Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics
Abstract
:1. Introduction
1.1. Graphene Oxide (GO)
1.2. Reduced Graphene Oxide (rGO)
1.3. Graphene Oxide Based Biosensors: Background
1.3.1. Graphene Oxide–Biomolecule Interactions
1.3.2. Electrochemical Techniques for Cancer Markers Detection
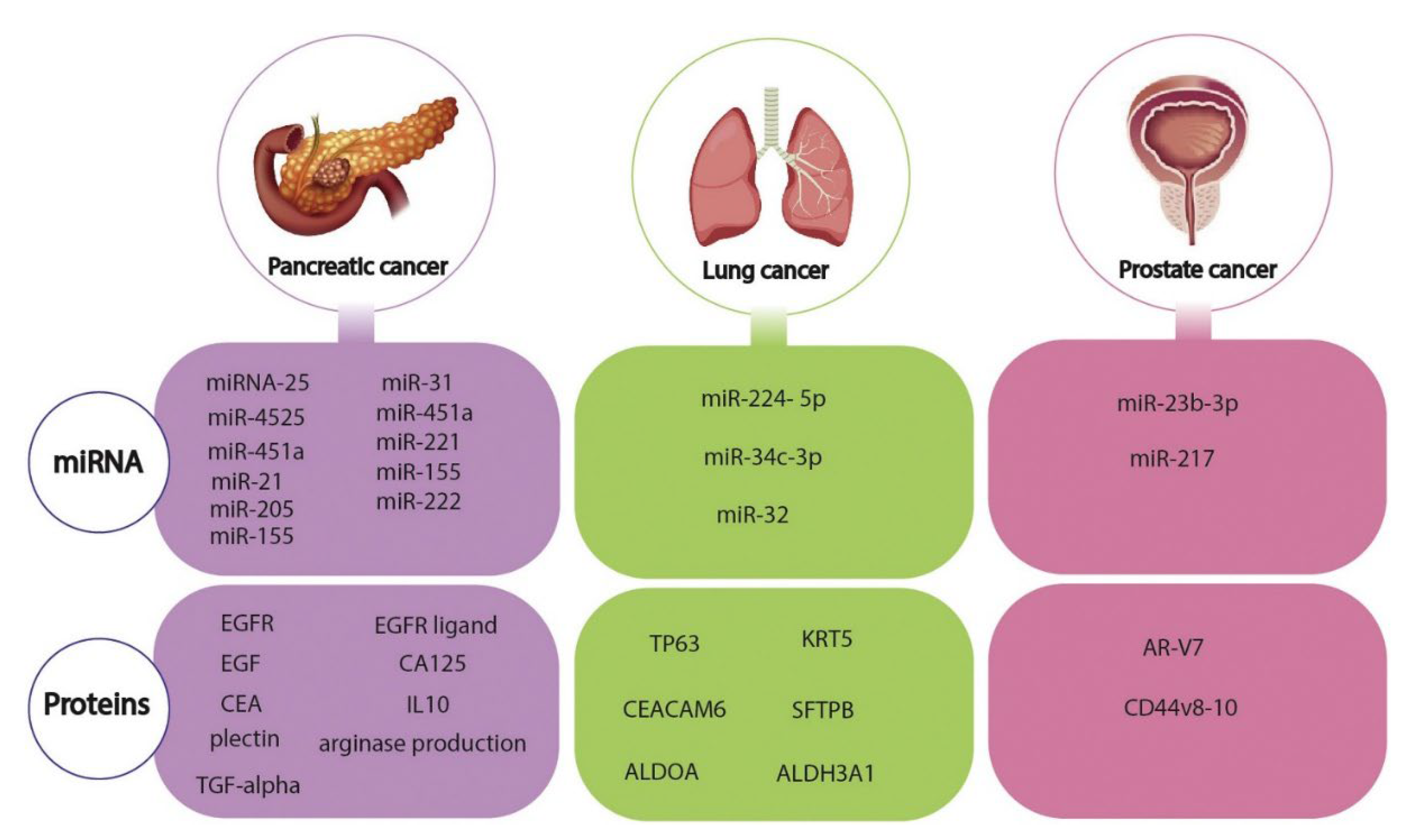
1.4. Graphene-Based Electrochemical Biosensor Strategies for Cancer Cells or Biomarker Detection
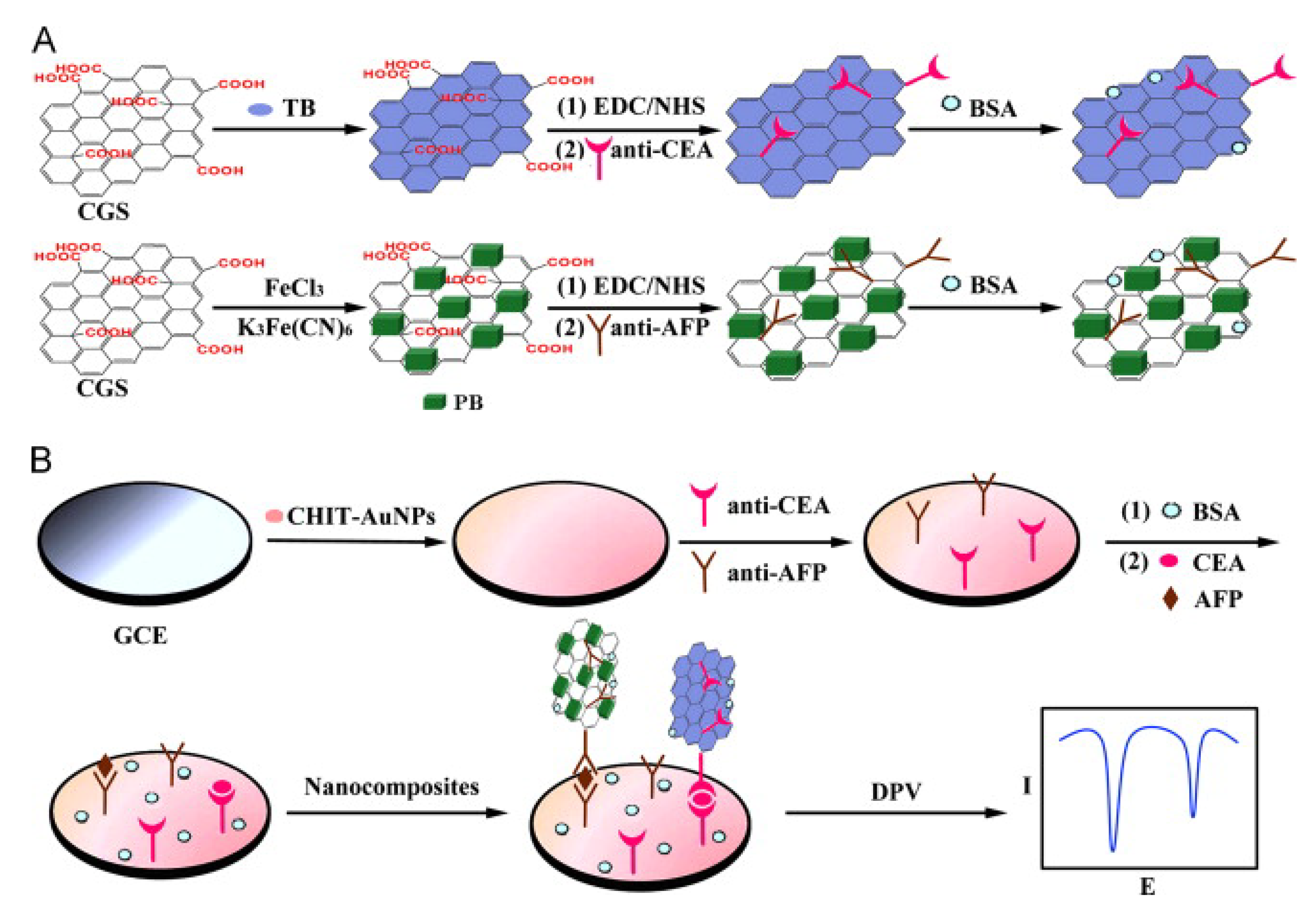
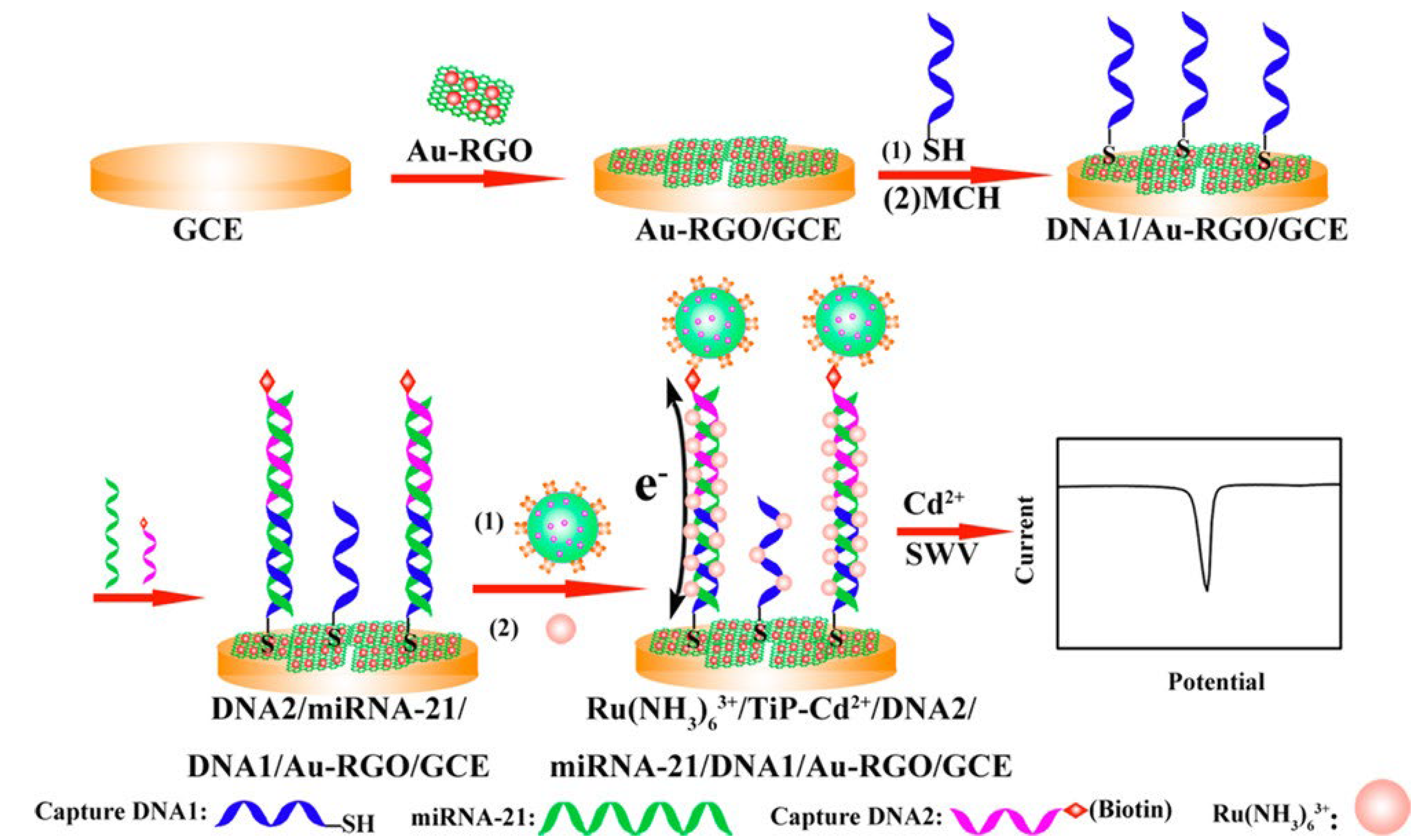
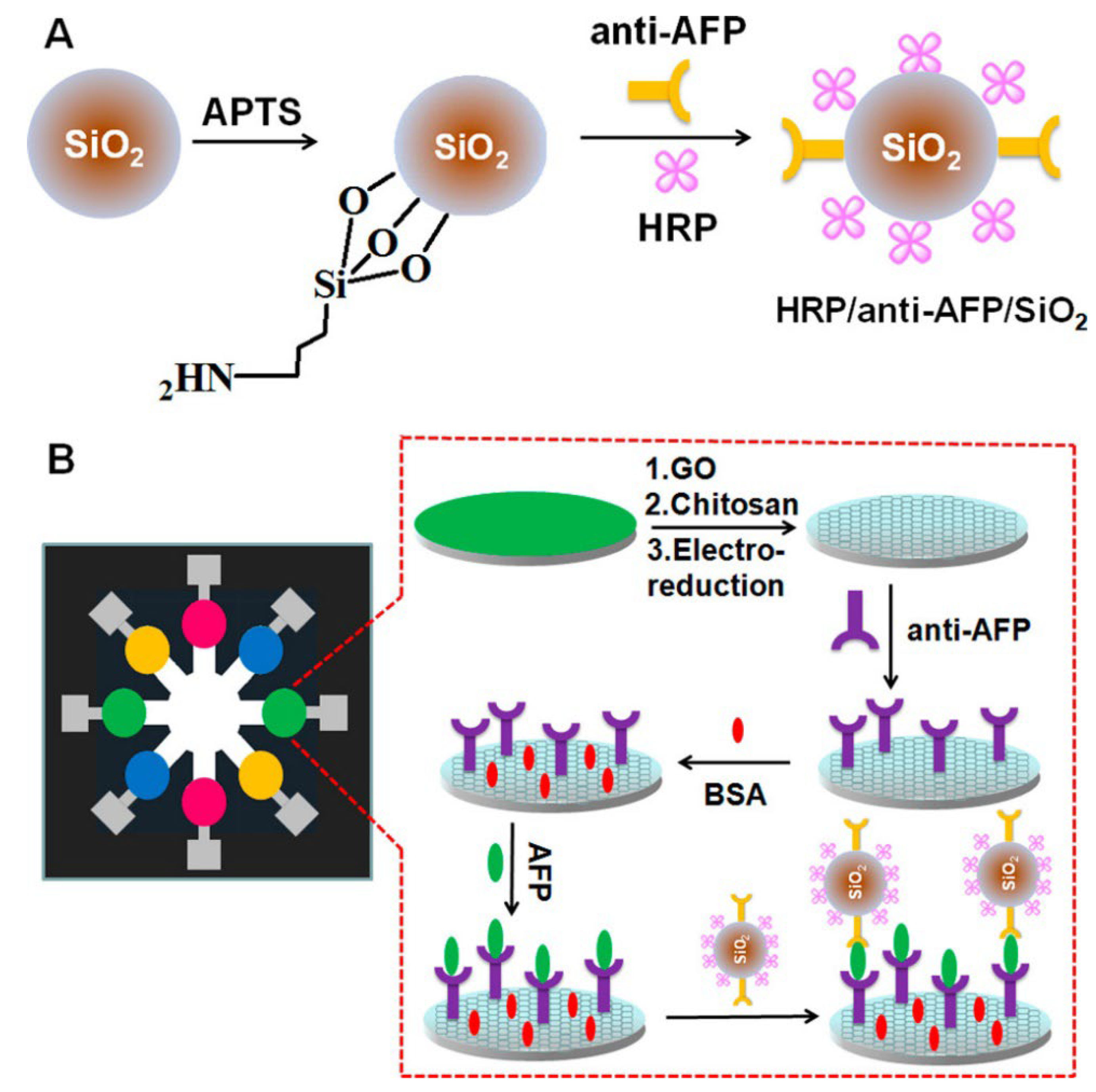
1.4.1. Label/Redox Mediator/Indicator-Based Electrochemical Biosensors
1.4.2. Label-Free Electrochemical Biosensors
Label-Free FET-Based Biosensors
Label-Free Amperometric/Potentiometric Biosensors
Label-Free but Redox Probe [Fe(CN)6]3−/4−-Based Voltammetric/Impedimetric Biosensors
| Sensor Surface Modification | Capture Biomolecule for Sensing | Detected Cancer Biomarker | Electrochemical Technique Involved in the Marker Determination | Linear Range and LOD | Reference |
|---|---|---|---|---|---|
| Glassy carbon electrode (GCE) functionalized with porous GO/Au composites and porous PtFe alloy | MUC1- Aptamer | MCF-7 cells (breast cancer) | EIS, CV, DPV | 100–5.0 × 107 cells/mL; 38 cells/mL | [133] |
| Au/Ti coated Si/SiO2 substrate modified with trimetallic Pd@Au@Pt nanocomposites platform on -COOH terminated reduced graphene oxide (COOH-rGO) | Antibody | CEA (different cancers) PSA (Prostate cancer) | CV, DPV | 12 pg/mL–85 ng/mL; 8 pg/mL (CEA) 3 pg/mL–60 ng/mL; 2 pg/mL (PSA) | [134] |
| Screen-printed carbon electrodes (SPCEs) functionalized with a water-soluble reduced graphene oxide− carboxymethylcellulose (rGO-CMC) hybrid nanomaterial | Hairpin capture DNA probes | p53 tumor suppressor (TP53) gene (different cancers) | Amperometry | 0.01−0.1 μM; 3.4 and 2.9 nM for two capture probes | [135] |
| Screen-printed carbon electrodes (SPCEs) functionalized with superparamag- netic graphene-loaded iron oxide nanoparticles (GO-NPFe2O3). | None (based on graphene-RNA affinity interaction) | FGFR2:FAM76A fusion gene (ovarian cancer) | Coulometry | Unspecified; 1 fM | [136] |
| Electrophoretic deposition (EPD) of reduced graphene oxide (rGO) onto a gold electrode and post-functionalization of rGO with folic acid (Au/rGO-FA) | none | Folic acid protein (human epithelial-derived cancers) | DPV | 1–200 pM ; 1 pM | [137] |
| Screen-printed carbon electrodes (SPCEs) functionalized with graphene oxide-loaded iron oxide (GO/IO hybrid material) | None (via RNA- graphene oxide (GO) affinity interaction) | miR-21 (ovarian cancer) | Coulometry, Amperometry, CV | 1.0 fM–1.0 nM; 1 fM | [138] |
| Glassy carbon electrode (GCE) functionalized with IL-rGO–Au | Antibody | CEA (different cancers) AFP (Hepatocellular cancer) | SWV | 0.01–100 ng/mL; 0.003 and 0.002 ng/mL | [139] |
| Screen-printed carbon electrodes (SPCEs) functionalized with carboxylic group (-COOH) rich graphene oxide (GO) | Antibody | MUC1 (variety of cancers) | DPV | 0.1–2 U/mL; 0.04 U/mL | [140] |
| Au electrode functionalized with graphene oxide/ polyaniline nanostructures (GO-PANI) and Au NPs. | Antibody | Nuclear matrix protein 22 (NMP22), CEA (different cancers) | SWV | 0.1 pg/mL–0.3 ng/mL; 25 and 30 fg/mL | [141] |
| Glassy carbon electrode functionalized with sulfur-doped reduced graphene oxide (SrGO) | None (direct 8-hydroxy-2′- deoxyguanosine (8-OHdG) signal measurement) | 8-OHdG molecule (cancer) | CV, EIS, DPV | 2 nM–20 μM; 1 nM | [142] |
| Glassy carbon electrode functionalized with magnesium oxide (MgO) nanoflower and gold nanoparticles (AuNPs). (GO is in the label, not in the surface modification) | Thiolated capture DNA probe | miRNA-21 (different cancers) | CV, EIS, DPV | 0.1–100 fM; 50 aM | [143] |
| Glassy carbon electrode functionalized with reduced graphene oxide-chitosan (rGO-Chit) film | Aptamer | HER2 (breast cancer) | DPV | 0.5–2 ng/mL and 2–75 ng/mL (two linear concentration ranges); 0.21 ng/mL | [144] |
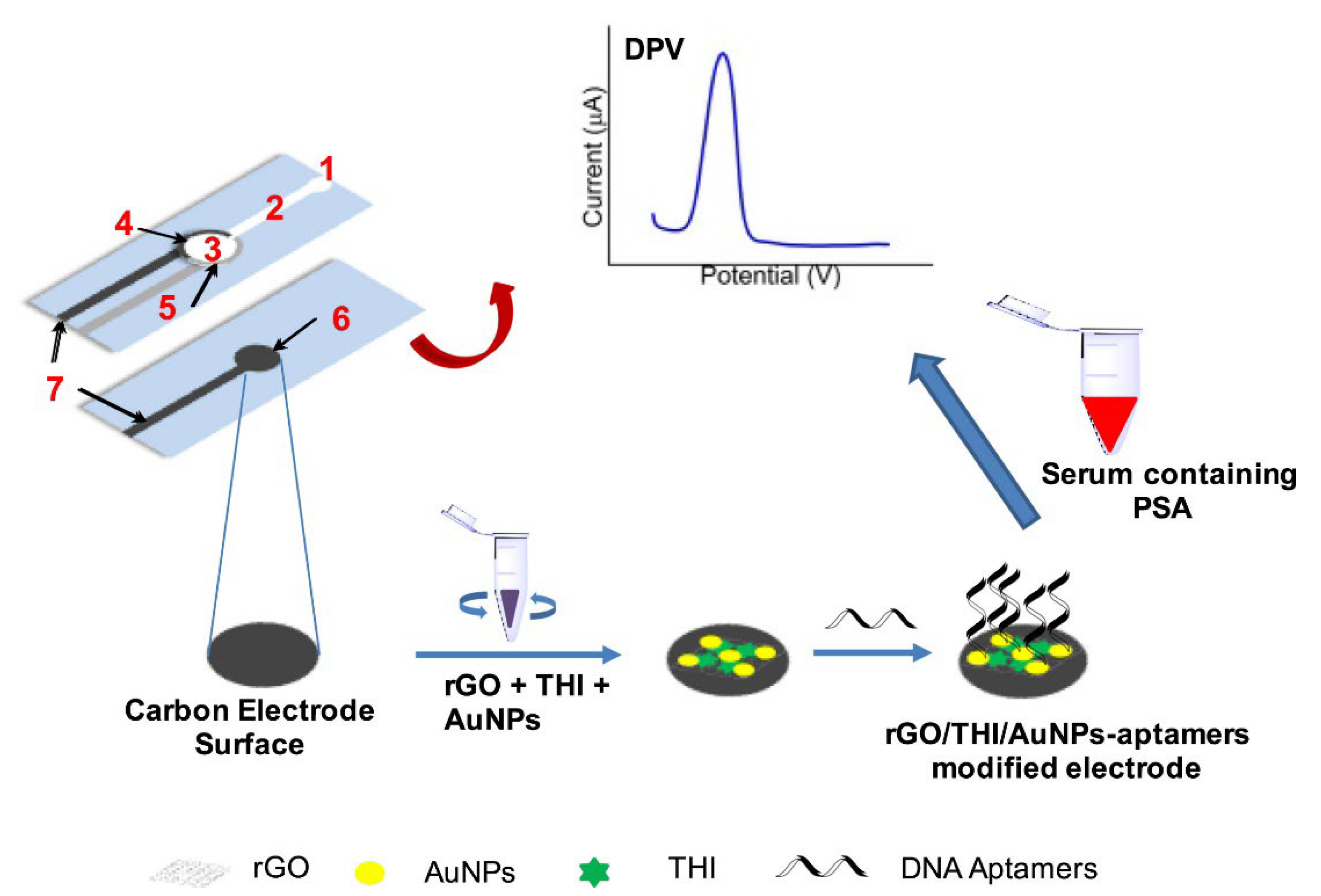
| Glassy carbon electrode functionalized with Au- poly(methylene blue) (Au-PMB) and reduced graphene oxide-Au nanocomposites (Au-rGO) | Peptides (CEHSSKLQLAK-NH2) | PSA (Prostate cancer) | SWV | 1.0 fg/mL–100 ng/mL; 0.11 fg/mL | [145] |
| Gold electrode functionalized with MPA and capture antibody (GO is in the label, not in the surface modification) | Antibody | Du-145 cells (prostate metastatic cancer) | DPV | 102–106 cells/mL; 20 cells/mL | [146] |
| Screen-printed carbon electrodes (SPCEs) functionalized with graphene oxide (GO) and ex-situ prepared silver nanoparticles (AgNPs) | Antibody | PSA (Prostate cancer) | CV, EIS, DPV | 0.75–100 ng/mL; 0.27 ng/mL | [147] |
| Sensor Surface Modification | Capture Biomolecule for Sensing | Detected Cancer Biomarker | Electrochemical Technique Involved in the Marker Determination | Linear Range and LOD | Reference |
|---|---|---|---|---|---|
| Paper-based electrode functionalized with silver nanoparticles-reduced graphene oxide nanocomposite (Ag/RGO) ink and cysteamine coped gold nanoparticles (CysA/Au NPs) | Antibody | CA15-3 protein (breast cancer) | Amperometry | 15–125 U/mL; 15 U/mL (LLOQ) | [148] |
| ITO coated glass electrode functionalized with palladium nanoparticle decorated-reduced graphene oxide (Pd@rGO) | Antibody | PSA (Prostate cancer) | CV, EIS, Amperometry | 0.01–12.5 ng/mL; 10 pg/mL | [149] |
| Glassy carbon electrode functionalized with palladium-reduced graphene oxide (Pd–rGO) | Antibody | AFP (Hepatocellular cancer) | Amperometry, DPV | 0.01–12 ng/mL; 5 pg/mL | [31] |
