Image-Guided Therapy. Biomacromolecules 2019, 20, 3767–3777. [Google Scholar] [CrossRef]
- Bao, C.; Beziere, N.; del Pino, P.; Pelaz, B.; Estrada, G.; Tian, F.; Ntziachristos, V.; de la Fuente, J.M.; Cui, D. Gold Nanoprisms as Optoacoustic Signal Nanoamplifiers for In Vivo Bioimaging of Gastrointestinal Cancers. Small 2013, 9, 68–74. [Google Scholar] [CrossRef]
- Song, J.; Wang, F.; Yang, X.; Ning, B.; Harp, M.G.; Culp, S.H.; Hu, S.; Huang, P.; Nie, L.; Chen, J. Gold Nanoparticle Coated Carbon Nanotube Ring with Enhanced Raman Scattering and Photothermal Conversion Property for Theranostic Applications. J. Am. Chem. Soc. 2016, 138, 7005–7015. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Lyu, Y.; Zhang, L.; Xiong, Q.; Pramanik, M.; Pu, K. Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second Near-Infrared Window. Nano Lett. 2017, 17, 4964–4969. [Google Scholar] [CrossRef]
- Jiang, Y.; Upputuri, P.K.; Xie, C.; Zeng, Z.; Sharma, A.; Zhen, X.; Li, J.; Huang, J.; Pramanik, M.; Pu, K. Metabolizable Semiconducting Polymer Nanoparticles for Second Near-Infrared Photoacoustic Imaging. Adv. Mater. 2019, 31, 1808166. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Huang, T.; Pan, X.; Sun, P.; Li, J.; Zhang, H.; Lu, X.; Fan, Q.; Huang, W. 1300 nm Absorption Two-Acceptor Semiconducting Polymer Nanoparticles for NIR-II Photoacoustic Imaging System Guided NIR-II Photothermal Therapy. Chem. Commun. 2019, 55, 9487–9490. [Google Scholar] [CrossRef]
- Guo, B.; Chen, J.; Chen, N.; Middha, E.; Xu, S.; Pan, Y.; Wu, M.; Li, K.; Liu, C.; Liu, B. High-Resolution 3D NIR-II Photoacoustic Imaging of Cerebral and Tumor Vasculatures Using Conjugated Polymer Nanoparticles as Contrast Agent. Adv. Mater. 2019, 31, 1808355. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Zhang, Y.; Chitgupi, U.; Geng, J.; Wang, Y.; Zhang, Y.; Cook, T.R.; Xia, J.; Lovell, J.F. A Phosphorus Phthalocyanine Formulation with Intense Absorbance at 1000 nm for Deep Optical Imaging. Theranostics 2016, 6, 688. [Google Scholar] [CrossRef]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1902279. [Google Scholar] [CrossRef]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep Tissue Photoacoustic Imaging of Nickel (II) Dithiolene-Containing Polymeric Nanoparticles in the Second Near-Infrared Window. Theranostics 2020, 10, 2509–2521. [Google Scholar] [CrossRef]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper Sulfide Nanoparticles as a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [Green Version]
- Yim, W.; Zhou, J.; Mantri, Y.; Creyer, M.N.; Moore, C.A.; Jokerst, J.V. Gold Nanorod–Melanin Hybrids for Enhanced and Prolonged Photoacoustic Imaging in the Near-Infrared-II Window. ACS Appl. Mater. Interfaces 2021, 13, 14974–14984. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature Gold Nanorods for Photoacoustic Molecular Imaging in the Second Near-Infrared Optical Window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Hou, S.; Upputuri, P.K.; Wu, D.; Li, J.; Wang, P.; Zhen, X.; Pramanik, M.; Pu, K. Compact Plasmonic Blackbody for Cancer Theranosis in the Near-Infrared II Window. ACS Nano 2018, 12, 2643–2651. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhao, J.; Hou, L.; Wen, C.; Liang, H.; Zhao, S. Hydrogen Sulfide Dual-Activated NIR-II Photoacoustic Probes for Accurate Imaging and Efficient Photothermal Therapy of Colon Cancer. ACS Appl. Bio Mater. 2021, 4, 974–983. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Kalva, S.K.; Dean-Ben, X.L.; Razansky, D. Single-Sweep Volumetric Optoacoustic Tomography of Whole Mice. Photonics Res. 2021, 9, 899–908. [Google Scholar] [CrossRef]
- Xia, J.; Chen, W.; Maslov, K.I.; Anastasio, M.A.; Wang, L.V. Retrospective Respiration-Gated Whole-Body Photoacoustic Computed Tomography of Mice. J. Biomed. Opt. 2014, 19, 016003. [Google Scholar] [CrossRef]
- Kye, H.; Song, Y.; Ninjbadgar, T.; Kim, C.; Kim, J. Whole-Body Photoacoustic Imaging Techniques for Preclinical Small Animal Studies. Sensors 2022, 22, 5130. [Google Scholar] [CrossRef]
