Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. Preparation of Ti3C2 MXene
2.4. Preparation of OMC@Ti3C2 MXene
2.5. Fabrication of the Aptasensor
2.6. Pretreatment of Milk Samples
3. Results and Discussion
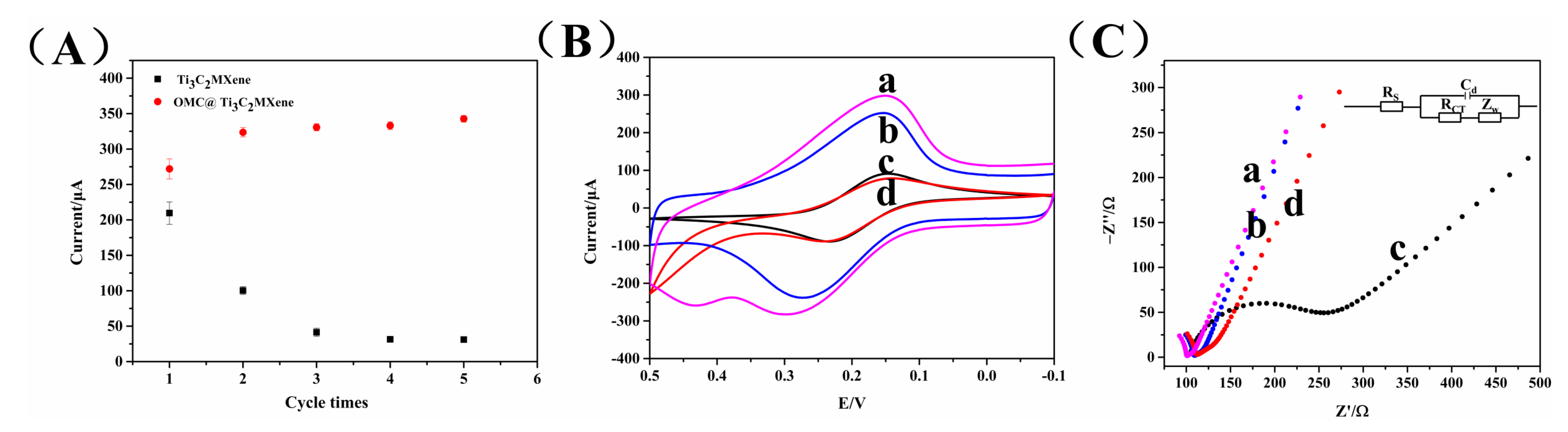
3.1. Characterization of Nanomaterials
3.2. Electrochemical Characterization of Assembly Fabrication of the Aptasensor
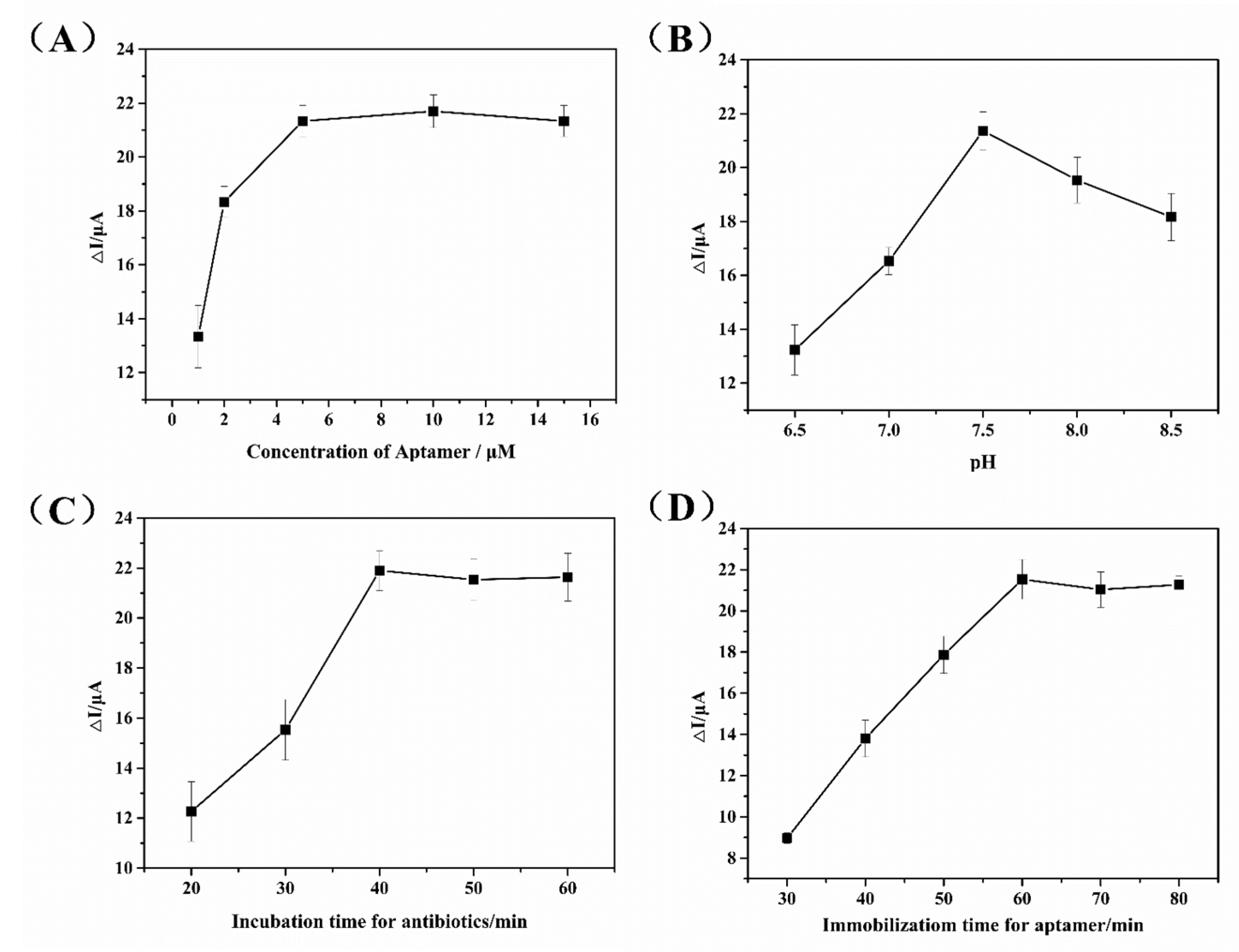
3.3. Optimization of Experimental Conditions
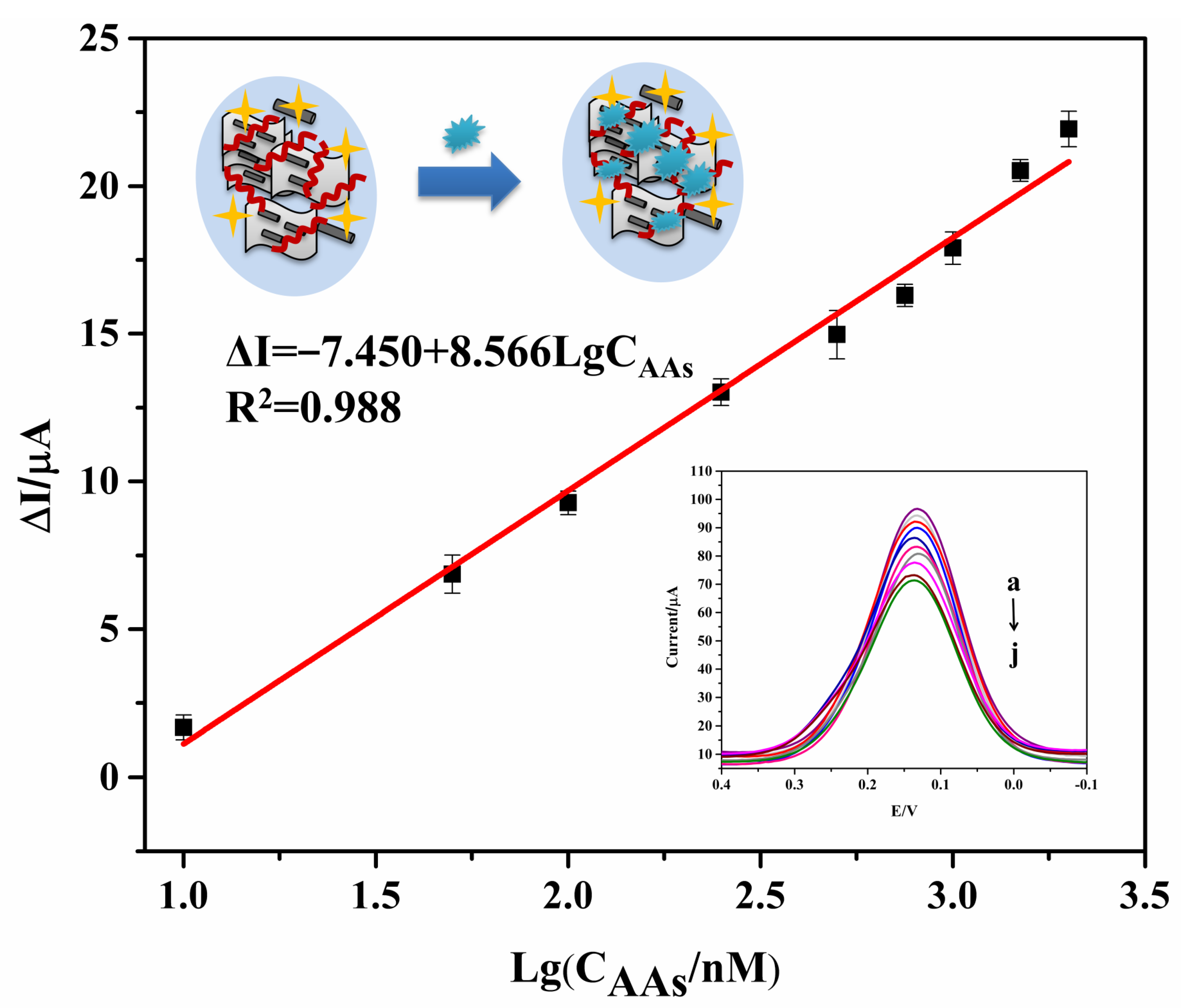
3.4. Electrochemical Analysis of the Aptasensor
3.5. Specificity, Stability and Reproducibility of the Aptasensor
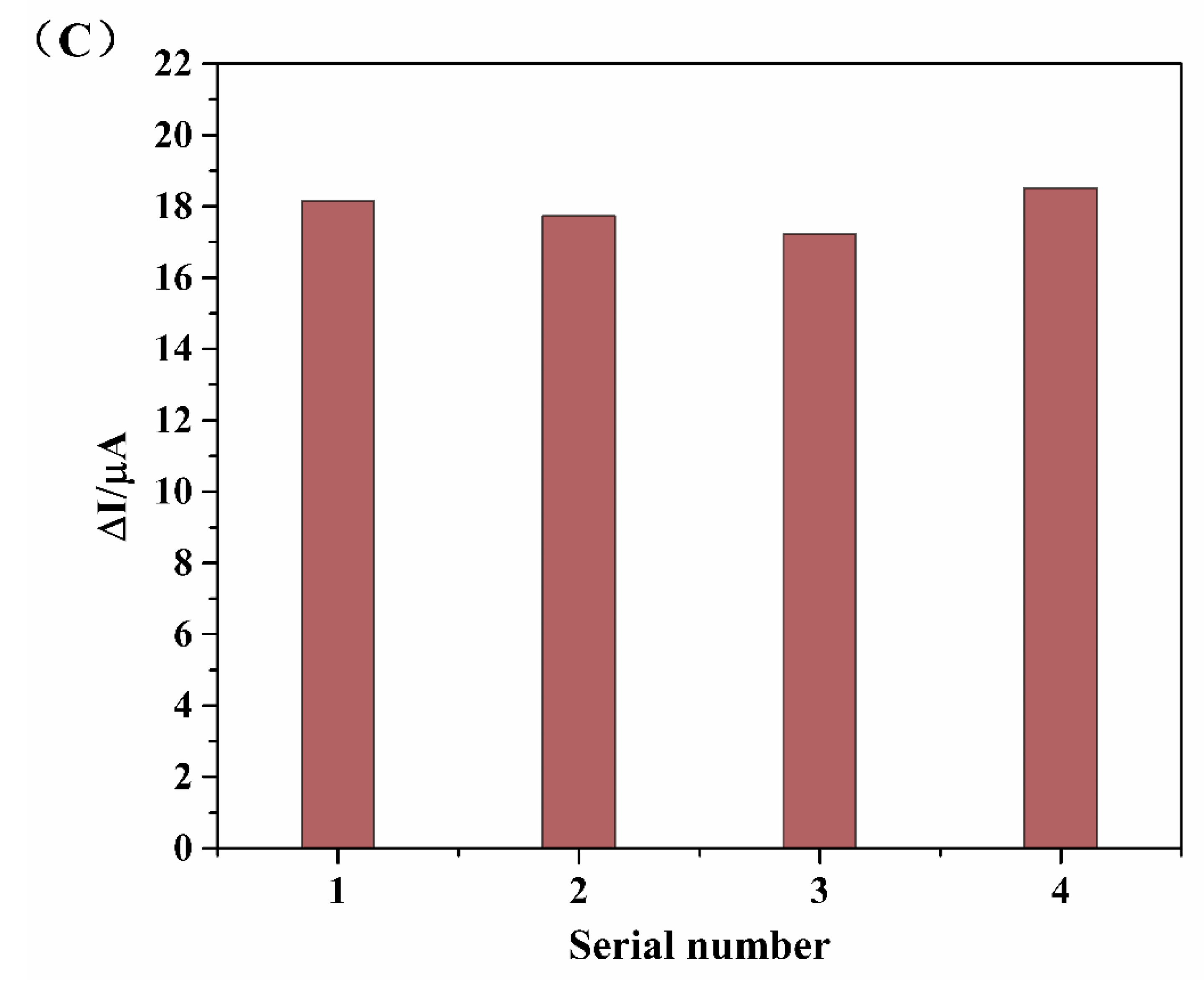
3.6. Analysis in Milk Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Faria, L.V.; Lisboa, T.P.; Campos, N.d.S.; Alves, G.F.; Matos, M.A.C.; Matos, R.C.; Munoz, R.A.A. Electrochemical methods for the determination of antibiotic residues in milk: A critical review. Anal. Chim. Acta 2021, 1173, 338569. [Google Scholar] [CrossRef] [PubMed]
- Jospe-Kaufman, M.; Siomin, L.; Fridman, M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 2020, 30, 127218. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.K.; Wu, C.Z.; Saunders, J.E.; McMahon, C.M. A scoping review of global aminoglycoside antibiotic overuse: A potential opportunity for primary ototoxicity prevention. Res. Soc. Adm. Pharm. 2022, 18, 3220–3229. [Google Scholar]
- Derbyshire, N.; White, S.J.; Bunka, D.; Song, L.; Stead, S.; Tarbin, J.; Sharman, M.; Zhou, D.; Stockley, P.G. Toggled RNA Aptamers Against Aminoglycosides Allowing Facile Detection of Antibiotics Using Gold Nanoparticle Assays. Anal. Chem. 2012, 84, 6595–6602. [Google Scholar] [CrossRef]