| Glassy carbon electrode functionalized with nanocomposite of Au NPs decorated on aminated reduced graphene oxide (Au–NH2–rGO) | Antibody | PSA (Prostate cancer) | Amperometry, EIS | 0.5 pg/mL–15 ng/mL; 0.17 pg/mL | [150] |
| Fluorine tin oxide (FTO) sheets coated with carboxylated graphene oxide followed by deposition of gold- platinum bimetallic nanoparticles | Capture DNA probe | miRNA-21 (breast cancer) | CV, EIS, DPV | 1 fM–1 μM; 1 fM | [151] |
| Fluorine-doped tin oxide (FTO) electrodes functionalized with facet-controlled Au nanorods-functionalized reduced graphene oxide (Au NRs/rGO) | Antibody | PSA (Prostate cancer) | CV, EIS, DPV | 0.1–150 ng/mL; 0.016 ng/mL | [152] |
| Pencil graphite electrode functionalized with graphene oxide (GO) | Capture DNA probe | miRNA-34a (different cancers) | CV, EIS | 0–10 μg/mL; 1.84 μg/mL (261.7 nM) | [153] |
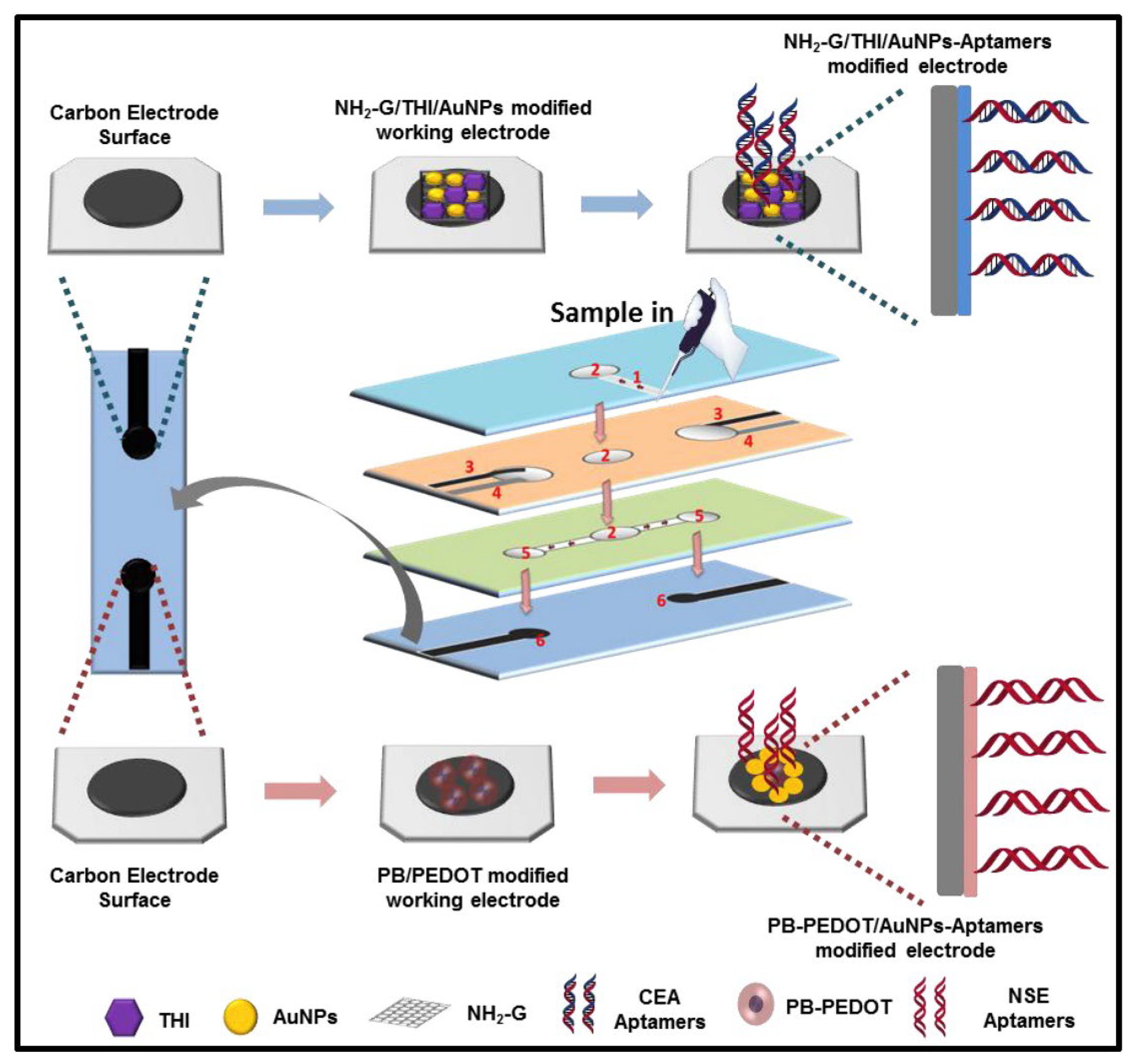
| Glassy carbon electrode functionalized with rhombic dodecahedral Cu2O nanocrystals– graphene oxide– gold nanoparticles (rCu2O-GO-AuNPs) | Antibody | CEA (different cancers) | CV, EIS | 0.01–120 ng/mL; 0.004 ng mL | [154] |
| Glassy carbon electrode functionalized with Au loaded on thionine functionalized graphene oxide (Au@Th/GO) | Antibody | PSA (Prostate cancer) | Amperometry, CV, EIS | 50 fg/mL–40 ng/mL; 16.6 fg/mL | [155] |
| Glassy carbon electrode functionalized with reduced graphene oxide /gold nanoparticles (GO/AuNPs) and antibody. | Antibody | PSA (Prostate cancer) | CV, EIS, SWV | Unspecified; 0.2 and 0.07 ng/mL for total and free PSA antigen | [156] |
| ITO coated glass electrode functionalized with cerium oxide nanocubes (ncCeO2)– reduced graphene oxide (RGO)-based nanocomposite. | Antibody | Cyfra-21-1 (oral cancer) | CV, EIS, DPV | 0.625 pg/mL–15 ng/mL; 0.625 pg/mL | [157] |
| Platinum electrode functionalized with GO layers and AuNPs. | Antibody | PSA (Prostate cancer) | CV, DPV, EIS | 0.001 fg/mL–0.02 μg/mL; 0.24 fg/mL | [158] |
| Glassy carbon electrode functionalized with herceptin-conjugated graphene. | Antibody | HER2 (breast cancer) | EIS, CV, DPV | 1–80 cells ; Unspecified | [159] |
| Graphite electrode functionalized with reduced graphene oxide nano-sheets (rGONs) and rhodium nanoparticles (Rh-NPs). | Aptamer | HER2-ECD (breast cancer) | CV, DPV, EIS | 10.0–500 ng/mL; 0.667 ng/mL | [160] |
| Au film electrode functionalized with Fe3O4@graphene oxide(GO)@molecularly imprinted polymer (MIP) nanoparticles. | Special sensor surface structure originating from MIP | IL-8 (oral cancer) | CV | 0.1–10 pM ; 0.04 pM | [161] |
| ITO coated glass electrode functionalized with zinc oxide–reduced graphene oxide (ZnO–rGO) nanocomposite. | Antibody | IL-8 (oral cancer) | CV, DPV | 100 fg/mL– 5 ng/mL; 51.53 ± 0.43 pg/mL | [162] |
| Pencil graphite electrodes modified with carbon black, multi-walled carbon nanotubes, and graphene oxide nanomaterials. | Capture DNA probe | microRNA-125a (different cancers) | CV, EIS | 0.008 and 15 μg/mL; 10 pM | [163] |
| FET chip functionali-zed with gold nano- particles (AuNPs)- decorated reduced graphene oxide. | Peptide nucleic acid (PNA) probe | miRNA (type not specified) | FET | Unspecified ; 10 fM | [164] |
| FET channel functionalized with tetra(4-aminophenyl) porphyrin mediated reduced graphene oxide. | Aptamer | CTCs (different cancers) | FET | 10–106 cells/mL ; Unspecified | [165] |
| Graphene FET chip functionalized with 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE). | Antibody | AFP (hepatocellular cancer) | FET | Unspecified; 0.1 ng/mL in PBS, 12.9 ng/mL in plasma | [166] |
| Pentacene-based FET with a graphene oxide support system (GOSS), composed of functionalized graphene oxide (GO) ink. | Single stranded DNA/ Antibody | Target DNA/ CTCs (basically HER2, breast cancer) | FET | Unspecified; 0.1 pmoles for DNA and 100 cancer cells/mL | [167] |
| FET functionalized with carboxylated multiwalled carbon nanotubes (MWCNTs)/ reduced graphene oxide. | Aptamer | CA 125 (ovarian cancer) | FET | 1.0 × 10−9–1.0 U/mL ; 5.0 × 10−10 U/mL | [168] |
| FET functionalized with Polymethyl methacrylate(PMMA) and graphene films | Antibody | CEA (different cancers) | FET | Unspecified; less than 100 pg/mL | [169] |
2. Challenges and Future Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Geim, A.; Novoselov, K. The rise of graphene (editorial). Nat. Mat. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Muazim, K.; Hussain, Z. Graphene oxide—A platform towards. theranostics. Mater. Sci. Eng. C 2017, 76, 1274–1288. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Ago, H. CVD Growth of High-Quality Single-Layer Graphene. In Frontiers of Graphene and Carbon Nanotubes: Devices and Applications; Matsumoto, K., Ed.; Springer: Tokyo, Japan, 2015; pp. 3–20. [Google Scholar]
- Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V.; Chen, G.-Y. Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications, Int. J. Mol. Sci. 2019, 20, 2975. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Terse-Thakoor, T.; Badhulika, S.; Mulchandani, A. Graphene based biosensors for healthcare. J. Mater. Res. 2017, 32, 2905–2929. [Google Scholar] [CrossRef]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Bustos, A.R.M.; Reipa, V.; Riley, K.R.; Bryant, C.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Nguyen, P.; Berry, V. Graphene Interfaced with Biological Cells:Opportunities and Challenges. J. Phys. Chem. Lett. 2012, 3, 1024–1029. [Google Scholar] [CrossRef]
