Nanosensor Applications in Plant Science
Abstract
:1. Introduction
2. The Designs and Principles of Nanosensors Used in Plant Science
2.1. Förster Resonance Energy Transfer-Based Nanosensors
2.1.1. Genetically Encoded FRET-Based Nanosensors
2.1.2. Exogenously Applied FRET-Based Nanosensors
2.2. Surface-Enhanced Raman Scattering Nanosensors
2.3. Electrochemical Nanosensors
2.4. Piezoelectric Nanosensors
2.5. Nanoparticles in a Living Plant or Plant Organelles
3. Nanosensor Applications in Plants
3.1. Detection of Molecular Oxygen
3.2. Water and Humidity Nanosensors
3.3. Detection of Adenosine Triphosphate
3.4. Detection of Calcium Ions
3.5. Detection of Reactive Oxygen Species
3.6. Detection of Nitric Oxide
3.7. Detection of Plant Hormones
3.8. Determination of Fruit Ripening
3.9. Plant Pathogen Detection
3.10. Fertiliser and Pesticide Management
3.11. Future Directions
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, A.; Liebisch, F.; Hund, A. Plant Phenotyping: From Bean Weighing to Image Analysis. Plant Methods 2015, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Mendel, G. Verhandlungen Des Naturforschenden Vereines in Brünn, Bd. IV Für Das Jahr, 1865, Abhandlungen. In Proceedings of the Versuche über Pflanzenhybriden, Brno, Czech Republic; 1865. Available online: https://www.biodiversitylibrary.org/part/175272 (accessed on 12 August 2022).
- Mendel, G.; Bateson, W. Experiments in Plant Hybridization (EN Transl.). J. R. Hortic. Soc. 1901, 26, 1–32. [Google Scholar]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Chen, D.; Gillani, Z.; Klukas, C.; Chen, M. Advanced Phenotyping and Phenotype Data Analysis for the Study of Plant Growth and Development. Front. Plant Sci. 2015, 6, 619. [Google Scholar] [CrossRef] [Green Version]
- Sagadevan, S.; Periasamy, M. Recent Trends in Nanobiosensors and Their Applications—A Review. Rev. Adv. Mater. Sci. 2014, 36, 62–69. [Google Scholar]
- Kosaka, P.M.; Pini, V.; Ruz, J.J.; Da Silva, R.A.; González, M.U.; Ramos, D.; Calleja, M.; Tamayo, J. Detection of Cancer Biomarkers in Serum Using a Hybrid Mechanical and Optoplasmonic Nanosensor. Nat. Nanotechnol. 2014, 9, 1047–1053. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Hörmann, F.; Lalonde, S.; Brady, S.M.; Orlando, D.A.; Benfey, P.; Frommer, W.B. Protonophore- and PH-Insensitive Glucose and Sucrose Accumulation Detected by FRET Nanosensors in Arabidopsis Root Tips. Plant J. 2008, 56, 948–962. [Google Scholar] [CrossRef] [Green Version]
- Bagal-Kestwal, D.; Kestwal, R.M.; Chiang, B.-H. Invertase-Nanogold Clusters Decorated Plant Membranes for Fluorescence-Based Sucrose Sensor. J. Nanobiotechnol. 2015, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. Probing Strigolactone Receptors in Striga Hermonthica with Fluorescence. Science 2015, 349, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan Nanoparticles: A Positive Modulator of Innate Immune Responses in Plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, M.; Krishnamurthy, S.; Shukla, D.; Kiiskila, J.; Jain, A.; Datta, R.; Sharma, N.; Sahi, S.V. Comparative Transcriptome and Proteome Analysis to Reveal the Biosynthesis of Gold Nanoparticles in Arabidopsis. Sci. Rep. 2016, 6, 21733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The New Perspective in Precision Agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.S.; Li, S.J.; Tzeng, K.C.; Cheng, T.C.; Chang, C.Y.; Chiu, C.Y.; Liao, C.Y.; Hsu, J.J.; Lin, Z.P. Fluorescence Silica Nanoprobe as a Biomarker for Rapid Detection of Plant Pathogens. Adv. Mater. Res. 2009, 79–82, 513–516. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wu, H.; Wee, E.J.H.; Trau, M.; Wang, Y.; Botella, J.R. Specific and Sensitive Isothermal Electrochemical Biosensor for Plant Pathogen DNA Detection with Colloidal Gold Nanoparticles as Probes. Sci. Rep. 2017, 7, 38896. [Google Scholar] [CrossRef] [PubMed]
- Firrao, G.; Moretti, M.; Ruiz Rosquete, M.; Gobbi, E.; Locci, R. Nanobiotransducer for Detecting Flavescence Dorée Phytoplasma. J. Plant Pathol. 2005, 87, 101–107. [Google Scholar]
- Lattanzio, V.M.T.; Nivarlet, N. Multiplex Dipstick Immunoassay for Semiquantitative Determination of Fusarium Mycotoxins in Oat. In Methods in Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 137–142. [Google Scholar]
- Zhang, Q.; Ying, Y.; Ping, J. Recent Advances in Plant Nanoscience. Adv. Sci. 2022, 9, 2103414. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.; Zhou, J. The Applications of Nanotechnology in Crop Production. Molecules 2021, 26, 7070. [Google Scholar] [CrossRef]
- Johnson, M.S.; Sajeev, S.; Nair, R.S. Role of Nanosensors in Agriculture. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 58–63. [Google Scholar]
- Kulabhusan, P.K.; Tripathi, A.; Kant, K. Gold Nanoparticles and Plant Pathogens: An Overview and Prospective for Biosensing in Forestry. Sensors 2022, 22, 1259. [Google Scholar] [CrossRef]
- Ang, M.C.-Y.; Lew, T.T.S. Non-Destructive Technologies for Plant Health Diagnosis. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Voke, E.; Pinals, R.L.; Goh, N.S.; Landry, M.P. In Planta Nanosensors: Understanding Biocorona Formation for Functional Design. ACS Sens. 2021, 6, 2802–2814. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.-O.; Kim, J. Engineering Plants with Carbon Nanotubes: A Sustainable Agriculture Approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum Dots-Fluorescence Resonance Energy Transfer-Based Nanosensors and Their Application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, F.; Xiong, X.; Peng, X. Fluorescent Nanosensors Based on Fluorescence Resonance Energy Transfer (FRET). Ind. Eng. Chem. Res. 2013, 52, 11228–11245. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.; Kang, W.P.; Davidson, J.L.; Gurbuz, Y. Review on Carbon-Derived, Solid-State, Micro and Nano Sensors for Electrochemical Sensing Applications. Diam. Relat. Mater. 2009, 18, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Wujcik, E.K.; Wei, H.; Zhang, X.; Guo, J.; Yan, X.; Sutrave, N.; Wei, S.; Guo, Z. Antibody Nanosensors: A Detailed Review. RSC Adv. 2014, 4, 43725–43745. [Google Scholar] [CrossRef]