- Park, E.-Y.; Lee, H.; Han, S.; Kim, C.; Kim, J. Photoacoustic Imaging Systems Based on Clinical Ultrasound Platform. Exp. Biol. Med. 2022, 247, 551–560. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical Photoacoustic Imaging Platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M. Visualization of Tumor-Related Blood Vessels in Human Breast by Photoacoustic Imaging System with a Hemispherical Detector Array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, G.L.; Pijnappel, R.M.; Meeuwis, C.; Bisschops, R.; Veltman, J.; Lavin, P.T.; Van De Vijver, M.J.; Mann, R.M. Downgrading of Breast Masses Suspicious for Cancer by using Optoacoustic Breast Imaging. Radiology 2018, 288, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, A.; Tsujita, K.; Irisawa, K.; Kasamatsu, T.; Hirota, K.; Kawaguchi, M.; Shinchi, M.; Ito, K.; Asano, T.; Shinmoto, H. A Pilot Study of Photoacoustic Imaging System for Improved Real-Time Visualization of Neurovascular Bundle During Radical Prostatectomy. Prostate 2016, 76, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.-R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous Transrectal Ultrasound and Photoacoustic Human Prostate Imaging. Sci. Transl Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]



| MB | EB | ICG | |
|---|---|---|---|
| (m) | 667 | 626 | 790 |
| (%) | 0.04 | 0.4 | <1 in water 10 in DMSO |
| Ref. | [81] | [82] | [83] |
| Range | Type | Base Material | (nm) | (nm) | Main Application | Ref. |
|---|---|---|---|---|---|---|
| Low NIR-I | Organic | MB | 677 | 635 | SLN in rats | [76] |
| Organic | EB | 620 | 600 | SLN in rats | [77] | |
| Organic | ICG | ~700 | 668 | SLN in rats | [79] | |
| Organic | SO with BODIPY dye | 680 | 680 | HeLa xenograft in mice | [84] | |
| Organic | aza-BODIPY dye | 673 | 680 | LPS-induced inflammation in mice | [85] | |
| Organic | PDI-based SPN | 650 | 700 | FeCl3-induced thrombus in mice | [86] | |
| Organic | PDI-based SPN | 790 | 790 | U87MG xenograft in mice | [87] | |
| Inorganic | AuNR | 756 | 756 | MDA-435S xenograft in mice | [89] | |
| Inorganic | Chain vesicle with AuNP | 780 | 780 | Subcutaneous layer in mice | [90] | |
| Inorganic | Au/Ag hybrid NP | 750 | 800 | Subcutaneous layer in mice | [91] | |
| Inorganic | SW-CNT | 690 | 690 | U87MG xenograft in mice | [92] | |
| Inorganic | WS2 | ~400 | 700 | 4T1 xenograft in mice | [93] | |
| Inorganic | rNGO | 780 | 780 | HeLa xenograft in mice | [94] | |
| Inorganic | MoS2 | ~500 | 675 | U87 xenograft in mice | [95] | |
| High NIR-I | Organic | Nc | 863 | 860 | GI tracts in mice | [97] |
| Organic | Nc | 860 | 860 | SLN in rats | [98] | |
| Organic | Nc | 860 | 860 | 4T1 xenograft in mice | [99] | |
| Organic | Nc | 850 | 850 | MDA-MB-231 xenograft in mice | [100] | |
| Inorganic | AuNPr | 830 | 830 | HT-29 xenograft in mice | [101] | |
| Inorganic | AuNP-coated CNTR | ~800 | 808 | U87MG xenograft in mice | [102] | |
| NIR-II | Organic | SPN | 1253 | 1064 | Brain in rats | [103] |
| Organic | SPN | 1079 | 1064 | 4T1 xenograft and brain in mice | [104] | |
| Organic | SPN | 1300 | 1280 | 4T1 xenograft in mice | [105] | |
| Organic | SPN | 1160 | 1064 | HepG2 xenografted ears in mice | [106] | |
| Organic | Pc | ~1000 | 1064 | Dye-containing tube through human arms | [107] | |
| Organic | CyFaP | 1040 | 1064 | Dye-containing tube through human breasts | [108] | |
| Organic | NiPNP | 1064 | 1064 | SLN, GI tracts, and bladder in rats | [109] | |
| Inorganic | Copper sulfide NP | 990 | 1064 | Brain and SLN in rats | [110] | |
| Inorganic | AuNR | ~1280 | 1064 | Subcutaneous layer in mice | [111] | |
| Inorganic | AuNR | ~1050 | 1064 | Prostate cancer xenograft in mice | [112] | |
| Inorganic | AuNP | ~1300 | 1064 | 4T1 xenograft in mice | [113] | |
| Inorganic | MoO3 | 1080 | 1080 | HCT116 xenograft in mice | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. https://doi.org/10.3390/bios12080594
Han S, Lee D, Kim S, Kim H-H, Jeong S, Kim J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors. 2022; 12(8):594. https://doi.org/10.3390/bios12080594
Chicago/Turabian StyleHan, Seongyi, Dakyeon Lee, Sungjee Kim, Hyung-Hoi Kim, Sanghwa Jeong, and Jeesu Kim. 2022. "Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range" Biosensors 12, no. 8: 594. https://doi.org/10.3390/bios12080594
APA StyleHan, S., Lee, D., Kim, S., Kim, H.-H., Jeong, S., & Kim, J. (2022). Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors, 12(8), 594. https://doi.org/10.3390/bios12080594