- Janegitz, B.C.; Silva, T.A.; Wong, A.; Ribovski, L.; Vicentini, F.C.; Sotomayor, M.D.P.T.; Fatibello-Filho, O. The application of graphene for in vitro and in vivo electrochemical biosensing. Biosens. Bioelectron. 2017, 89, 224–233. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Johns, J.E.; Hersam, M.C. Atomic Covalent Functionalization of Graphene. Acc. Chem. Res. 2013, 46, 77–86. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Hao, L.-Y.; Shao, X.-R.; Zhang, Q.; Jia, X.-Q.; Zhang, Z.-R.; Lin, Y.-F.; Peng, Q. Insight into the interaction of graphene oxide with serum proteins and the impact of the degree of reduction and concentration. ACS Appl. Mater. Interfaces 2015, 7, 13367–13374. [Google Scholar] [CrossRef]
- Balaji, A.; Zhang, J. Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nano 2017, 8, 10. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Chen, K.; Liu, F.; Song, S.; Xue, D. Water crstallization to create ice spacersbetween graphene oxide sheets for highly electroactive graphene paper. Cryst. Eng. Commun. 2014, 16, 7771–7776. [Google Scholar] [CrossRef]
- Liu, F.; Xue, D. An electrochemical route to quantitative oxidation of graphene frameworks with controllable C/O ratios and added pseudocapacitances. Chem. A Eur. J. 2013, 19, 10716–10722. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Xue, D.; Zhang, H. Folded structured graphene paper for Highperformance electrode materials. Adv. Mater. 2012, 24, 1089–1094. [Google Scholar] [CrossRef]
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview. Molecules 2015, 20, 14155–14190. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Gao, L.; Lian, C.; Zhou, Y.; Yan, L.; Li, Q.; Zhang, C.; Chen, L.; Guo, K.C.; Yang, F.; Zhang, Y.; et al. Graphene oxide–DNA based sensors. Biosens. Bioelectron. 2014, 60, 22–29. [Google Scholar] [CrossRef]
- Jamali, A.A.; Pourhassan-Moghaddam, M.; Dolatabadi, J.E.N.; Omidi, Y. Nanomaterials on the road to microRNA detection with optical and electrochemical nanobiosensors. TrAC 2014, 55, 24–42. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Graphene-DNA electrochemical sensor for the sensitive detection of BRCA1 gene. Sens. Act B 2014, 204, 777–782. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Ghodsi, E.; Abdollahi, S.; Nadri, S. Porous graphene oxide nanostructure as an excellent scaffold for label-free electrochemical biosensor: Detection of cardiac troponin I. Mater. Sci. Eng. C 2016, 69, 447–452. [Google Scholar] [CrossRef]
- Qi, T.T.; Liao, J.F.; Li, Y.S.; Peng, J.R.; Li, W.T.; Chu, B.Y.; Li, H.; Wei, Y.Q.; Qian, Z.Y. Label-free alpha fetoprotein immunosensor established by the facile synthesis of a palladium–graphene nanocomposite. Biosens. Bioelectron. 2014, 61, 245–250. [Google Scholar] [CrossRef]
- Oghli, H.; Soleymanpour, A. Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, C.; Wang, F.; Liu, G. A polyethyleneimine reduced graphene oxide/gold nanocubes based electrochemical aptasensor for chloramphenicol detection using single-stranded DNA-binding protein. Mater. Des. 2021, 199, 109409. [Google Scholar] [CrossRef]
- Teradala, N.L.; Narayana, P.S.; Satpatib, A.K.; Seetharamappa, J. Fabrication of electrochemical sensor based on green reduction of graphene oxide for an antimigraine drug, rizatriptan benzoate. Sens. Actuators B 2014, 196, 596–603. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Liu, Y.; Li, L.-J.; Chen, P. Graphene based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011, 21, 12358. [Google Scholar] [CrossRef]
- Morales-Narvaez, E.; Hassan, A.-R.; Merkoci, A. Graphene oxide as a pathogen-revealing agent: Sensing with a digital-like response. Angew. Chem. Int. Ed. 2013, 52, 13779–13783. [Google Scholar] [CrossRef]
- Liu, F.; Kim, Y.H.; Cheon, D.S.; Seo, T.S. Micropatterned reduced graphene oxide based field-effect transistor for real-time virus detection. Sens. Actuators B 2013, 186, 252. [Google Scholar] [CrossRef]
- Mishra, R.K.; Nawaz, M.H.; Hayat, A.; Nawaz, M.A.H.; Sharma, V.; Marty, J.L. Electrospinning of graphene-oxide onto screen printed electrodes for heavy metal biosensor. Sens. Actuators B 2017, 247, 366–373. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B.; Reisberg, S.; HuyNguyen, L.; DungNguyen, T.; Duc, H.T.; Pham, M.C. An electrochemical ELISA-like immunosensor for miRNAs detection based on screen-printed gold electrodes modified with reduced Graphene oxide and carbon nanotubes. Biosens. Bioelectron. 2014, 62, 25–30. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Fiorani, A.; Merino, J.P.; Zanut, A.; Criado, A.; Valenti, G.; Prato, M.; Paolucci, F. Advanced carbon nanomaterials for electrochemiluminescent biosensor applications. Curr. Opin. Electrochem. 2019, 16, 66–74. [Google Scholar] [CrossRef]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized Graphene Oxide as a Nanocarrier in a Multienzyme Labeling Amplification Strategy for Ultrasensitive Electrochemical Immunoassay of Phosphorylated p53 (S392). Anal. Chem. 2011, 83, 746–752. [Google Scholar] [CrossRef]
- Tang, Z.W.; Wu, H.; Cort, J.R.; Buchko, G.W.; Zhang, Y.Y.; Shao, Y.Y.; Aksay, I.A.; Liu, J.; Lin, Y.H. Constraint of DNA on Functionalized Graphene Improves its Biostability and Specificity. Small 2010, 6, 1205–1209. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Turner, A.P.F.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectron. 2004, 19, 515–530. [Google Scholar] [CrossRef]
- Jarczewska, M.; Górski, L.; Malinowska, E. Electrochemical aptamer-based biosensors as potential tools for clinical diagnostics. Anal. Meth. 2016, 8, 3861–3877. [Google Scholar] [CrossRef]