- Dillard, L.K.; Martinez, R.X.; Perez, L.L.; Fullerton, A.M.; Chadha, S.; McMahon, C.M. Prevalence of aminoglycoside-induced hearing loss in drug-resistant tuberculosis patients: A systematic review. J. Infect. 2021, 83, 27–36. [Google Scholar] [PubMed]
- Iqbal, M.O.; Yahya, E.B. In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell 2021, 72, 101525. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Evanescent wave aptasensor for continuous and online aminoglycoside antibiotics detection based on target binding facilitated fluorescence quenching. Biosens. Bioelectron. 2018, 102, 646–651. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, F.; Lu, Y.; Yang, B.; Zhang, F.; Liang, X. Study of matrix effects for liquid chromatography–electrospray ionization tandem mass spectrometric analysis of 4 aminoglycosides residues in milk. J. Chromatogr. A 2016, 1437, 8–14. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kang, H.S. Multi-residue determination of twenty aminoglycoside antibiotics in various food matrices by dispersive solid phase extraction and liquid chromatography-tandem mass spectrometry. Food Control 2021, 130, 108374. [Google Scholar] [CrossRef]
- Guironnet, A.; Sanchez-Cid, C.; Vogel, T.M.; Wiest, L.; Vulliet, E. Aminoglycosides analysis optimization using ion pairing liquid chromatography coupled to tandem mass spectrometry and application on wastewater samples. J. Chromatogr. A 2021, 1651, 462133. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, Y.; Zhang, J.; Jin, Y.; Zhao, W.; Zhang, Y.; Huang, J.; Wang, P.; Wu, C.; et al. Optimization and application of parallel solid-phase extraction coupled with ultra-high performance liquid chromatography–tandem mass spectrometry for the determination of 11 aminoglycoside residues in honey and royal jelly. J. Chromatogr. A 2018, 1542, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Glinka, M.; Wojnowski, W.; Wasik, A. Determination of aminoglycoside antibiotics: Current status and future trends. TrAC Trends Anal. Chem. 2020, 131, 116034. [Google Scholar] [CrossRef]
- Paul, P.; Sänger-van de Griend, C.; Adams, E.; Van Schepdael, A. Recent advances in the capillary electrophoresis analysis of antibiotics with capacitively coupled contactless conductivity detection. J. Pharm. Biomed. Anal. 2018, 158, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Duan, N.; Sun, Y.; Zhou, Y.; Ma, P.; Wu, S.; Wang, Z. High-affinity aptamer of allergen β-lactoglobulin: Selection, recognition mechanism and application. Sens. Actuators B Chem. 2021, 340, 129956. [Google Scholar] [CrossRef]
- Feagin, T.A.; Nicolò, M.; Tom, S.H. Strategies for Creating Structure-Switching Aptamers. ACS Sens. 2018, 3, 1611–1615. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.S.; Huang, Q. Aptamer-functionalized Au nanoparticles array as the effective SERS biosensor for label-free detection of interleukin-6 in serum. Sens. Actuators B Chem. 2021, 334, 129607. [Google Scholar] [CrossRef]