- Medintz, I.L. Recent Progress in Developing FRET-Based Intracellular Sensors for the Detection of Small Molecule Nutrients and Ligands. Trends Biotechnol. 2006, 24, 539–542. [Google Scholar] [CrossRef]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Postma, M.; Zhou, W.; Goedhart, J.; Sánchez-Pérez, M.-I.; Gadella, T.W.J.; Simon, R.; Scheres, B.; et al. In Vivo FRET–FLIM Reveals Cell-Type-Specific Protein Interactions in Arabidopsis Roots. Nature 2017, 548, 97–102. [Google Scholar] [CrossRef]
- Jones, A.M.; Grossmann, G.; Danielson, J.Å.; Sosso, D.; Chen, L.-Q.; Ho, C.-H.; Frommer, W.B. In Vivo Biochemistry: Applications for Small Molecule Biosensors in Plant Biology. Curr. Opin. Plant Biol. 2013, 16, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Okumoto, S.; Jones, A.; Frommer, W.B. Quantitative Imaging with Fluorescent Biosensors. Annu. Rev. Plant Biol. 2012, 63, 663–706. [Google Scholar] [CrossRef] [PubMed]
- Bajar, B.; Wang, E.; Zhang, S.; Lin, M.; Chu, J. A Guide to Fluorescent Protein FRET Pairs. Sensors 2016, 16, 1488. [Google Scholar] [CrossRef] [PubMed]
- Krukenberg, K.A.; Street, T.O.; Lavery, L.A.; Agard, D.A. Conformational Dynamics of the Molecular Chaperone Hsp90. Q. Rev. Biophys. 2011, 44, 229–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krusiński, T.; Ożyhar, A.; Dobryszycki, P. Dual FRET Assay for Detecting Receptor Protein Interaction with DNA. Nucleic Acids Res. 2010, 38, e108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Immink, R.G.H.; Gadella, T.W.J.; Ferrario, S.; Busscher, M.; Angenent, G.C. Analysis of MADS Box Protein-Protein Interactions in Living Plant Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 2416–2421. [Google Scholar] [CrossRef] [Green Version]
- Boeneman, K.; Mei, B.C.; Dennis, A.M.; Bao, G.; Deschamps, J.R.; Mattoussi, H.; Medintz, I.L. Sensing Caspase 3 Activity with Quantum Dot−Fluorescent Protein Assemblies. J. Am. Chem. Soc. 2009, 131, 3828–3829. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Smet, W.; Du, Y.; Gadella, T.W.J.; Goedhart, J.; Scheres, B.; Blilou, I. Optimizing FRET-FLIM Labeling Conditions to Detect Nuclear Protein Interactions at Native Expression Levels in Living Arabidopsis Roots. Front. Plant Sci. 2018, 9, 639. [Google Scholar] [CrossRef] [Green Version]
- Camborde, L.; Jauneau, A.; Brière, C.; Deslandes, L.; Dumas, B.; Gaulin, E. Detection of Nucleic Acid-Protein Interactions in Plant Leaves Using Fluorescence Lifetime Imaging Microscopy. Nat. Protoc. 2017, 12, 1933–1950. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Hormann, F.; Frommer, W.B. Dynamic Imaging of Glucose Flux Impedance Using FRET Sensors in Wild-Type Arabidopsis Plants. J. Exp. Bot. 2011, 62, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Wang, L.; Dong, Q.; Chang, S.; Wen, K.; Jia, S.; Chu, Z.; Wang, H.; Gao, P.; Zhao, H.; et al. FRET-Based Glucose Imaging Identifies Glucose Signalling in Response to Biotic and Abiotic Stresses in Rice Roots. J. Plant Physiol. 2017, 215, 65–72. [Google Scholar] [CrossRef]
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent Proteins for High-Speed Single-Cell and Whole-Body Imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-Based Genetically Encoded Sensors Allow High-Resolution Live Cell Imaging of Ca2+ Dynamics. Plant J. 2012, 69, 181–192. [Google Scholar] [CrossRef]
- Rizza, A.; Walia, A.; Lanquar, V.; Frommer, W.B.; Jones, A.M. In Vivo Gibberellin Gradients Visualized in Rapidly Elongating Tissues. Nat. Plants 2017, 3, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Fluorometric Immunoassay for Detecting the Plant Virus Citrus Tristeza Using Carbon Nanoparticles Acting as Quenchers and Antibodies Labeled with CdTe Quantum Dots. Microchim. Acta 2016, 183, 2277–2287. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO Films Formed by Atomic Layer Deposition as an Optical Biosensor Platform for the Detection of Grapevine Virus A-Type Proteins. Biosens. Bioelectron. 2017, 92, 763–769. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Qian, J.; Wang, C.; Liu, Q.; Han, E.; Hao, N.; Zhang, L.; Cai, J.; Wang, K. A Homogeneous Assay for Highly Sensitive Detection of CaMV35S Promoter in Transgenic Soybean by Förster Resonance Energy Transfer between Nitrogen-Doped Graphene Quantum Dots and Ag Nanoparticles. Anal. Chim. Acta 2016, 948, 90–97. [Google Scholar] [CrossRef]
- Uslu, V.V.; Grossmann, G. The Biosensor Toolbox for Plant Developmental Biology. Curr. Opin. Plant Biol. 2016, 29, 138–147. [Google Scholar] [CrossRef]
- Moseyko, N.; Feldman, L.J. Expression of PH-Sensitive Green Fluorescent Protein in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 557–563. [Google Scholar] [CrossRef]
- Miesenböck, G.; De Angelis, D.A.; Rothman, J.E. Visualizing Secretion and Synaptic Transmission with PH-Sensitive Green Fluorescent Proteins. Nature 1998, 394, 192–195. [Google Scholar] [CrossRef]
- Hanson, G.T.; Aggeler, R.; Oglesbee, D.; Cannon, M.; Capaldi, R.A.; Tsien, R.Y.; Remington, S.J. Investigating Mitochondrial Redox Potential with Redox-Sensitive Green Fluorescent Protein Indicators. J. Biol. Chem. 2004, 279, 13044–13053. [Google Scholar] [CrossRef] [Green Version]
- Wierer, S.; Peter, S.; Elgass, K.; Mack, H.-G.; Bieker, S.; Meixner, A.J.; Zentgraf, U.; Schleifenbaum, F. Determination of the in vivo Redox Potential by One-Wavelength Spectro-Microscopy of RoGFP. Anal. Bioanal. Chem. 2012, 403, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A Protocol for Combining Fluorescent Proteins with Histological Stains for Diverse Cell Wall Components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.T.; Carroll, A. Identification and Use of Fluorescent Dyes for Plant Cell Wall Imaging Using High-Throughput Screening. Methods Mol. Biol. 2014, 1056, 103–109. [Google Scholar] [PubMed]
- Deuschle, K.; Chaudhuri, B.; Okumoto, S.; Lager, I.; Lalonde, S.; Frommer, W.B. Rapid Metabolism of Glucose Detected with FRET Glucose Nanosensors in Epidermal Cells and Intact Roots of Arabidopsis RNA-Silencing Mutants. Plant Cell 2006, 18, 2314–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escouboué, M.; Camborde, L.; Jauneau, A.; Gaulin, E.; Deslandes, L. Preparation of Plant Material for Analysis of Protein–Nucleic Acid Interactions by FRET-FLIM. Methods Mol. Biol. 2019, 1991, 69–77. [Google Scholar]