- Ozkan, D.; Erdem, A.; Kara, P.; Kerman, K.; Meric, B.; Hassmann, J.; Ozsoz, M. Allele-Specific Genotype Detection of Factor V Leiden Mutation from Polymerase Chain Reaction Amplicons Based on Label-Free Electro-chemical Genosensor. Anal. Chem. 2002, 74, 5931–5936. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Voltammetric aptasensors for protein diseasebiomarkers detection: A review. Biotechnol. Adv. 2016, 34, 941–953. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Rivas, G.; Shiraishi, H.; Farias, P.A.; Dontha, N. DNA electrochemical biosensor for the detection of short DNAsequences related to the human immunodeficiency virus. Anal. Chem. 1996, 68, 2629–2634. [Google Scholar] [CrossRef]
- Odenthal, K.J.; Gooding, J.J. An introduction to electrochemical DNA biosensors. Analyst 2007, 132, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Millan, K.M.; Mikkelsen, S.R. Sequence-selective biosensor for DNA Based on electroactive hybridization indicators. Anal. Chem. 1993, 65, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Millan, K.M.; Spurmanis, A.J.; Mikkelsen, S.R. Covalent immobilization of DNA onto glassy carbon electrodes. Electroanalysis 1992, 4, 929–932. [Google Scholar] [CrossRef]
- Yang, M.; McGovern, M.E.; Thompson, M. Genosensor technology and the detection of interfacial nucleic acid chemistry. Anal. Chim. Acta 1997, 346, 259–275. [Google Scholar] [CrossRef]
- Ozsoz, M.; Erdem, A.; Kerman, K.; Ozkan, D.; Tugrul, B.; Topcuoglu, N.; Ekren, H.; Taylan, M. Electrochemical Genosensor Based on Colloidal Gold Nanoparticles for the Detection of Factor V Leiden Mutation Using Disposable Pencil Graphite Electrodes. Anal. Chem. 2003, 75, 2181–2187. [Google Scholar] [CrossRef]
- Nirmalya, K.; Vijayamohanan, C.K. Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron. 2002, 17, 1–12. [Google Scholar]
- Zammatteo, N.; Jeanmart, L.; Hamels, S.; Courtois, S.; Louette, P.; Hevesi, L.; Remacle, J. Comparison between different strate-gies of covalent attachment of DNA to glass surfaces to build DNA microarrays. Anal. Biochem. 2000, 280, 143–150. [Google Scholar] [CrossRef]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA probes immobilized on gold surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar] [CrossRef]
- Gooding, J.J.; Mearns, F.; Yang, W.; Liu, J. Self-Assembled Monolayers into the 21st Century: Recent Advances and Applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Kelley, S.O.; Barton, J.K. Electrochemistry of methylene blue bound to a DNA-modified electrode. Bioconjug. Chem. 1997, 8, 31–37. [Google Scholar] [CrossRef]
- Pividori, M.I.; Merkoçi, A.; Alegret, S. Electrochemical genosensor design: Immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens. Bioelectron. 2000, 15, 291–303. [Google Scholar] [CrossRef]
- Carpini, G.; Lucarelli, F.; Marrazza, G.; Mascini, M. Oligonucleotide-modified screen-printed gold electrodes for enzyme-amplified sensing of nucleic acids. Biosens. Bioelectron. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, H.; Dong, Y.; Zhang, W. A sensitive impedimetric DNA biosensor for the determination of the HIV gene based on electrochemically reduced graphene oxide. Anal. Methods 2015, 7, 2554–2562. [Google Scholar] [CrossRef]
- Haque, A.J.; Park, H.; Sung, D.; Jon, S.; Choi, S.-Y.; Kim, K. An Electrochemically Reduced Graphene Oxide-Based Electrochemical Immunosensing Platform for Ultrasensitive Antigen Detection. Anal. Chem. 2012, 84, 1871–1878. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; ISBN -0-471-28272-3. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley &Sons: New York, NY, USA, 2001. [Google Scholar]
- Ozkan, S.A.; Uslu, B.; Aboul-Enein, H.Y. Analysis of Pharmaceuticals and Biological Fluids Using Modern Electroanalytical Techniques. Crit. Rev. Anal. Chem. 2003, 33, 155–181. [Google Scholar] [CrossRef]
- Chandra, P.; Tan, Y.N.; Singh, S.P. Next Generation Point-of-Care Biomedical Sensors Technologies for Cancer Diagnosis, 1st ed.; Springer: Singapore, 2017. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Mahato, K.; Kumar, A.; Maurya, P.K.; Chandra, P. Shifting paradigm of cancer diagnoses in clinically relevant samples based on miniaturized electrochemical nanobiosensors and microfluidic devices. Biosens. Bioelectron. 2018, 100, 411–428. [Google Scholar] [CrossRef]
- Hanjani, A.N.; Esmaelizad, N.; Zanganeh, S.; Gharavi, A.T.; Heidarizadeh, P.; Radfar, M.; Omidi, F.; MacLoughlin, R.; Doroudian, M. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit. Rev. Oncol. Hematol. 2022, 169, 103565. [Google Scholar] [CrossRef]
- Ecsedy, J.; Hunter, D. The origin of cancer. In Textbook of Cancer Epidemiology, 2nd. ed.; Adami, H.O., Hunter, D., Trichopoulos, D., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 61–85. [Google Scholar]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Recent advances in enhancement strategies for electrochemical ELISA-based immunoassays for cancer biomarker detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Biosensing in Cancer Diagnostics and Follow-up. Electroanalysis 2018, 30, 1584–1603. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Zhao, F.; Jiang, Y.; Chen, Z. A disposable paper-based microfluidic immunosensor based on reduced graphene oxide-tetraethylene pentamine/Au nanocomposite decorated carbon screen-printed electrodes. Sens. Actuators B 2017, 252, 44–54. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Saadati, A.; Hassanpour, S.; Hasanzadeh, M.; Shadjou, N.; Hassanzadeh, A. Paper based immunosensing of ovarian cancer tumor protein CA 125 using novel nano-ink: A new platform for effi-cient diagnosis of cancer and biomedical analysis using microfluidic paper-based ana-lytical devices (μPAD). Int. J. Biol. Macromol. 2019, 138, 744–754. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Kithva, P.H.; Rauf, S.; Trau, M. Femtomolar detection of a cancer biomarker protein in serum with ultralow background current by anodic stripping voltammetry. Chem. Commun. 2012, 48, 6411–6413. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.D.; Polsky, R.; Merkoci, A. Electrochemical stripping detection of DNA hybridization based on cadmium sulfide nanoparticle tags. Electrochem. Commun. 2002, 4, 722–726. [Google Scholar] [CrossRef]