- Yue, F.; Li, H.; Kong, Q.; Liu, J.; Wang, G.; Li, F.; Yang, Q.; Chen, W.; Guo, Y.; Sun, X. Selection of broad-spectrum aptamer and its application in fabrication of aptasensor for detection of aminoglycoside antibiotics residues in milk. Sens. Actuators B Chem. 2022, 351, 130959. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Chen, S.; Neis, M.; Zhou, L.; Zhang, Y.; Lo, Y.; Tanner, J.A.; Kreidenweiss, A.; Offenhäusser, A.; Mayer, D. Multi-target electrochemical malaria aptasensor on flexible multielectrode arrays for detection in malaria parasite blood samples. Sens. Actuators B Chem. 2021, 349, 130812. [Google Scholar] [CrossRef]
- Forouzanfar, S.; Alam, F.; Pala, N.; Wang, C. Highly sensitive label-free electrochemical aptasensors based on photoresist derived carbon for cancer biomarker detection. Biosens. Bioelectron. 2020, 170, 112598. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Zhu, B.; Liu, J.; Zhu, X.; Xu, M.; Travas-Sejdic, J. Electrochemical aptasensor for 17β-estradiol using disposable laser scribed graphene electrodes. Biosens. Bioelectron. 2021, 185, 113247. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Sonne, C.; Kim, K.H.; Kumar, V. Nanomaterial-based aptasensors as an efficient substitute for cardiovascular disease diagnosis: Future of smart biosensors. Biosens. Bioelectron. 2021, 193, 113617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Liu, B.; Zhang, D.; Zhang, C.; Guo, Y.; Chu, X.; Wang, W.; Wang, H.; Yan, X.; et al. Signal enhancing strategies in aptasensors for the detection of small molecular contaminants by nanomaterials and nucleic acid amplification. Talanta 2022, 236, 122866. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.; Jia, H.; Tian, Q.; Liu, P.; Chen, S.; Cai, Y.; Lu, X.; Duan, X.; Lu, L. Hierarchical Ti3C2 MXene-derived sodium titanate nanoribbons/PEDOT for signal amplified electrochemical immunoassay of prostate specific antigen. J. Electroanal. Chem. 2020, 860, 113869. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Koyappayil, A.; Kim, T.; Cheon, Y.P.; Lee, M.H. A MoS2@Ti3C2Tx MXene hybrid-based electrochemical aptasensor (MEA) for sensitive and rapid detection of Thyroxine. Bioelectrochemistry 2021, 137, 107674. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, F.; Zhang, Y.; Wang, H.; Liu, Y. Ti3C2 MXene mediated Prussian blue in situ hybridization and electrochemical signal amplification for the detection of exosomes. Talanta 2021, 224, 121879. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, Q.; Wang, F.; Liu, Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens. Bioelectron. 2019, 124–125, 184–190. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Sun, X.; Guo, Y. Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles. Sens. Actuators B Chem. 2018, 265, 217–226. [Google Scholar] [CrossRef]
- Wen, J.; Fu, Q.; Wu, W.; Hong, G.; Zhang, X.; Wang, B. Understanding the Different Diffusion Mechanisms of Hydrated Protons and Potassium Ions in Titanium Carbide MXene. Acs Appl. Mater. Inter. 2019, 11, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- Fei, A.; Liu, Q.; Huang, J.; Qian, J.; Dong, X.; Qiu, B.; Mao, H.; Wang, K. Label-free impedimetric aptasensor for detection of femtomole level acetamiprid using gold nanoparticles decorated multiwalled carbon nanotube-reduced graphene oxide nanoribbon composites. Biosens. Bioelectron. 2015, 70, 122–129. [Google Scholar] [CrossRef] [PubMed]




| Analyte | GEN | NEO | KAN | AMI | PAR | APR | SPE | TOB | DH-STR | STR |