- Anjum, N.A.; Amreen; Tantray, A.Y.; Khan, N.A.; Ahmad, A. Reactive Oxygen Species Detection-Approaches in Plants: Insights into Genetically Encoded FRET-Based Sensors. J. Biotechnol. 2020, 308, 108–117. [Google Scholar] [CrossRef]
- Ahmad, M.; Mohsin, M.; Iqrar, S.; Manzoor, O.; Siddiqi, T.O.; Ahmad, A. Live Cell Imaging of Vitamin B12 Dynamics by Genetically Encoded Fluorescent Nanosensor. Sens. Actuators B Chem. 2018, 257, 866–874. [Google Scholar] [CrossRef]
- Chen, N.-T.; Cheng, S.-H.; Liu, C.-P.; Souris, J.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling. Int. J. Mol. Sci. 2012, 13, 16598–16623. [Google Scholar] [CrossRef]
- Pang, C.; Gong, Y. Current Status and Future Prospects of Semiconductor Quantum Dots in Botany. J. Agric. Food Chem. 2019, 67, 7561–7568. [Google Scholar] [CrossRef]
- Santos, A.R.; Miguel, A.S.; Macovei, A.; Maycock, C.; Balestrazzi, A.; Oliva, A.; Fevereiro, P. CdSe/ZnS Quantum Dots Trigger DNA Repair and Antioxidant Enzyme Systems in Medicago Sativa Cells in Suspension Culture. BMC Biotechnol. 2013, 13, 111. [Google Scholar] [CrossRef] [Green Version]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size Exclusion Limits and Lateral Heterogeneity of the Stomatal Foliar Uptake Pathway for Aqueous Solutes and Water-Suspended Nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and Distribution of Ultrasmall Anatase TiO2 Alizarin Red S Nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichiriccò, G.; Poma, A. Penetration and Toxicity of Nanomaterials in Higher Plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Al-Salim, N.; Barraclough, E.; Burgess, E.; Clothier, B.; Deurer, M.; Green, S.; Malone, L.; Weir, G. Quantum Dot Transport in Soil, Plants, and Insects. Sci. Total Environ. 2011, 409, 3237–3248. [Google Scholar] [CrossRef]
- Hong, S.; Lee, C. The Current Status and Future Outlook of Quantum Dot-Based Biosensors for Plant Virus Detection. Plant Pathol. J. 2018, 34, 85–92. [Google Scholar] [CrossRef]
- Stewart, C.N. Monitoring the Presence and Expression of Transgenes in Living Plants. Trends Plant Sci. 2005, 10, 390–396. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Wang, L.; Jiao, T.; Chen, Q. Ratiometric Upconversion Fluorometric Turn-off Nanosensor for Quantification of Furfural in Foods. Sens. Actuators B Chem. 2022, 350, 130843. [Google Scholar] [CrossRef]
- Smekal, A. Zur Quantentheorie Der Dispersion. Naturwissenschaften 1923, 11, 873–875. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, Y.; Wang, L.; Guo, C.; Sun, Y.; Li, Z.; Liu, Z. Ethanol-Induced Formation of Silver Nanoparticle Aggregates for Highly Active SERS Substrates and Application in DNA Detection. J. Phys. Chem. C 2008, 112, 1415–1422. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Alvarez-Puebla, R.A. Surface-Enhanced Raman Scattering (SERS) Nanoparticle Sensors for Biochemical and Environmental Sensing. In Nanosensors for Chemical and Biological Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 197–230. ISBN 9780857096609. [Google Scholar]
- Moskovits, M.; Suh, J.S. Surface Selection Rules for Surface-Enhanced Raman Spectroscopy: Calculations and Application to the Surface-Enhanced Raman Spectrum of Phthalazine on Silver. J. Phys. Chem. 1984, 88, 5526–5530. [Google Scholar] [CrossRef]
- Otto, A. What Is Observed in Single Molecule SERS, and Why? J. Raman Spectrosc. 2002, 33, 593–598. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy—A Practical Approach; John Wiley & Sons, Ltd.: Chichester, UK, 2005; ISBN 9780471497943. [Google Scholar]
- Le Ru, E.C.; Etchegoin, P.G. EM Enhancements and Plasmon Resonances: Examples and Discussion. In Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 299–365. [Google Scholar]
- Otto, A. Theory of First Layer and Single Molecule Surface Enhanced Raman Scattering (SERS). Phys. Status Solidi 2001, 188, 1455–1470. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780470035641. [Google Scholar]
- Zeiri, L. SERS of Plant Material. J. Raman Spectrosc. 2007, 38, 950–955. [Google Scholar] [CrossRef]
- Du, S.; Yu, C.; Tang, L.; Lu, L. Applications of SERS in the Detection of Stress-Related Substances. Nanomaterials 2018, 8, 757. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gu, X.; Zheng, C.; Dong, F.; Zhang, L.; Cai, Y.; You, Z.; You, J.; Du, S.; Zhang, Z. Ehrlich Reaction Evoked Multiple Spectral Resonances and Gold Nanoparticle Hotspots for Raman Detection of Plant Hormone. Anal. Chem. 2017, 89, 8836–8843. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Liu, M.; Qiu, C.; Yang, H.; Chen, X. In Situ Fabrication of Label-Free Optical Sensing Paper Strips for the Rapid Surface-Enhanced Raman Scattering (SERS) Detection of Brassinosteroids in Plant Tissues. Talanta 2017, 165, 313–320. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.M.; Zheng, D.W.; Lin, T.F.; Wei, X.D.; Liu, X.Y.; Wang, H.Q. Surface-Enhanced Raman Spectroscopic Analysis of N6-Benzylaminopurine Residue Quantity in Sprouts with Gold Nanoparticles. J. Environ. Sci. Health Part B 2018, 53, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Merkl, P.; Sommertune, J.; Thersleff, T.; Sotiriou, G.A. SERS Hotspot Engineering by Aerosol Self-Assembly of Plasmonic Ag Nanoaggregates with Tunable Interparticle Distance. Adv. Sci. 2022, 2201133. [Google Scholar] [CrossRef] [PubMed]
- Yotova, L.; Yaneva, S.; Marinkova, D. Biomimetic Nanosensors for Determination of Toxic Compounds in Food and Agricultural Products (Review). J. Univ. Chem. Technol. Metall. 2013, 48, 215–227. [Google Scholar]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold Nanoparticle Based Optical and Electrochemical Sensing of Dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Tan, X.; He, S.; Liu, X.; Zhao, G.; Huang, T.; Yang, L. Ultrasensitive Electrochemical Sensing of Dopamine by Using Dihydroxylatopillar [5]Arene-Modified Gold Nanoparticles and Anionic Pillar [5]Arene-Functionalized Graphitic Carbon Nitride. Microchim. Acta 2019, 186, 703. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene Based Sensors and Biosensors. TrACTrends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Janssen, S.; Schmitt, K.; Blanke, M.; Bauersfeld, M.L.; Wöllenstein, J.; Lang, W. Ethylene Detection in Fruit Supply Chains. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130311. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Mandon, J.; Arslanov, D.; De Pessemier, J.; Hermans, C.; Harren, F.J.M. Current Methods for Detecting Ethylene in Plants. Ann. Bot. 2013, 111, 347–360. [Google Scholar] [CrossRef] [Green Version]