- Ozkan-Ariksoysal, D. Electrochemical DNA Biosensors based on quantum dots. In Electroanalytical Applications of Quantum Dot-Based Biosensors; Uslu, B., Ed.; Elsevier: London, UK, 2021. [Google Scholar]
- Cheng, F.-F.; He, T.-T.; Miao, H.-T.; Shi, J.-J.; Jiang, L.-P.; Zhu, J.-J. Electron Transfer Mediated Electrochemical Biosensor for MicroRNAs Detection Based on Metal Ion Functionalized Titanium Phosphate Nanospheres at Attomole Level. ACS Appl. Mater. Interfaces 2015, 7, 2979–2985. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Rahaiea, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B.; Reisberg, S.; Duc, H.T.; Pham, M.C. Antibodies Directed to RNA/DNA Hybrids: An Electrochemical Immunosensor for MicroRNAs Detection using Graphene-Composite Electrodes. Anal. Chem. 2013, 85, 8469–8474. [Google Scholar] [CrossRef]
- Elshafey, R.; Siaj, M.; Tavares, A.C. Au nanoparticle decorated graphene nanosheets for electrochemical immunosensing of p53 antibodies for cancer prognosis. Analyst 2016, 141, 2733–2740. [Google Scholar] [CrossRef]
- Dou, B.; Xu, L.; Jiang, B.; Yuan, R.; Xiang, Y. Aptamer-functionalized and Gold Nanoparticle Array-Decorated Magnetic Graphene Nanosheets Enable Multiplexed and Sensitive Electrochemical Detection of Rare Circulating Tumor Cells in Whole Blood. Anal. Chem. 2019, 91, 10792–10799. [Google Scholar] [CrossRef]
- Pereira, C.; Parolo, C.; Idili, A.; Gomis, R.R.; Rodrigues, L.; Sales, G.; Merkoci, A. Paper-based biosensors for cancer diagnostics. Trends Chem. 2022, 4, 554–567. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-Based Microfluidic Electrochemical Immunodevice Integrated with Nanobioprobes onto Graphene Film for Ultrasensitive Multiplexed Detection of Cancer Biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Hui, K.M.; Kang, Y. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014, 52, 180–187. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, C.; Sun, X.; Jian, Y.; Kong, Q.; Cui, K.; Ge, S.; Yu, J. Polyhedral-AuPd nanoparticles-based dual-mode cytosensor with turn on enable signal for highly sensitive cell evalution on lab-on-paper device. Biosens. Bioelectron. 2018, 117, 651–658. [Google Scholar] [CrossRef]
- Wei, B.; Mao, K.; Liu, N.; Zhang, M.; Yang, Z. Graphene nanocomposites modified electrochemical aptamer sensor for rapid and highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 121, 41–46. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
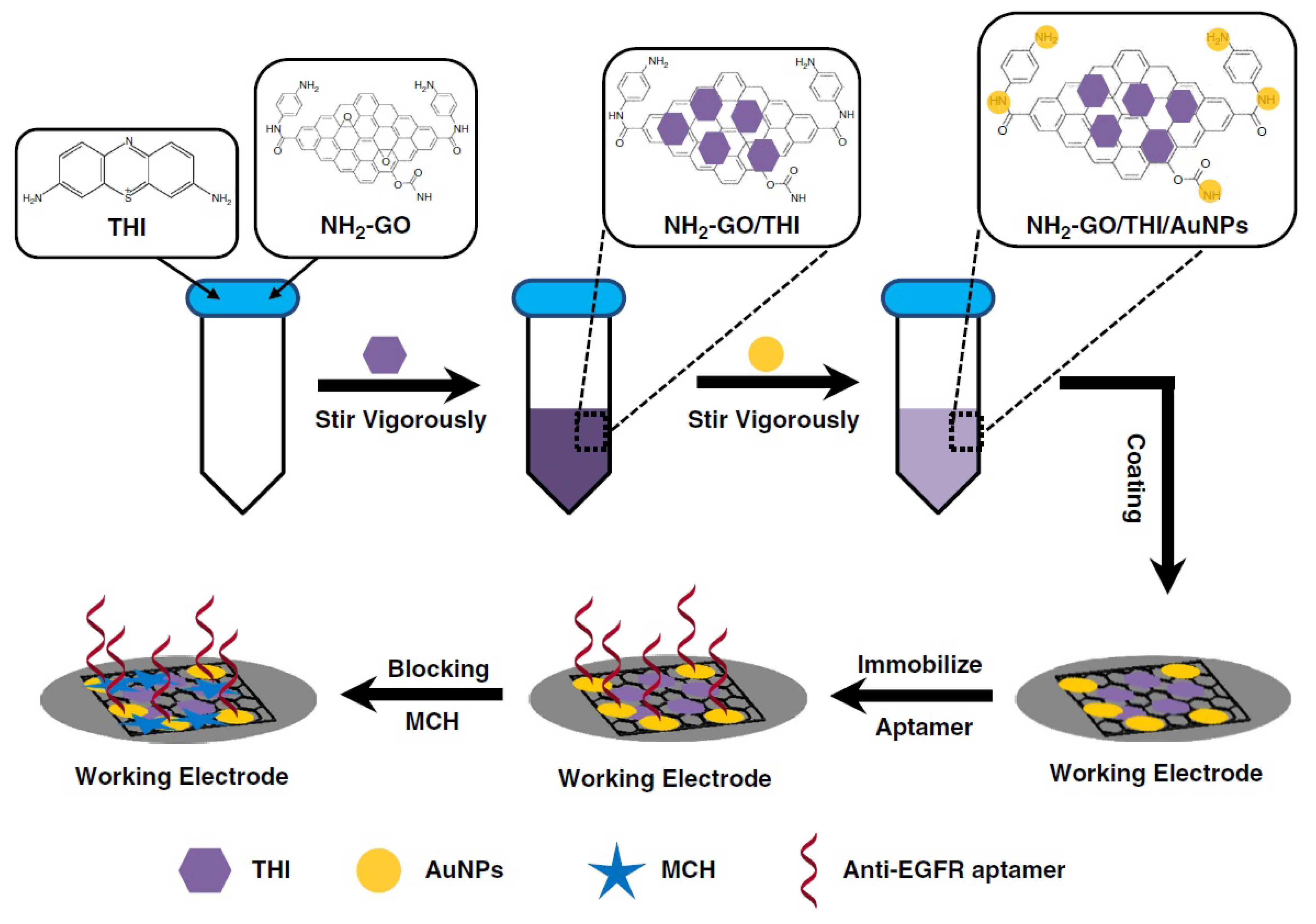
- Wang, Y.; Sun, S.; Luo, J.; Xiong, Y.; Ming, T.; Liu, J.; Ma, Y.; Yan, S.; Yang, Y.; Yang, Z.; et al. Low sample volume origami-paper-based graphene-modified aptasensors for label-free electrochemical detection of cancer biomarker-EGFR. Microsyst. Nanoeng. 2020, 6, 32. [Google Scholar] [CrossRef]
- Ortega, F.G.; Regiart, M.D.; Rodríguez-Martínez, A.; Miguel-Pérez, D.; Serrano, M.J.; Lorente, J.A.; Tortella, G.; Rubilar, O.; Sapag, K.; Bertotti, M.; et al. Sandwich-Type Electrochemical Paper-Based Immunosensor for Claudin 7 and CD81 Dual Determination on Extracellular Vesicles from Breast Cancer Patients. Anal. Chem. 2021, 93, 1143–1153. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, Y.; Ma, H.; Du, B.; Wei, Q. Facile synthesis of cuprous oxide nanowires decorated graphene oxide nanosheets nanocomposites and its application in label-free electrochemical Immunosensor. Biosens. Bioelectron. 2017, 87, 745–751. [Google Scholar] [CrossRef]
- Gao, Y.-S.; Zhu, X.-F.; Xu, J.-K.; Lu, L.-M.; Wang, W.-M.; Yang, T.-T.; Xing, H.-K.; Yu, Y.-F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Fan, L.; Guo, Y. Electrochemical prostate specific antigen aptasensor based on hemin functionalized graphene-conjugated palladium nanocomposites. Microchim. Acta 2018, 185, 159. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An electrochemical biosensor for simultaneous detection of breast cancer clinically related microRNAs based on a gold nanoparticles/ graphene quantum dots/graphene oxide film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef]
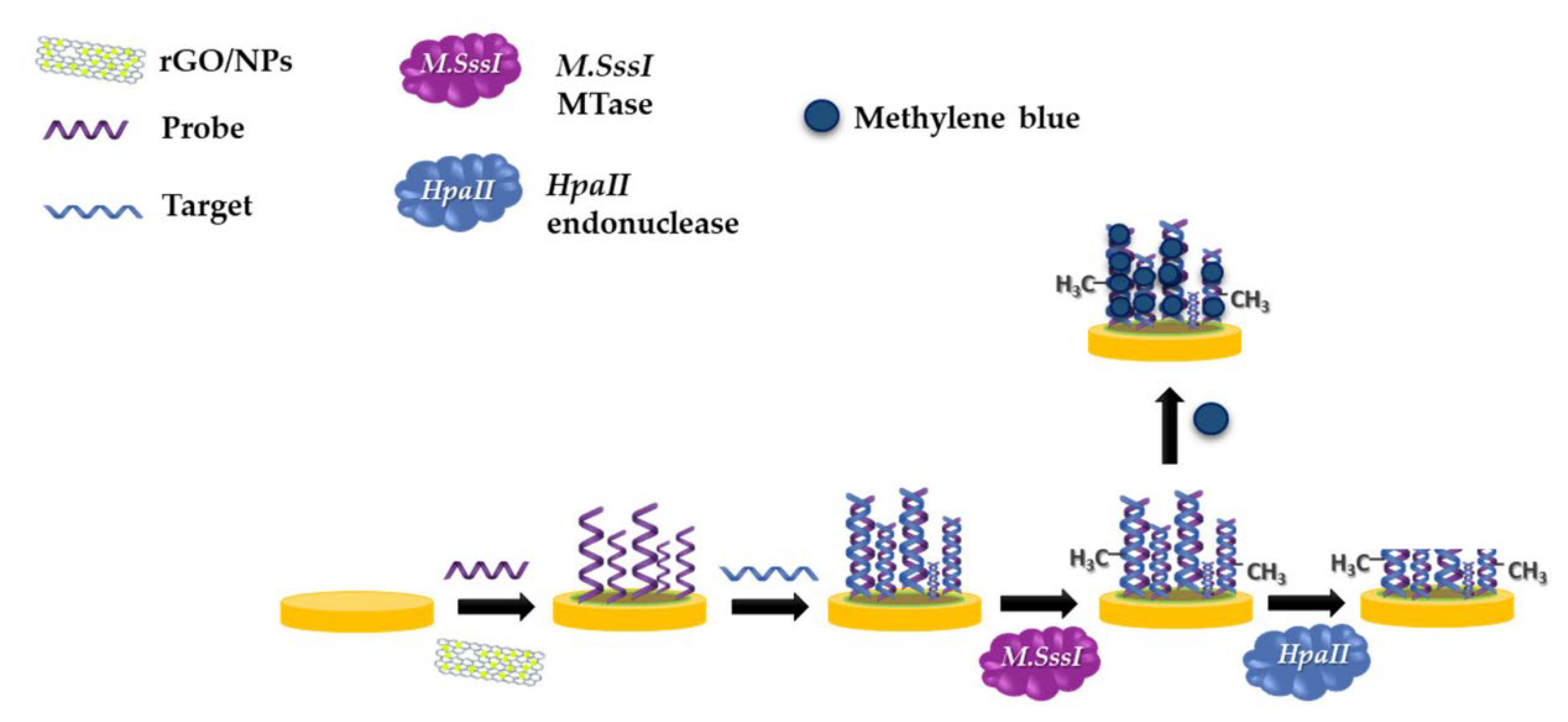
- Sedlackova, E.; Bytesnikova, Z.; Birgusova, E.; Svec, P.; Ashrafi, A.M.; Estrela, P.; Richtera, L. Label-Free DNA Biosensor Using Modified Reduced Graphene Oxide Platform as a DNA Methylation Assay. Materials 2020, 13, 4936. [Google Scholar] [CrossRef]
- Pothipor, C.; Wiriyakun, N.; Putnin, T.; Ngamaroonchote, A.; Jakmunee, J.; Ounnunkad, K.; Laocharoensuk, R.; Aroonyadet, N. Highly sensitive biosensor based on graphene–poly (3-aminobenzoic acid) modified electrodes and porous-hollowed-silver-gold nanoparticle labelling for prostate cancer detection. Sens. Actuators B Chem. 2019, 296, 126657. [Google Scholar] [CrossRef]