|---|---|---|---|---|---|---|---|---|---|---|
| Precursor ion (m/z) | 478.4 | 615.4 | 485.3 | 586.4 | 616.0 | 540.5 | 333.3 | 468.4 | 584.3 | 582.4 |
| Product ion (m/z) | 160.2 a 157.3 | 323.3 293.3 a | 324.2 163.2 a | 425.3 a 324.2 | 455.1 160.9 a | 378.2 a 217.2 | 189.2 140.2 a | 205.2 163.2 a | 409.3 263.3 a | 407.4 263.3 a |
| Sample | Background (nM) | Added (nM) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0.00 | 0.00 | 0.00 | -- | -- |
| 2 | 0.00 | 50 | 53.45 | 106.90 | 1.59 |
| 3 | 0.00 | 100 | 101.35 | 101.35 | 1.67 |
| 4 | 0.00 | 500 | 485.06 | 97.01 | 4.53 |
| 5 | 0.00 | 1000 | 1023.32 | 102.33 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, F.; Liu, M.; Bai, M.; Hu, M.; Li, F.; Guo, Y.; Vrublevsky, I.; Sun, X. Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk. Biosensors 2022, 12, 626. https://doi.org/10.3390/bios12080626
Yue F, Liu M, Bai M, Hu M, Li F, Guo Y, Vrublevsky I, Sun X. Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk. Biosensors. 2022; 12(8):626. https://doi.org/10.3390/bios12080626
Chicago/Turabian StyleYue, Fengling, Mengyue Liu, Mengyuan Bai, Mengjiao Hu, Falan Li, Yemin Guo, Igor Vrublevsky, and Xia Sun. 2022. "Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk" Biosensors 12, no. 8: 626. https://doi.org/10.3390/bios12080626
APA StyleYue, F., Liu, M., Bai, M., Hu, M., Li, F., Guo, Y., Vrublevsky, I., & Sun, X. (2022). Novel Electrochemical Aptasensor Based on Ordered Mesoporous Carbon/2D Ti3C2 MXene as Nanocarrier for Simultaneous Detection of Aminoglycoside Antibiotics in Milk. Biosensors, 12(8), 626. https://doi.org/10.3390/bios12080626