- McLamore, E.S.; Diggs, A.; Calvo Marzal, P.; Shi, J.; Blakeslee, J.J.; Peer, W.A.; Murphy, A.S.; Porterfield, D.M. Non-Invasive Quantification of Endogenous Root Auxin Transport Using an Integrated Flux Microsensor Technique. Plant J. 2010, 63, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Hu, L.S.; Liu, Y.L.; Chen, R.S.; Cheng, Z.; Chen, S.J.; Amatore, C.; Huang, W.H.; Huo, K.F. Real-Time Monitoring of Auxin Vesicular Exocytotic Efflux from Single Plant Protoplasts by Amperometry at Microelectrodes Decorated with Nanowires. Angew. Chem.-Int. Ed. 2014, 53, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.U.; Ibupoto, Z.H.; Salman, S.; Nur, O.; Willander, M.; Danielsson, B. Selective Determination of Urea Using Urease Immobilized on ZnO Nanowires. Sens. Actuators B Chem. 2011, 160, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; et al. Spectrometric and Voltammetric Analysis of Urease—Nickel Nanoelectrode as an Electrochemical Sensor. Sensors 2007, 7, 1238–1255. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Bohner, A.; Von Wirén, N. Molecular Mechanisms of Urea Transport in Plants. J. Membr. Biol. 2006, 212, 83–91. [Google Scholar] [CrossRef]
- Milakin, K.A.; Korovin, A.N.; Moroz, E.V.; Levon, K.; Guiseppi-Elie, A.; Sergeyev, V.G. Polyaniline-Based Sensor Material for Potentiometric Determination of Ascorbic Acid. Electroanalysis 2013, 25, 1323–1330. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J.; Liu, M.; Li, D.; Lu, L.; Yue, R.; He, H. A Vitamin C Electrochemical Biosensor Based on One-Step Immobilization of Ascorbate Oxidase in the Biocompatible Conducting Poly(3,4-Ethylenedioxythiophene)-Lauroylsarcosinate Film for Agricultural Application in Crops. J. Electroanal. Chem. 2012, 674, 71–82. [Google Scholar] [CrossRef]
- Uchiyama, S.; Umetsu, Y. Concentration-Step Amperometric Sensor of l-Ascorbic Acid Using Cucumber Juice. Anal. Chim. Acta 1991, 255, 53–57. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Gilbert, L.; Hart, J.P. Electrocatalytic Behaviour of Citric Acid at a Cobalt Phthalocyanine- Modified Screen-Printed Carbon Electrode and Its Application in Pharmaceutical and Food Analysis. Anal. Bioanal. Chem. 2010, 396, 3103–3111. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Gorton, L.; Antiochia, R. An Overview of the Latest Graphene-Based Sensors for Glucose Detection: The Effects of Graphene Defects. Electroanalysis 2015, 27, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Tian, K.; Alex, S.; Siegel, G.; Tiwari, A. Enzymatic Glucose Sensor Based on Au Nanoparticle and Plant-like ZnO Film Modified Electrode. Mater. Sci. Eng. C 2015, 46, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Nasirizadeh, N.; Shekari, Z.; Nazari, A.; Tabatabaee, M. Fabrication of a Novel Electrochemical Sensor for Determination of Hydrogen Peroxide in Different Fruit Juice Samples. J. Food Drug Anal. 2016, 24, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, F.; Chen, H.; Zhang, S.-H.; Liu, S.-Y.; Wei, F.; Dong, X.-Y.; Cheng, J.-K.; Huang, W.-H. Real-Time Monitoring of Oxidative Burst from Single Plant Protoplasts Using Microelectrochemical Sensors Modified by Platinum Nanoparticles. Anal. Chem. 2009, 81, 8453–8458. [Google Scholar] [CrossRef]
- Armstrong, W.; Cousins, D.; Armstrong, J.; Turner, D.W.; Beckett, P.M. Oxygen Distribution in Wetland Plant Roots and Permeability Barriers to Gas-Exchange with the Rhizosphere: A Microelectrode and Modelling Study with Phragmites Australis. Ann. Bot. 2000, 86, 687–703. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.-J.; Ryu, W.; Fasching, R.J.; Grossman, A.R.; Prinz, F.B. In Vivo O2 Measurement inside Single Photosynthetic Cells. Biotechnol. Lett. 2011, 33, 1675–1681. [Google Scholar] [CrossRef]
- Alova, A.; Erofeev, A.; Gorelkin, P.; Bibikova, T.; Korchev, Y.; Majouga, A.; Bulychev, A. Prolonged Oxygen Depletion in Microwounded Cells of Chara Corallina Detected with Novel Oxygen Nanosensors. J. Exp. Bot. 2020, 71, 386–398. [Google Scholar] [CrossRef]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; et al. Electroanalysis of Plant Thiols. Sensors 2007, 7, 932–959. [Google Scholar] [CrossRef] [Green Version]
- Alberich, A.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Multivariate Curve Resolution Applied to the Simultaneous Analysis of Electrochemical and Spectroscopic Data: Study of the Cd(II)/Glutathione-Fragment System by Voltammetry and Circular Dichroism Spectroscopy. Anal. Chim. Acta 2007, 584, 403–409. [Google Scholar] [CrossRef]
- Anik, Ü.; Çubukçu, M.; Ertaş, F.N. An Effective Electrochemical Biosensing Platform for the Detection of Reduced Glutathione. Artif. Cells Nanomed. Biotechnol. 2016, 44, 971–977. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, G.; Xu, J.; Wen, Y.; Lu, B.; Zhang, J.; Ding, W. Novel Highly Selective Fluorescent Sensor Based on Electrosynthesized Poly(9-Fluorenecarboxylic Acid) for Efficient and Practical Detection of Iron(III) and Its Agricultural Application. Sens. Actuators B Chem. 2016, 230, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Roy, E.; Patra, S.; Madhuri, R.; Sharma, P.K. Simultaneous Determination of Heavy Metals in Biological Samples by a Multiple-Template Imprinting Technique: An Electrochemical Study. RSC Adv. 2014, 4, 56690–56700. [Google Scholar] [CrossRef]
- Li, S.; Simonian, A.; Chin, B.A. Sensors for Agriculture and the Food Industry. Electrochem. Soc. Interface 2010, 19, 41–46. [Google Scholar] [CrossRef]
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective Detection of Ethylene Gas Using Carbon Nanotube-Based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef]
- Chauhan, R.; Moreno, M.; Banda, D.M.; Zamborini, F.P.; Grapperhaus, C.A. Chemiresistive Metal-Stabilized Thiyl Radical Films as Highly Selective Ethylene Sensors. RSC Adv. 2014, 4, 46787–46790. [Google Scholar] [CrossRef]
- Krivec, M.; Mc Gunnigle, G.; Abram, A.; Maier, D.; Waldner, R.; Gostner, J.; Überall, F.; Leitner, R. Quantitative Ethylene Measurements with MOx Chemiresistive Sensors at Different Relative Air Humidities. Sensors 2015, 15, 28088–28098. [Google Scholar] [CrossRef] [Green Version]