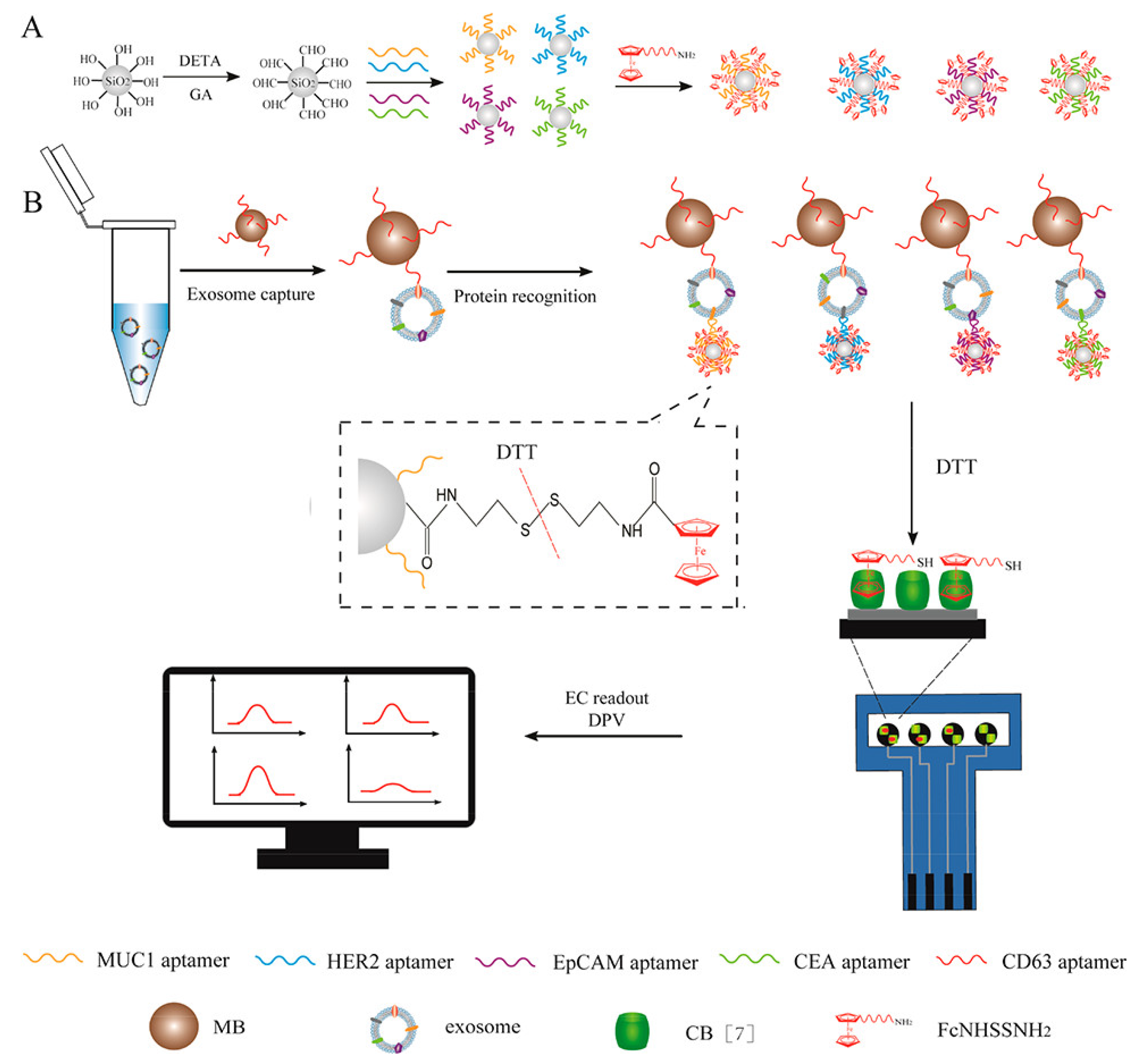
- An, Y.; Li, R.; Zhang, F.; He, P. Magneto-Mediated Electrochemical Sensor for Simultaneous Analysis of Breast Cancer Exosomal Proteins. Anal. Chem. 2020, 92, 5404–5410. [Google Scholar] [CrossRef] [PubMed]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide–based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2021, 413, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, H.; Jin, D.; Yu, Y.; Pang, D.-W.; Xiao, M.-M.; Zhang, Z.-L.; Zhang, Z.-Y.; Zhang, G.-J. Microvesicle detection by a reduced graphene oxide field-effect transistor biosensor based on a membrane biotinylation strategy. Analyst 2019, 144, 6055–6063. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, Y.; Han, Y.; Pang, J.; Wang, C.; Wang, Y.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive Label-free MiRNA Sensing Based on a Flexible Graphene Field-Effect Transistor without Functionalization. ACS Appl. Electron. Mater. 2020, 2, 1090–1098. [Google Scholar] [CrossRef]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawya, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.K.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhotra, B.D. Reduced graphene oxide modified smart conducting paper for cancer. Biosens. Bioelectron. 2015, 73, 114–122. [Google Scholar] [CrossRef]
- Yen, Y.-K.; Chao, C.-H.; Yeh, Y.-S. A Graphene-PEDOT:PSS Modified Paper-Based Aptasensor for Electrochemical Impedance Spectroscopy Detection of Tumor Marker. Sensors 2020, 20, 1372. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Manoj, D.; Wang, T.; Sun, Y.; Zhang, Y.; Xiao, F. Functionalized graphene fiber modified by dual nanoenzyme: Towards high performance flexible nanohybrid microelectrode for electrochemical sensing in live cancer cells. Sens. Actuators B 2020, 310, 127861. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to ap-plications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. CuO/WO3 nanoparticles decorated graphene oxide nanosheets with enhanced peroxidase-like activity for electrochemical cancer cell detection and targeted therapeutics. Mater. Sci. Eng. C 2019, 99, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liu, L.; Li, Y.; Feng, X.; Wei, Q.; Cao, W. A novel label-free electrochemical immunosensor for the detection of hepatitis B surface antigen. Anal. Methods 2016, 8, 7380–7386. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Omidinia, E.; Zargartalebi, H.; Majidzadeh, A.K.; Naghib, S.M.; Sanati-Nezhad, A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens. Bioelectron. 2018, 120, 129–136. [Google Scholar] [CrossRef]
- Lu, X.; Miodek, A.; Munief, W.-M.; Jolly, P.; Pachauri, V.; Chen, X.; Estrela, P.; Ingebrandt, S. Reduced graphene-oxide transducers for biosensing applications beyond the Debye-screening limit. Biosens. Bioelectron. 2019, 130, 352–359. [Google Scholar] [CrossRef]
- Aayanifard, Z.; Alebrahim, T.; Pourmadadi, M.; Yazdian, F.; Dinani, H.S.; Rashedi, H.; Omidi, M. Ultra pH-sensitive detection of total and free prostate-specific antigen using electrochemical aptasensor based on reduced graphene oxide/gold nanoparticles emphasis on TiO2/carbon quantum dots as a redox probe. Eng. Life Sci. 2021, 21, 739–752. [Google Scholar] [CrossRef]
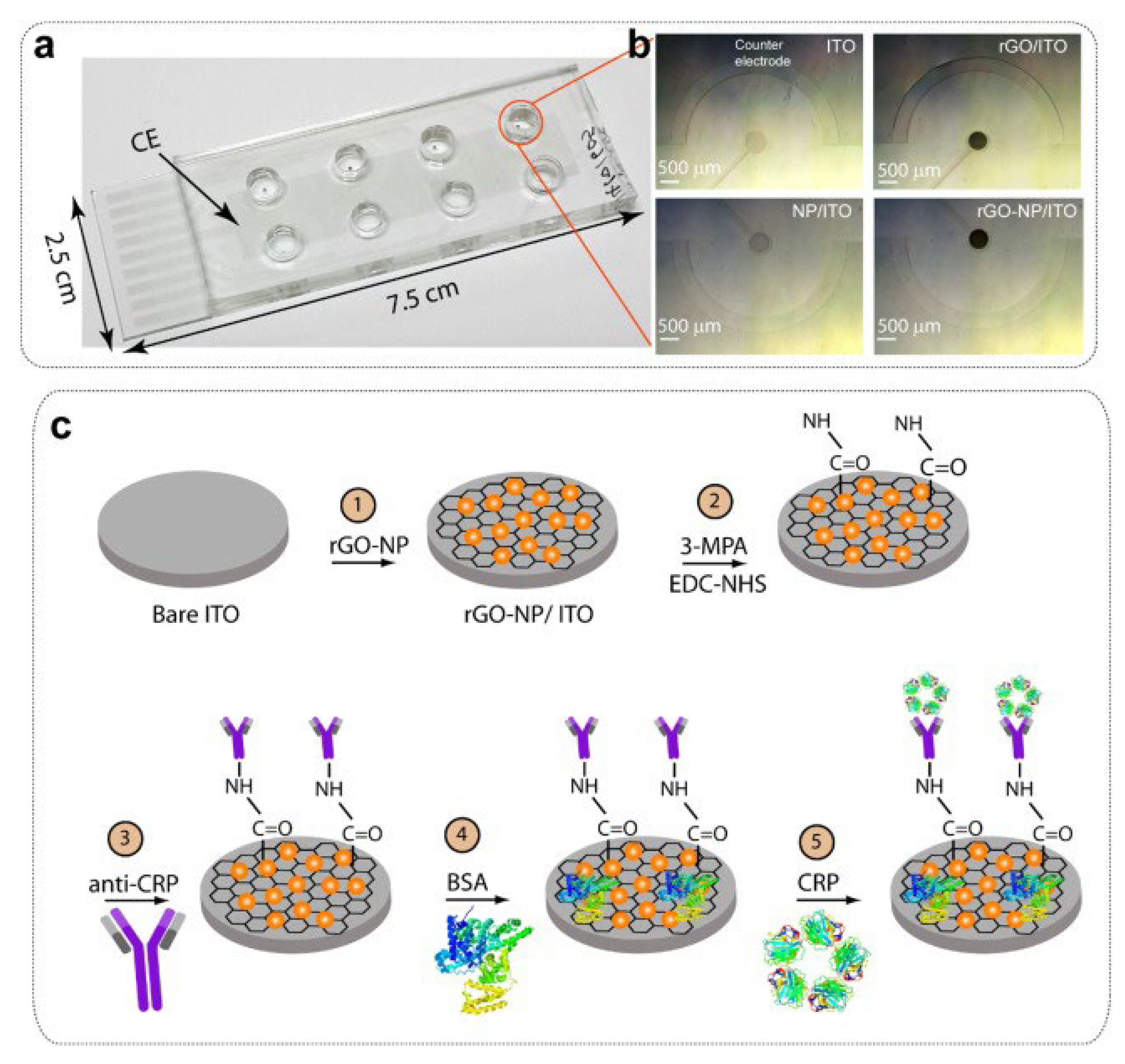
- Yagati, A.K.; Pyun, Y.-C.; Min, J.; Cho, S. Label-free and direct detection of C-reactive protein using reduced graphene oxide-nanoparticle hybrid impedimetric sensor. Bioelectrochemistry 2016, 107, 37–44. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection, Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
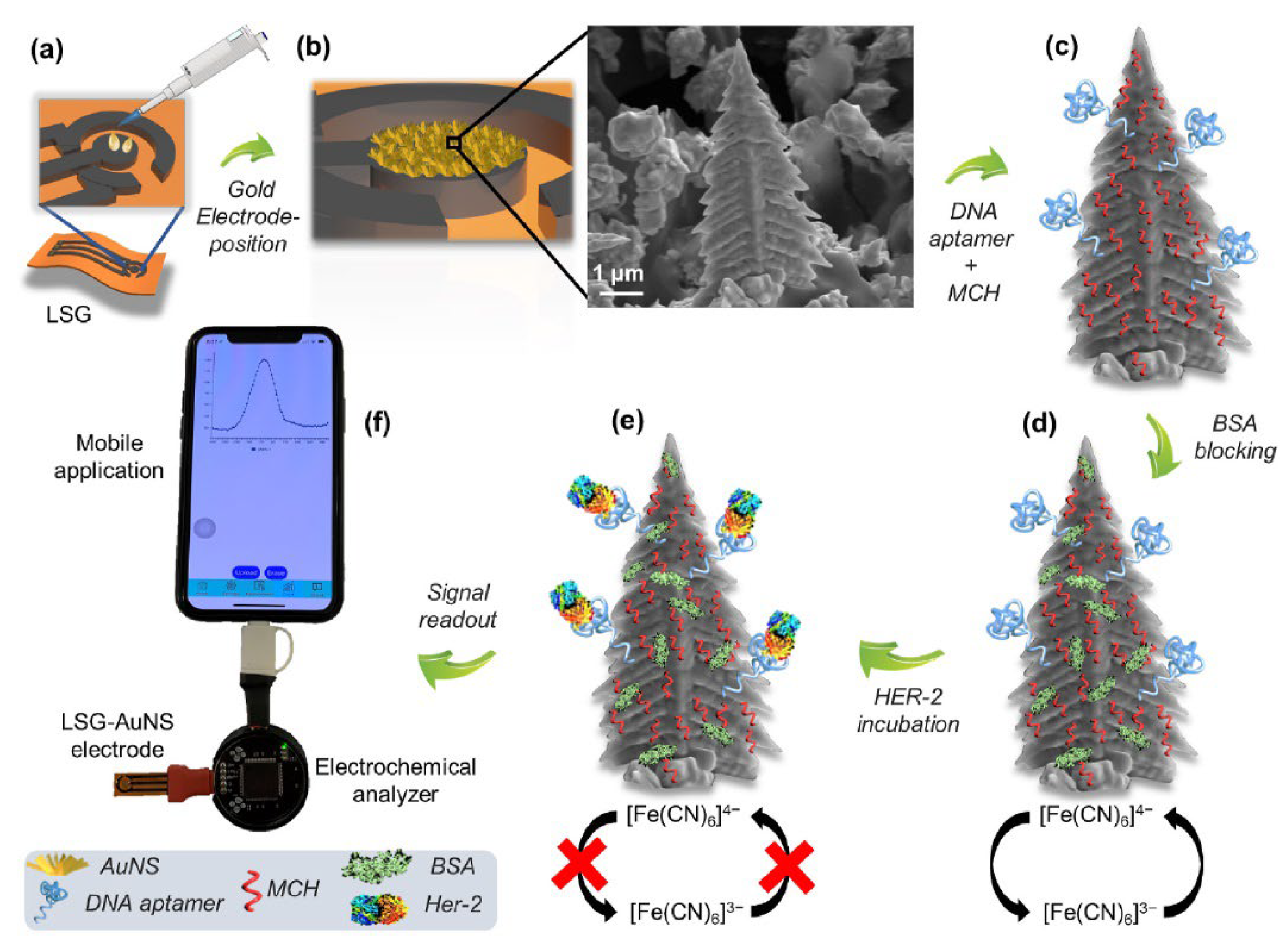
- Rauf, S.; Lahcen, A.A.; Aljedaibi, A.; Beduk, T.; Filho, J.I.O.; Salama, K.N. Gold nanostructured laser-scribed graphene: A new electrochemical biosensing platform for potential point-of-care testing of disease biomarkers. Biosens. Bioelectron. 2021, 180, 113116. [Google Scholar] [CrossRef]
- Shuai, H.-L.; Huang, K.-J.; Xing, L.-L.; Chen, Y.-X. Ultrasensitive electrochemical sensing platform for microRNA based on tungsten oxide-graphene composites coupling with catalyzed hairpin assembly target recycling and enzyme signal amplification. Biosens. Bioelectron. 2016, 86, 337–345. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Li, X.; Li, R.; Du, B.; Wei, Q. Electrochemical immunosensor for detecting typical bladder cancer biomarker based on reduced graphene oxide–tetraethylenepentamine and trimetallic AuPdPt nanoparticles. Talanta 2015, 143, 77–82. [Google Scholar] [CrossRef]
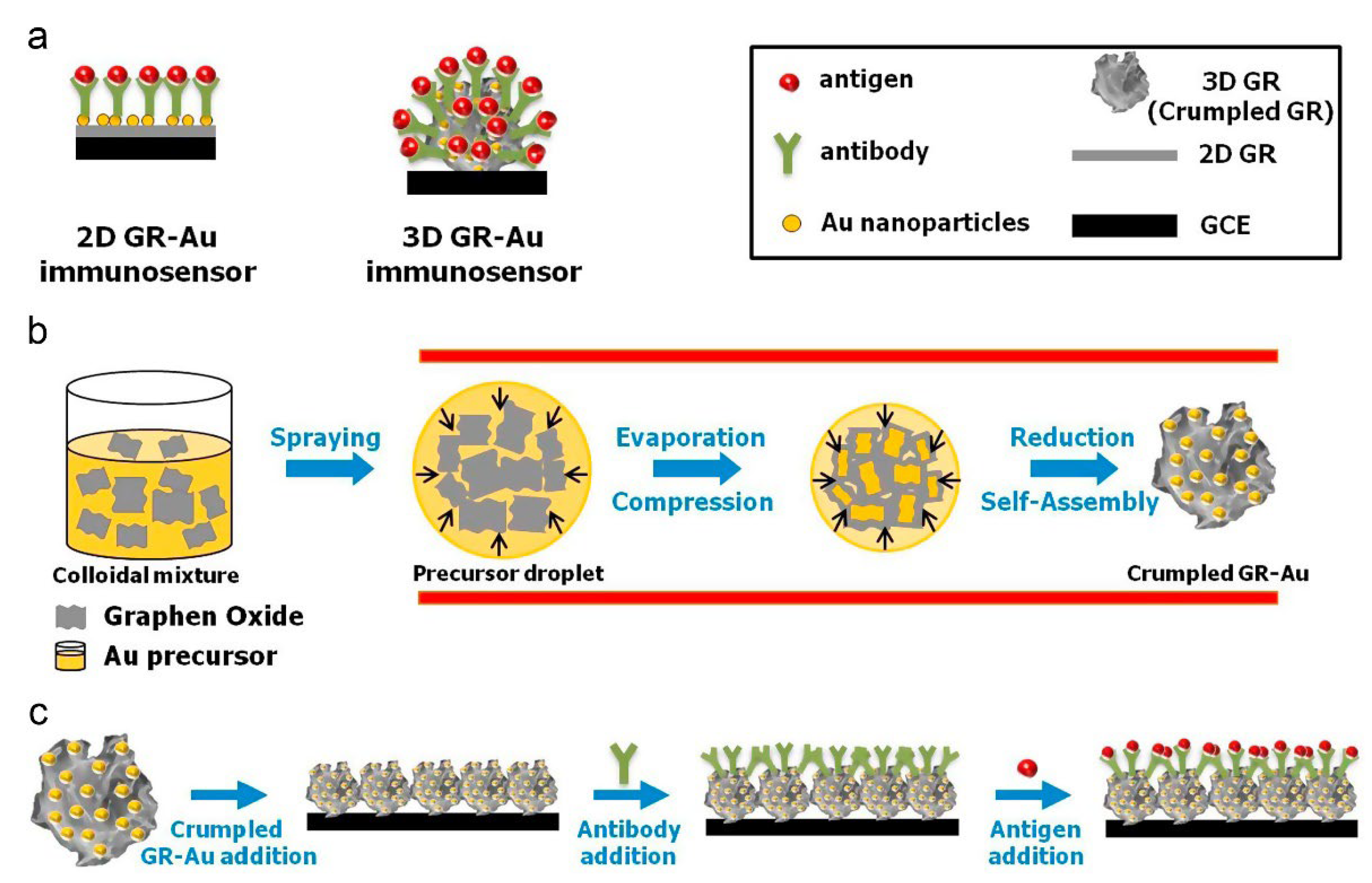
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.-W. 3D label-free prostate specific antigen (PSA) immunosensor based on graphene–gold composites. Biosens. Bioelectron. 2015, 63, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Darvishi, S.; Preet, A.; Huang, T.-Y.; Lin, S.-H.; Girault, H.H.; Wang, L.; Lin, T.-E. A Review: Electrochemical Biosensors for Oral Cancer. Chemosensors 2020, 8, 54. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Dehghani, S.; Nosrati, R.; Yousefi, M.; Nezamid, A.; Soltani, F.; Taghdisif, S.M.; Abnous, K.; Alibolandi, M.; Ramezani, M. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosens. Bioelectron. 2018, 110, 23–37. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Pillozzi, S.; Palchetti, I. Electrochemical and PEC platforms for miRNA and other epigenetic markers of cancer diseases: Recent updates. Electrochem. Commun. 2021, 124, 106929. [Google Scholar] [CrossRef]