- Mirica, K.A.; Azzarelli, J.M.; Weis, J.G.; Schnorr, J.M.; Swager, T.M. Rapid Prototyping of Carbon-Based Chemiresistive Gas Sensors on Paper. Proc. Natl. Acad. Sci. USA 2013, 110, E3265–E3270. [Google Scholar] [CrossRef] [Green Version]
- Weerakoon, K.A.; Shu, J.H.; Chin, B.A. A Chemiresistor Sensor with a Poly3-Hexylthiophene Active Layer for the Detection of Insect Infestation at Early Stages. IEEE Sens. J. 2011, 11, 1617–1622. [Google Scholar] [CrossRef]
- Degenhardt, D.C.; Greene, J.K.; Khalilian, A. Temporal Dynamics and Electronic Nose Detection of Stink Bug-Induced Volatile Emissions from Cotton Bolls. Psyche A J. Entomol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Krystofova, O.; Trnkova, L.; Adam, V.; Zehnalek, J.; Hubalek, J.; Babula, P.; Kizek, R. Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants. Sensors 2010, 10, 5308–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieling, T.H.; Van Straten, G.; Janssen, H.J.J.; Wouters, H. ISE and Chemfet Sensors in Greenhouse Cultivation. Sens. Actuators B Chem. 2005, 105, 74–80. [Google Scholar] [CrossRef]
- Wang, L.; Han, D.; Ni, S.; Ma, W.; Wang, W.; Niu, L. Photoelectrochemical Device Based on Mo-Doped BiVO4 Enables Smart Analysis of the Global Antioxidant Capacity in Food. Chem. Sci. 2015, 6, 6632–6638. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L. Nanopiezotronics. Adv. Mater. 2007, 19, 889–892. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric Field Effect Transistor and Nanoforce Sensor Based on a Single ZnO Nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Murphy, V.A.; Clemmons, J.I.; Curley, M.J.; Markin, V.S. Energetics and Forces of the Dionaea Muscipula Trap Closing. J. Plant Physiol. 2012, 169, 55–64. [Google Scholar] [CrossRef]
- Volkov, A.G.; Harris, S.L.; Vilfranc, C.L.; Murphy, V.A.; Wooten, J.D.; Paulicin, H.; Volkova, M.I.; Markin, V.S. Venus Flytrap Biomechanics: Forces in the Dionaea Muscipula Trap. J. Plant Physiol. 2013, 170, 25–32. [Google Scholar] [CrossRef]
- Maraldo, D.; Mutharasan, R. Detection and Confirmation of Staphylococcal Enterotoxin B in Apple Juice and Milk Using Piezoelectric-Excited Millimeter-Sized Cantilever Sensors at 2.5 Fg/ML. Anal. Chem. 2007, 79, 7636–7643. [Google Scholar] [CrossRef]
- Eun, A.J.C.; Huang, L.; Chew, F.T.; Li, S.F.Y.; Wong, S.M. Detection of Two Orchid Viruses Using Quartz Crystal Microbalance-Based DNA Biosensors. Phytopathology 2002, 92, 654–658. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, Translocation, and Accumulation of Manufactured Iron Oxide Nanoparticles by Pumpkin Plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.-J.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina, C.; Ibarra, M.R.; de la Fuente, J.M.; Rubiales, D.; Pérez-de-Luque, A.; et al. Nanoparticle Penetration and Transport in Living Pumpkin Plants: In Situ Subcellular Identification. BMC Plant Biol. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant Nanobionics Approach to Augment Photosynthesis and Biochemical Sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.H.; Misra, R.P.; Giraldo, J.P.; Kwak, S.-Y.; Son, Y.; Landry, M.P.; Swan, J.W.; Blankschtein, D.; Strano, M.S. Lipid Exchange Envelope Penetration (LEEP) of Nanoparticles for Plant Engineering: A Universal Localization Mechanism. Nano Lett. 2016, 16, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Mohammad, F. (Eds.) Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319145020. [Google Scholar]
- Dietz, K.J.; Herth, S. Plant Nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate Material Delivery to Plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and Titanium Dioxide Nanoparticle Toxicity in Plants: A Review of Current Research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Ast, C.; Schmälzlin, E.; Löhmannsröben, H.-G.; van Dongen, J.T. Optical Oxygen Micro- and Nanosensors for Plant Applications. Sensors 2012, 12, 7015–7032. [Google Scholar] [CrossRef] [Green Version]
- Considine, M.J.; Diaz-Vivancos, P.; Kerchev, P.; Signorelli, S.; Agudelo-Romero, P.; Gibbs, D.J.; Foyer, C.H. Learning to Breathe: Developmental Phase Transitions in Oxygen Status. Trends Plant Sci. 2017, 22, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous Recording of Blood Oxygen Tensions by Polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [CrossRef]
- Bykova, N.V.; Keerberg, O.; Pärnik, T.; Bauwe, H.; Gardeström, P. Interaction between Photorespiration and Respiration in Transgenic Potato Plants with Antisense Reduction in Glycine Decarboxylase. Planta 2005, 222, 130–140. [Google Scholar] [CrossRef]
- Day, D.; Neuburger, M.; Douce, R. Biochemical Characterization of Chlorophyll-Free Mitochondria from Pea Leaves. Funct. Plant Biol. 1985, 12, 219. [Google Scholar] [CrossRef]
- Shaw, D.S.; Meitha, K.; Considine, M.J.; Foyer, C.H. Mitochondrial Respiration and Oxygen Tension. Methods Mol. Biol. 2017, 1670, 97–113. [Google Scholar]
- Kearns, A.; Whelan, J.; Young, S.; Elthon, T.E.; Day, D.A. Tissue-Specific Expression of the Alternative Oxidase in Soybean and Siratro. Plant Physiol. 1992, 99, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, S.M.; Xu, H.; Brasuel, M.; Philbert, M.A.; Kopelman, R. Nanoscale Probes Encapsulated by Biologically Localized Embedding (PEBBLEs) for Ion Sensing and Imaging in Live Cells. Talanta 2004, 63, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 0387312781. [Google Scholar]
- Schmälzlin, E.; Van Dongen, J.T.; Klimant, I.; Marmodée, B.; Steup, M.; Fisahn, J.; Geigenberger, P.; Löhmannsröben, H.G. An Optical Multifrequency Phase-Modulation Method Using Microbeads for Measuring Intracellular Oxygen Concentrations in Plants. Biophys. J. 2005, 89, 1339–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tian, Y.; Wang, X.; Dong, L. Miniaturized Soil Sensor for Continuous, In-Situ Monitoring of Soil Water Potential. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2025–2028. [Google Scholar]
- Leone, M.; Consales, M.; Passeggio, G.; Buontempo, S.; Zaraket, H.; Youssef, A.; Persiano, G.V.; Cutolo, A.; Cusano, A. Fiber Optic Soil Water Content Sensor for Precision Farming. Opt. Laser Technol. 2022, 149, 107816. [Google Scholar] [CrossRef]