- Campuzano, S.; Barderas, R.; Pedrero, M.; Yanez-Sedeno, P.; Pingarron, J.M. Electrochemical biosensing to move forward in cancer epigenetics and metastasis: A review, Anal. Chim. Acta 2020, 1109, 169–190. [Google Scholar] [CrossRef]
- Yan, M.; Sun, G.; Liu, F.; Lu, J.; Yu, J.; Song, X. An aptasensor for sensitive detection of human breast cancer cells byusing porous GO/Au composites and porous PtFe alloy as effectivesensing platform and signal amplification labels. Anal. Chim. Acta 2013, 798, 33–39. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Yoon, H.; Park, J.Y. Trimetallic Pd@Au@Pt nanocomposites platform on -COOH terminated reduced graphene oxide for highly sensitive CEA and PSA biomarkers detection. Biosens.Bioelectron. 2018, 100, 16–22. [Google Scholar] [CrossRef]
- Esteban-Fernandez de Ávila, B.; Araque, E.; Campuzano, S.; Pedrero, M.; Dalkiran, B.; Barderas, B.; Villalonga, R.; Kiliç, E.; Pingarrón, J.M. Dual Functional Graphene Derivative-Based Electrochemical Platforms for Detection of the TP53 Gene with Single Nucleotide Polymorphism Selectivity in Biological Samples. Anal. Chem. 2015, 87, 2290–2298. [Google Scholar] [CrossRef]
- Gorgannezhad, L.; Umer, M.; Masud, M.K.; Hossain, M.S.A.; Tanaka, S.; Yamauchi, Y.; Salomon, C.; Kline, R.; Nguyen, N.-T.; Shiddiky, M.J.A. Detection of FGFR2:FAM76A Fusion Gene in Circulating Tumor RNA Based on Catalytic Signal Amplification of Graphene Oxide-loaded Magnetic Nanoparticles. Electroanalysis 2018, 30, 2293–2301. [Google Scholar] [CrossRef]
- He, L.; Wang, Q.; Mandler, D.; Li, M.; Boukherroub, R.; Szunerits, S. Detection of folic acid protein in human serum using reduced graphene oxide electrodes modified by folic-acid. Biosens. Bioelectron. 2016, 75, 389–395. [Google Scholar] [CrossRef]
- Islam, M.N.; Gorgannezhad, L.; Masud, M.K.; Tanaka, S.; Hossain, M.S.A.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Graphene-Oxide-Loaded Superparamagnetic Iron Oxide Nanoparticles for Ultrasensitive Electrocatalytic Detection of MicroRNA. ChemElectroChem 2018, 5, 2488–2495. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Z.; Han, H.; Ma, Z. Graphene oxide reduced directly by redox probes for multiplexed detection of tumor markers. J. Mater. Chem. B 2014, 2, 3292–3298. [Google Scholar] [CrossRef]
- Rauf, S.; Mishra, G.K.; Azhar, J.; Mishra, R.K.; Goud, K.Y.; Nawaza, M.A.H.; Marty, J.L.; Hayat, A. Carboxylic group riched graphene oxide based disposable electrochemical immunosensor for cancer biomarker detection. Anal. Biochem. 2018, 545, 13–19. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Zhang, T.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. Sulfur-Doped Graphene-Based Immunological Biosensing Platform for Multianalysis of Cancer Biomarkers. ACS Appl. Mater. Interfaces 2017, 9, 37637–37644. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidic, S.A.; Koo, C.M. Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancerbiomarker detection. Sens. Actuators B 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Shuai, S.H.L.; Huang, K.-J.; Zhang, W.-J.; Cao, X.; Jia, M.-P. Sandwich-type microRNA biosensor based on magnesium oxidenanoflower and graphene oxide–gold nanoparticles hybrids couplingwith enzyme signal amplification. Sens. Actuators B 2017, 243, 403–411. [Google Scholar] [CrossRef]
- Tabasi, A.; Noorbakhsh, A.; Sharifi, E. Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens. Bioelectron. 2017, 95, 117–123. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, L.; Ma, Z. Triple sensitivity amplification for ultrasensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2017, 92, 577–582. [Google Scholar] [CrossRef]
- Yadegari, A.; Omidi, M.; Yazdian, F.; Zalia, H.; Tayebide, L. An electrochemical cytosensor for ultrasensitive detection of cancer cells using modified graphene–gold nanostructures. RSC Adv. 2017, 7, 2365–2372. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.-H.; Saianand, G.; Gopalan, A.-I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; Hasanzadeh, M.; Saadati, A.; Shadjou, N.; Soleymani, J.; Jouyban, A. A novel paper based immunoassay of breast cancer specific carbohydrate (CA 15.3) using silver nanoparticles-reduced graphene oxide nano-ink technology: A new platform to construction of microfluidic paper-based analytical devices (μPADs) towards biomedical analysis. Microchem. J. 2019, 146, 345–358. [Google Scholar]
- Kumar, V.; Srivastava, S.; Umrao, S.; Kumar, R.; Nath, G.; Sumana, G.; Saxena, P.S.; Srivastava, A. Nanostructured palladium-reduced graphene oxide platform for high sensitive, label free detection of a cancer biomarker. RSC Adv. 2014, 4, 2267–2273. [Google Scholar] [CrossRef]
- Tian, L.; Liu, L.; Li, Y.; Wei, Q.; Cao, W. 3D sandwich-type prostate specific antigen (PSA) immunosensor based on rGO–MWCNT–Pd nanocomposite. New J. Chem. 2015, 39, 5522–5528. [Google Scholar] [CrossRef]
- Bharti, A.; Agnihotri, N.; Prabhakar, N. A voltammetric hybridization assay for microRNA-21 using carboxylated graphene oxide decorated with gold-platinum bimetallic nanoparticles. Microchim. Acta 2019, 186, 185. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Sheng, K.; Zhou, Q.; Dong, B.; Bai, X.; Lu, G.; Song, H. A label-free electrochemical immunosensor based on facet-controlled Au nanorods/reduced graphene oxide composites for prostate specific antigen detection. Sens. Actuators B 2021, 336, 129748. [Google Scholar] [CrossRef]
- Congur, G.; Eksin, E.; Erdem, A. Impedimetric detection of miRNA-34a using graphene oxide modified chemically activated graphite electrodes. Sens. Actuators A 2018, 279, 493–500. [Google Scholar] [CrossRef]
- Feng, T.; Chen, X.; Qiao, X.; Sun, Z.; Wang, H.; Qi, Y. Chenglin Hong Graphene oxide supported rhombic dodecahedral Cu2O nanocrystals for the detection of carcinoembryonic antigen. Anal. Biochem. 2016, 494, 101–107. [Google Scholar] [CrossRef]
- Feng, J.; Li, Y.; Li, M.; Li, F.; Han, J.; Donga, Y.; Chen, Z.; Wang, P.; Liu, H.; Wei, Q. A novel sandwich-type electrochemical immunosensor for PSA detection based on PtCu bimetallic hybrid (2D/2D) rGO/g-C3N4. Biosens. Bioelectron. 2017, 91, 441–448. [Google Scholar] [CrossRef]
- Jonous, Z.A.; Shayeh, J.S.; Yazdian, F.; Yadegari, A.; Hashemi, M.; Omidi, M. An electrochemical biosensor for prostate cancer biomarker detection using graphene oxide–gold nanostructures. Eng. Life Sci. 2019, 19, 206–216. [Google Scholar] [CrossRef]
- Pachauri, N.; Dave, K.; Dindab, A.; Solanki, P.R. Cubic CeO2 implanted reduced graphene oxidebased highly sensitive biosensor for non-invasive oral cancer biomarker detection. J. Mater. Chem. B 2018, 6, 3000–3012. [Google Scholar] [CrossRef]
- Pal, M.; Khan, R. Graphene oxide layer decorated gold nanoparticles based immunosensor for the detection of prostate cancer risk factor. Anal. Biochem. 2017, 536, 51–58. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Askari, E.; Molaabasi, F.; Sadr, A.; Zare, Y.; Afsharpad, M.; Rhee, K.Y. A rapid nanobiosensing platform based on herceptin-conjugated graphene for ultrasensitive detection of circulating tumor cells in early breast cancer. Nanotechnol. Rev. 2021, 10, 744–753. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kashanian, S.; Naghib, S.M.; Arkan, E. A high-performance electrochemical aptasensor based on graphene-decorated rhodium nanoparticles to detect HER2-ECD oncomarker in liquid biopsy. Sci. Rep. 2022, 12, 3299. [Google Scholar] [CrossRef]
- Tang, P.; Zhang, H.; Huo, J.; Lin, X. An electrochemical sensor based on iron(II,III)@graphene oxide@molecularly imprinted polymer nanoparticles for interleukin-8 detection in saliva. Anal. Methods 2015, 7, 7784. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.P. Non-invasive oral cancer detection from saliva using zinc oxide–reduced graphene oxide nanocomposite based bioelectrode. MRS Commun. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Yammouri, G.; Mandli, J.; Mohammadi, H.; Amine, A. Development of an electrochemical label-free biosensor for microRNA-125a detection using pencil graphite electrode modified with different carbon nanomaterials. J. Electroanal. Chem. 2017, 806, 75–81. [Google Scholar] [CrossRef]
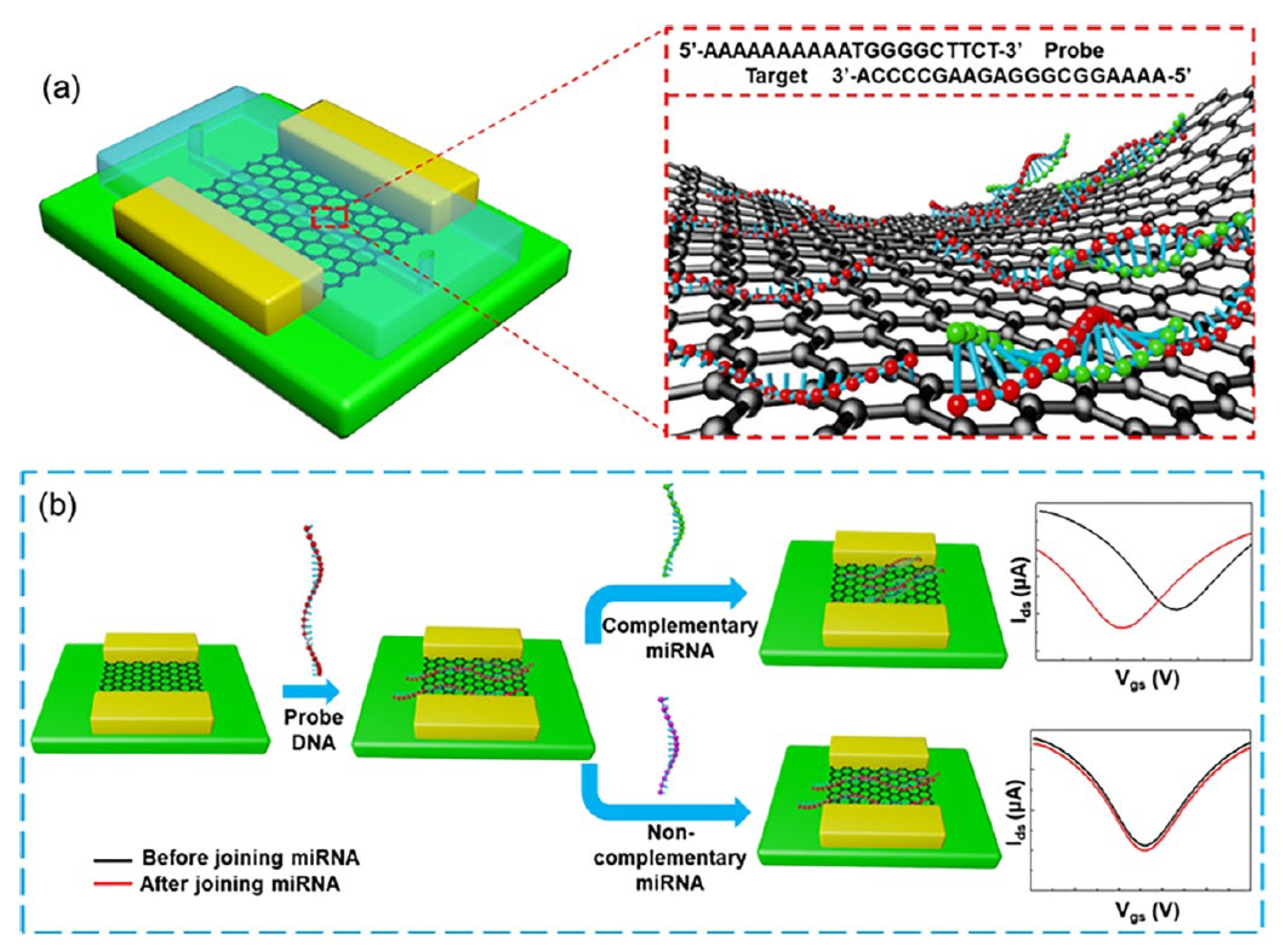
- Cai, B.; Huang, L.; Zhang, H.; Sun, Z.; Zhang, Z.; Zhang, G.-J. Gold nanoparticles-decorated graphene field-effect transistor biosensor for femtomolar Micro RNA detection. Biosens. Bioelectron. 2015, 74, 329–334. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Z.; Gu, Y.; Li, Y.; Jia, Y. Clinical available circulating tumor cell assay based on tetra(4-aminophenyl) porphyrin mediated reduced graphene oxide field effect transistor. Electrochim. Acta 2019, 313, 415–422. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, H.G.; Park, W.H.; Jeon, D.C.; Lim, K.M.; Kim, H.J.; Jang, B.K.; Song, K.S. Detection of Alpha-Fetoprotein in Hepatocellular Carcinoma Patient Plasma with Graphene Field-Effect Transistor. Sensors 2018, 18, 4032. [Google Scholar] [CrossRef]
- Lee, D.-H.; Cho, H.-S.; Han, D.; Chand, R.; Yoon, T.-J.; Kim, Y.-S. Highly selective organic transistor biosensor with inkjet printed graphene oxide support system. J. Mater. Chem. B 2017, 5, 3580. [Google Scholar] [CrossRef]
- Majd, S.M.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef]
- Zhou, L.; Mao, H.; Wu, C.; Tang, L.; Wu, Z.; Sun, H.; Zhang, H.; Zhou, H.; Jia, C.; Jin, Q.; et al. Label-free graphene biosensor targeting cancer molecules based on non-covalent modification. Biosens. Bioelectron. 2017, 87, 701–707. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Pumera, M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect? ACS Nano 2020, 14, 21–25. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart Biosensors for Cancer Diagnosis Based on Graphene Quantum Dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef]
- Ko, C.-N.; Li, G.; Leung, C.-H.; Ma, D.-L. Dual Function Luminescent Transition Metal Complexes for Cancer Theranostics: The Combination of Diagnosis and Therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Wong, X.-Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoci, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Ozkan-Ariksoysal, D. Electrochemical determination of Cystic Fibrosis gene mutation by magnetic beads-based disposable kit-type biosensor. J. Electrochem. Soc. 2017, 164, B258–B266. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 1, 418–464. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan-Ariksoysal, D. Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics. Biosensors 2022, 12, 607. https://doi.org/10.3390/bios12080607
Ozkan-Ariksoysal D. Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics. Biosensors. 2022; 12(8):607. https://doi.org/10.3390/bios12080607
Chicago/Turabian StyleOzkan-Ariksoysal, Dilsat. 2022. "Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics" Biosensors 12, no. 8: 607. https://doi.org/10.3390/bios12080607
APA StyleOzkan-Ariksoysal, D. (2022). Current Perspectives in Graphene Oxide-Based Electrochemical Biosensors for Cancer Diagnostics. Biosensors, 12(8), 607. https://doi.org/10.3390/bios12080607