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-Step and Large-Scale Fabrication of Flexible and Wearable Humidity Sensor Based on Laser-Induced Graphene for Real-Time Tracking of Plant Transpiration at Bio-Interface. Biosens. Bioelectron. 2020, 165, 112360. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjärvi, E. Reactive Oxygen Species: Reactions and Detection from Photosynthetic Tissues. J. Photochem. Photobiol. B Biol. 2015, 152, 176–214. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen Peroxide and Nitric Oxide as Signalling Molecules in Plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Schnaubelt, D.; Queval, G.; Dong, Y.; Diaz-Vivancos, P.; Makgopa, M.E.; Howell, G.; de Simone, A.; Bai, J.; Hannah, M.A.; Foyer, C.H. Low Glutathione Regulates Gene Expression and the Redox Potentials of the Nucleus and Cytosol in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 266–279. [Google Scholar] [CrossRef]
- García-Quirós, E.; Alché, J.d.D.; Karpinska, B.; Foyer, C.H. Glutathione Redox State Plays a Key Role in Flower Development and Pollen Vigour. J. Exp. Bot. 2020, 71, 730–741. [Google Scholar] [CrossRef]
- Jiang, K.; Schwarzer, C.; Lally, E.; Zhang, S.; Ruzin, S.; Machen, T.; Remington, S.J.; Feldman, L. Expression and Characterization of a Redox-Sensing Green Fluorescent Protein (Reduction-Oxidation-Sensitive Green Fluorescent Protein) in Arabidopsis. Plant Physiol. 2006, 141, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Rosenwasser, S.; Rot, I.; Meyer, A.J.; Feldman, L.; Jiang, K.; Friedman, H. A Fluorometer-Based Method for Monitoring Oxidation of Redox-Sensitive GFP (RoGFP) during Development and Extended Dark Stress. Physiol. Plant. 2010, 138, 493–502. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Fricker, M.D.; Müller, C.; Marty, L.; Brach, T.; Novak, J.; Sweetlove, L.J.; Hell, R.; Meyer, A.J. Confocal Imaging of Glutathione Redox Potential in Living Plant Cells. J. Microsc. 2008, 231, 299–316. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F. lo H2O2 in Plant Peroxisomes: An in Vivo Analysis Uncovers a Ca2+-Dependent Scavenging System. Plant J. 2010, 62, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Barrera, A.; Velarde-Buendía, A.; Zepeda, I.; Sanchez, F.; Quinto, C.; Sánchez-Lopez, R.; Cheung, A.Y.; Wu, H.M.; Cardenas, L. Hyper, a Hydrogen Peroxide Sensor, Indicates the Sensitivity of the Arabidopsis Root Elongation Zone to Aluminum Treatment. Sensors 2015, 15, 855–867. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yang, H.; Mu, J.; Lu, T.; Peng, J.; Deng, X.; Kong, Z.; Bao, S.; Cao, X.; Zuo, J. Nitric Oxide Regulates Protein Methylation during Stress Responses in Plants. Mol. Cell 2017, 67, 702–710.e4. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Pierre-Jerome, E.; Drapek, C.; Benfey, P.N. Regulation of Division and Differentiation of Plant Stem Cells. Annu. Rev. Cell Dev. Biol. 2018, 34, 289–310. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No Hormone to Rule Them All: Interactions of Plant Hormones during the Responses of Plants to Pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Umehara, M.; Cao, M.; Akiyama, K.; Akatsu, T.; Seto, Y.; Hanada, A.; Li, W.; Takeda-Kamiya, N.; Morimoto, Y.; Yamaguchi, S. Structural Requirements of Strigolactones for Shoot Branching Inhibition in Rice and Arabidopsis. Plant Cell Physiol. 2015, 56, 1059–1072. [Google Scholar] [CrossRef] [Green Version]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and Application of Strigolactones. New Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga Lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination Stimulants. II. Structure of Strigol, a Potent Seed Germination Stimulant for Witchweed (Striga Lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of Shoot Branching by New Terpenoid Plant Hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhong, S.; Grierson, D. Recent Advances in Ethylene Research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Xu, X.; Cheng, Y.; Tan, Y.; Xiao, L.; Tang, D.; Jiang, H.; Qin, X.; Wang, H. Chemical Pre-Reduction and Electro-Reduction Guided Preparation of a Porous Graphene Bionanocomposite for Indole-3-Acetic Acid Detection. Nanoscale 2019, 11, 962–967. [Google Scholar] [CrossRef]
- Sun, T. Gibberellin Metabolism, Perception and Signaling Pathways in Arabidopsis. Arab. Book 2008, 6, e0103. [Google Scholar] [CrossRef] [Green Version]
- Ueguchi-Tanaka, M.; Nakajima, M.; Katoh, E.; Ohmiya, H.; Asano, K.; Saji, S.; Hongyu, X.; Ashikari, M.; Kitano, H.; Yamaguchi, I.; et al. Molecular Interactions of a Soluble Gibberellin Receptor, GID1, with a Rice DELLA Protein, SLR1, and Gibberellin. Plant Cell 2007, 19, 2140–2155. [Google Scholar] [CrossRef] [Green Version]
- Sun, T. Gibberellin-GID1-DELLA: A Pivotal Regulatory Module for Plant Growth and Development. Plant Physiol. 2010, 154, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, S.; Zhou, M.; Ji, C.; Que, L.; Wang, W. Development of a Structure-Switching Aptamer-Based Nanosensor for Salicylic Acid Detection. Biosens. Bioelectron. 2019, 140, 111342. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Dubois, M.; van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Light, K.M.; Wisniewski, J.A.; Vinyard, W.A.; Kieber-Emmons, M.T. Perception of the Plant Hormone Ethylene: Known-Knowns and Known-Unknowns. JBIC J. Biol. Inorg. Chem. 2016, 21, 715–728. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Pranamornkith, T.; East, A.; Heyes, J. Influence of Exogenous Ethylene during Refrigerated Storage on Storability and Quality of Actinidia Chinensis (Cv. Hort16A). Postharvest Biol. Technol. 2012, 64, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B.H. Continuous Exposure to Ethylene in the Storage Environment Adversely Affects ‘Afourer’ Mandarin Fruit Quality. Food Chem. 2018, 242, 585–590. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.; Zhang, Y.; Wang, S.; Wong, M.W.; Ni, R.; Huang, D. Rapid and Visual Detection and Quantitation of Ethylene Released from Ripening Fruits: The New Use of Grubbs Catalyst. J. Agric. Food Chem. 2019, 67, 507–513. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene Control Technologies in Extending Postharvest Shelf Life of Climacteric Fruit. J. Agric. Food Chem. 2017, 65, 7308–7319. [Google Scholar] [CrossRef] [PubMed]
- Jedermann, R.; Praeger, U.; Geyer, M.; Lang, W. Remote Quality Monitoring in the Banana Chain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130303. [Google Scholar] [CrossRef]
- Spaniolas, S.; Bazakos, C.; Awad, M.; Kalaitzis, P. Exploitation of the Chloroplast Trn L (UAA) Intron Polymorphisms for the Authentication of Plant Oils by Means of a Lab-on-a-Chip Capillary Electrophoresis System. J. Agric. Food Chem. 2008, 56, 6886–6891. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, G.J.; Lévesque, C.A.; De Cock, A.W.A.M.; Duchaine, C.; Brière, S.; Uribe, P.; Martin, F.N.; Hamelin, R.C. Molecular Detection of Phytophthora Ramorum by Real-Time Polymerase Chain Reaction Using TaqMan, SYBR Green, and Molecular Beacons. Phytopathology 2007, 97, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Sanzani, S.M.; Li Destri Nicosia, M.G.; Faedda, R.; Cacciola, S.O.; Schena, L. Use of Quantitative PCR Detection Methods to Study Biocontrol Agents and Phytopathogenic Fungi and Oomycetes in Environmental Samples. J. Phytopathol. 2014, 162, 1–13. [Google Scholar] [CrossRef]
- Weller, S.A.; Elphinstone, J.G.; Smith, N.C.; Boonham, N.; Stead, D.E. Detection of Ralstonia Solanacearum Strains with a Quantitative, Multiplex, Real-Time, Fluorogenic PCR (TaqMan) Assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongman, M.; Carmichael, P.C.; Bill, M. Technological Advances in Phytopathogen Detection and Metagenome Profiling Techniques. Curr. Microbiol. 2020, 77, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Nivarlet, N.; Lippolis, V.; Della Gatta, S.; Huet, A.C.; Delahaut, P.; Granier, B.; Visconti, A. Multiplex Dipstick Immunoassay for Semi-Quantitative Determination of Fusarium Mycotoxins in Cereals. Anal. Chim. Acta 2012, 718, 99–108. [Google Scholar] [CrossRef]
- Medintz, I.L.; Sapsford, K.E.; Konnert, J.H.; Chatterji, A.; Lin, T.; Johnson, J.E.; Mattoussi, H. Decoration of Discretely Immobilized Cowpea Mosaic Virus with Luminescent Quantum Dots. Langmuir 2005, 21, 5501–5510. [Google Scholar] [CrossRef]
- Sun, W.; Zhong, J.; Qin, P.; Jiao, K. Electrochemical Biosensor for the Detection of Cauliflower Mosaic Virus 35 S Gene Sequences Using Lead Sulfide Nanoparticles as Oligonucleotide Labels. Anal. Biochem. 2008, 377, 115–119. [Google Scholar] [CrossRef]
- Moreau, A.L.D.; Janissen, R.; Santos, C.A.; Peroni, L.A.; Stach-Machado, D.R.; de Souza, A.A.; de Souza, A.P.; Cotta, M.A. Highly-Sensitive and Label-Free Indium Phosphide Biosensor for Early Phytopathogen Diagnosis. Biosens. Bioelectron. 2012, 36, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Detection of Citrus Tristeza Virus by Using Fluorescence Resonance Energy Transfer-Based Biosensor. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2016, 169, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, W.; Chen, X.; Zhang, Y.; Lin, X.; Wu, Z.; Li, M. Multiplex Immunoassays of Plant Viruses Based on Functionalized Upconversion Nanoparticles Coupled with Immunomagnetic Separation. J. Nanomater. 2013, 1–8. [Google Scholar] [CrossRef]
- Yazgan, I.; Osonga, F.J.; Miller, R.M.; Kariuki, V.M.; Zhang, J.; Feng, J.; Skeete, Z.; Crapo, H.; Schulte, J.; Sadik, O.A. Greener One-Pot Synthesis of Gold Nanoparticle Glycoconjugates Using Functionalized Sugars. ACS Agric. Sci. Technol. 2021, 1, 379–389. [Google Scholar] [CrossRef]
- Yazgan, I.; Zhang, J.; Kariuki, V.; Akgul, A.; Cronmiller, L.E.; Akgul, A.; Osonga, F.; McMahon, A.; Gao, Y.; Eshun, G.; et al. Selective Sensing and Imaging of Penicillium Italicum Spores and Hyphae Using Carbohydrate–Lectin Interactions. ACS Sens. 2018, 3, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, I.; Gümüş, A.; Gökkuş, K.; Demir, M.A.; Evecen, S.; Sönmez, H.A.; Miller, R.M.; Bakar, F.; Oral, A.; Popov, S.; et al. On the Effect of Modified Carbohydrates on the Size and Shape of Gold and Silver Nanostructures. Nanomaterials 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
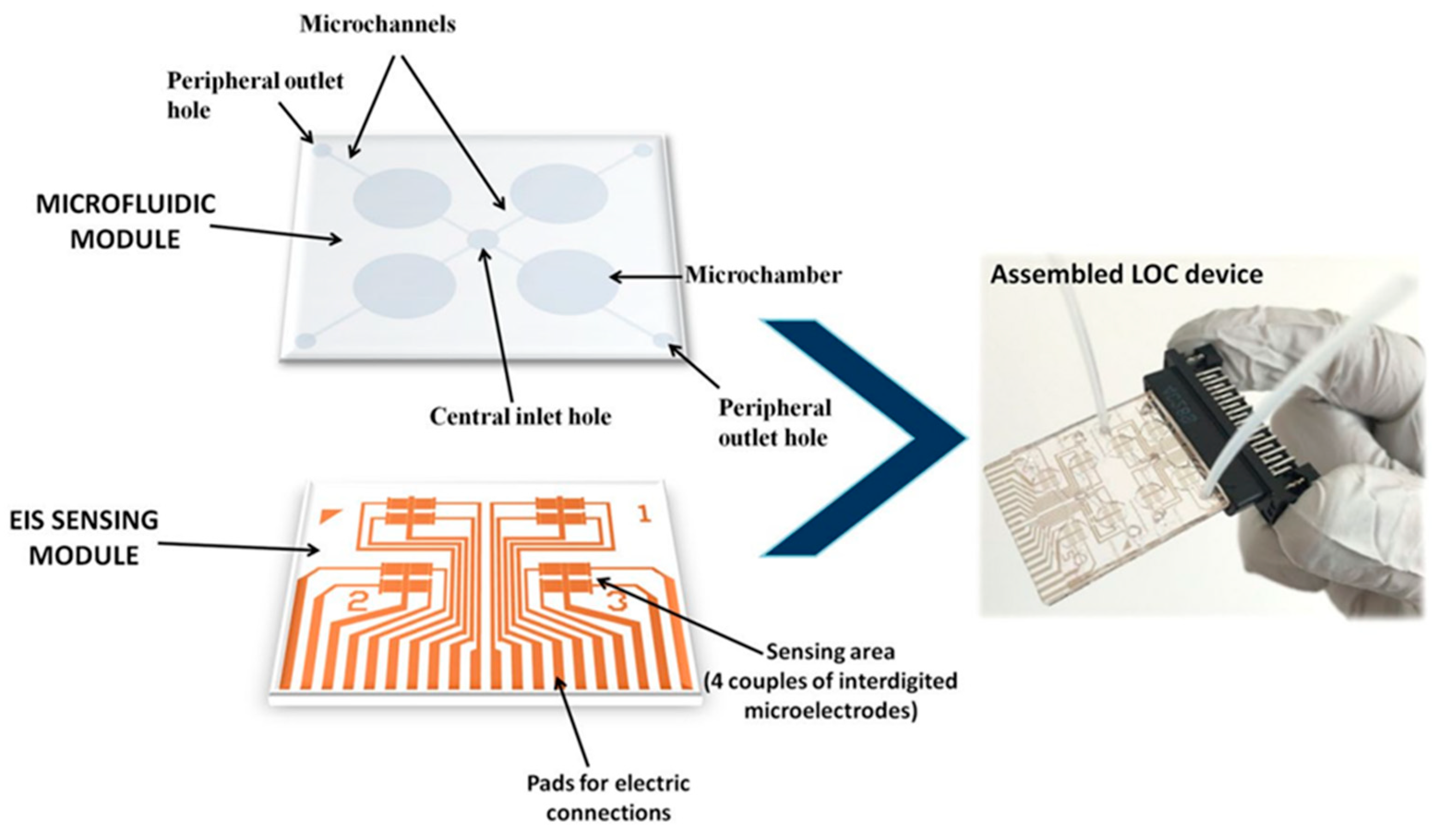
- Chiriacò, M.S.; Luvisi, A.; Primiceri, E.; Sabella, E.; de Bellis, L.; Maruccio, G. Development of a Lab-on-a-Chip Method for Rapid Assay of Xylella Fastidiosa Subsp. Pauca Strain CoDiRO. Sci. Rep. 2018, 8, 7376. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Z.; Aluru, M.R.; Dong, L. Plant Chip for High-Throughput Phenotyping of Arabidopsis. Lab Chip 2014, 14, 1281–1293. [Google Scholar] [CrossRef]
- Julich, S.; Riedel, M.; Kielpinski, M.; Urban, M.; Kretschmer, R.; Wagner, S.; Fritzsche, W.; Henkel, T.; Möller, R.; Werres, S. Development of a Lab-on-a-Chip Device for Diagnosis of Plant Pathogens. Biosens. Bioelectron. 2011, 26, 4070–4075. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [Green Version]
- Vitosh, M.L.; Silva, G.H. A Rapid Petiole Sap Nitrate-Nitrogen Test for Potatoes. Commun. Soil Sci. Plant Anal. 1994, 25, 183–190. [Google Scholar] [CrossRef]
- Errebhi, M.; Rosen, C.J.; Birong, D.E. Calibration of a Petiole Sap Nitrate Test for Irrigated “Russet Burbank” Potato. Commun. Soil Sci. Plant Anal. 1998, 29, 23–35. [Google Scholar] [CrossRef]
- Kubota, A.; Thompson, T.L.; Doerge, T.A.; Godin, R.E. A Petiole Sap Nitrate Test for Broccoli. J. Plant Nutr. 1997, 20, 669–682. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Alegret, S.; Cáceres, R.; Casadesús, J.; Marfà, O.; del Valle, M. Application of a Potentiometric Electronic Tongue to Fertigation Strategy in Greenhouse Cultivation. Comput. Electron. Agric. 2007, 57, 12–22. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A Worldwide Review of Currently Used Pesticides’ Monitoring in Agricultural Soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Guo, H.; Zhao, B.; Clark, J.M.; Xing, B.; Hou, R.; He, L. Real-Time Monitoring of Pesticide Translocation in Tomato Plants by Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 2093–2099. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Flame Aerosol Synthesis of Smart Nanostructured Materials. J. Mater. Chem. 2007, 17, 4743. [Google Scholar] [CrossRef]
- Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Long Term Stability of Cantilever Gas Nanosensors to Detect Euschistus Heros (F.) Pheromone Release by Rubber Septa. Sens. Actuators B Chem. 2022, 359, 131566. [Google Scholar] [CrossRef]
- MacVittie, K.; Conlon, T.; Katz, E. A Wireless Transmission System Powered by an Enzyme Biofuel Cell Implanted in an Orange. Bioelectrochemistry 2015, 106, 28–33. [Google Scholar] [CrossRef] [Green Version]


| Sensor Type/Detector | Mechanism | Analytes in Plants |
|---|---|---|
| Förster Resonance Energy Transfer (FRET) | A recognition element is fused to a reporter element (this is a fluorophore pair that have an overlapping emission spectra). The donor chromophore in its excited state may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. | ATP, calcium ions, metabolites, transgenes, and plant viruses. |
| Surface-Enhanced Ramen Scattering (SERS) | A technique that enhances Raman scattering by molecules adsorbed on rough metal surfaces or by nanostructures. The enhancement factor can be as much as 1014, and hence the technique may detect single molecules. | Hormones, e.g., cytokinins and brassinosteroids, as well as pesticides. |
| Electrochemical | Comprises a working electrode, counter electrode, and reference electrode. Reports the electrochemical response or electrical resistance change of materials resulting from a reaction with the analytes. | Hormones, enzymes, metabolites, ROS, and ions such as H+, K+, and Na+. |
| Piezoelectric | A reversible process in which mechanical stress is converted into an electric signal. | Morphogenesis. |
| Förster Resonance Energy Transfer-Based Nanosensors | ||||
|---|---|---|---|---|
| Plant Analyte | Sensor | Type | Plant Species | References |
| Nucleic acid | GFP-tagged proteins | Genetically encoded | Nicotiana benthamiana | Camborde et al., 2017 [41] |
| Glucose | FLIP: FRET between a cyan fluorescent protein and a yellow fluorescent protein | Genetically encoded | A. thaliana and Oryza sativa L. spp. japonica cv. Zhonghua11 | Chaudhuri et al., 2011 [42] and Zhu et al., 2017 [43] |
| ATP | Nano-lantern: a chimera of enhanced Renilla luciferase and the fluorescent protein Venus | Genetically encoded | A. thaliana | Saito et al., 2012 [44] |
| Ca2+ ions | Yellow cameleons: FRET between a cyan fluorescent protein and a yellow fluorescent protein | Genetically encoded | Lotus japonicus | Krebs et al., 2012 [45] |
| Plant hormone: Gibberellin | FRET between a cyan fluorescent protein and a yellow fluorescent protein | Genetically encoded | A. thaliana | Rizza et al., 2017 [46] |
| Plant virus: Citrus tristeza virus | Carbon nanoparticles acting as quenchers and antibodies labeled with CdTe quantum dots | Exogenously applied | Citrus sp. | Shojaei et al., 2016 [47] |
| Plant virus: Grapevine virus A-type | Films of zinc oxide deposited by atomic layer deposition | Exogenously applied | Vitis sp. | Tereshchenko et al., 2017 [48] |
| Transgenes/virus: Cauliflower mosaic virus 35s | DNA hybridization with probe modified nitrogen-doped graphene quantum dots and silver nanoparticles | Exogenously applied | Glycine max | Li et al., 2016 [49] |
| Surface-Enhanced Raman Spectroscopic-Based Nanosensors | ||||
|---|---|---|---|---|
| Plant Analyte | Nanomaterial | Detection Limit | Plant Species | References |
| Hormone: indole-3-butyric acid | Gold (Au) nanoparticles | 0.002 μM | Pea, mungbean, soybean, and black bean | Wang et al., 2017 [86] |
| Hormone: Brassinosteroids | Au nanoparticles | 1 × 10−11 M | Not specified | Chen et al., 2017 [87] |
| Pesticide: N6-benzylaminopurine | Au colloidal nanoparticles | 0.065 μg/g | Commercial bean sprouts and bean grains | Zhang et al., 2018 [88] |
| Pesticide: parathion-ethyl | Plasmonic silver nanoaggregates | 0.1 ppm | Apple | Li et al., 2022 [89] |
| Electrochemical Nanosensors | ||||
|---|---|---|---|---|
| Plant Analyte | Nanomaterial | Detection Method | Detection Limit | References |
| Hormone: indole-3-acetic acid | Multi-walled carbon nanotubes | Amperometry | 0.4 μM | McLamore et al., 2010 [98] |
| Hormone: indole-3-acetic acid | Microelectrodes decorated with nanowires | Amperometry | 1 nM | Liu et al., 2014 [99] |
| Hormone: Ethylene | Chemoresistive sensor modified with organo–copper complex and single-walled carbon nanotubes | Chemoresistivity | <0.5 ppm | Esser et al., 2012 [122] |
| Hormone: Ethylene | Metal-stabilized thiyl radical film chemoresistive sensor | Chemoresistivity | 30% | Chauhan et al., 2014 [123] |
| Enzyme: Urease | Nickel nanoelectrodes | Differential pulse voltammetry | 200 ng/mL | Hubalek et al., 2007 [101] |
| Vitamin C | Immobilized ascorbate oxidase in poly(3,4-ethylenedioxythio-phene)-lauroylsarcosinate film electrode | Amperometry and voltammetry | Amperometry 0.464 μM; voltammetry 56.1 μM | Wen et al., 2012 [104] |
| Molecular oxygen | Carbon-filled quartz micropipettes with Platinum-coated tips (tip diameter in the nanometre range) | Cyclic voltammetry | - | Alova et al., 2020 [114] |
| Oxidation: Hydrogen peroxide | Multi-walled carbon nanotubes | Amperometry | 0.27 μM | Nasirizadeh et al., 2016 [110] |
| Oxidation: Hydrogen peroxide | Platinum (Pt) nanoparticles | Amperometry | 5.0 × 10−9 M | Ai et al., 2009 [111] |
| Antioxidant: Glutathione | Glutathione peroxidase Pt nanoparticle glassy carbon paste electrode | Differential pulse voltammetry | - | Anik et al., 2016 [117] |
| Ions: Cd(II), Cu(II), and Pb(II) | Multi-walled carbon nanotubes | Cyclic voltammetry | Cd(II): 1.03 μg L−1 Cu(II): 2.12 μg L−1 Pb(II): 1.62 μg L−1 | Roy et al., 2014 [120] |
| Plant virus: Pseudomonas syringae | Gold nanoparticles | Differential pulse voltammetry | - | Lau et al., 2017 [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, D.S.; Honeychurch, K.C. Nanosensor Applications in Plant Science. Biosensors 2022, 12, 675. https://doi.org/10.3390/bios12090675
Shaw DS, Honeychurch KC. Nanosensor Applications in Plant Science. Biosensors. 2022; 12(9):675. https://doi.org/10.3390/bios12090675
Chicago/Turabian StyleShaw, Daniel S., and Kevin C. Honeychurch. 2022. "Nanosensor Applications in Plant Science" Biosensors 12, no. 9: 675. https://doi.org/10.3390/bios12090675
APA StyleShaw, D. S., & Honeychurch, K. C. (2022). Nanosensor Applications in Plant Science. Biosensors, 12(9), 675. https://doi.org/10.3390/bios12090675






