An Immunochromatographic Assay for the Rapid and Qualitative Detection of Mercury in Rice
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Instrumentation
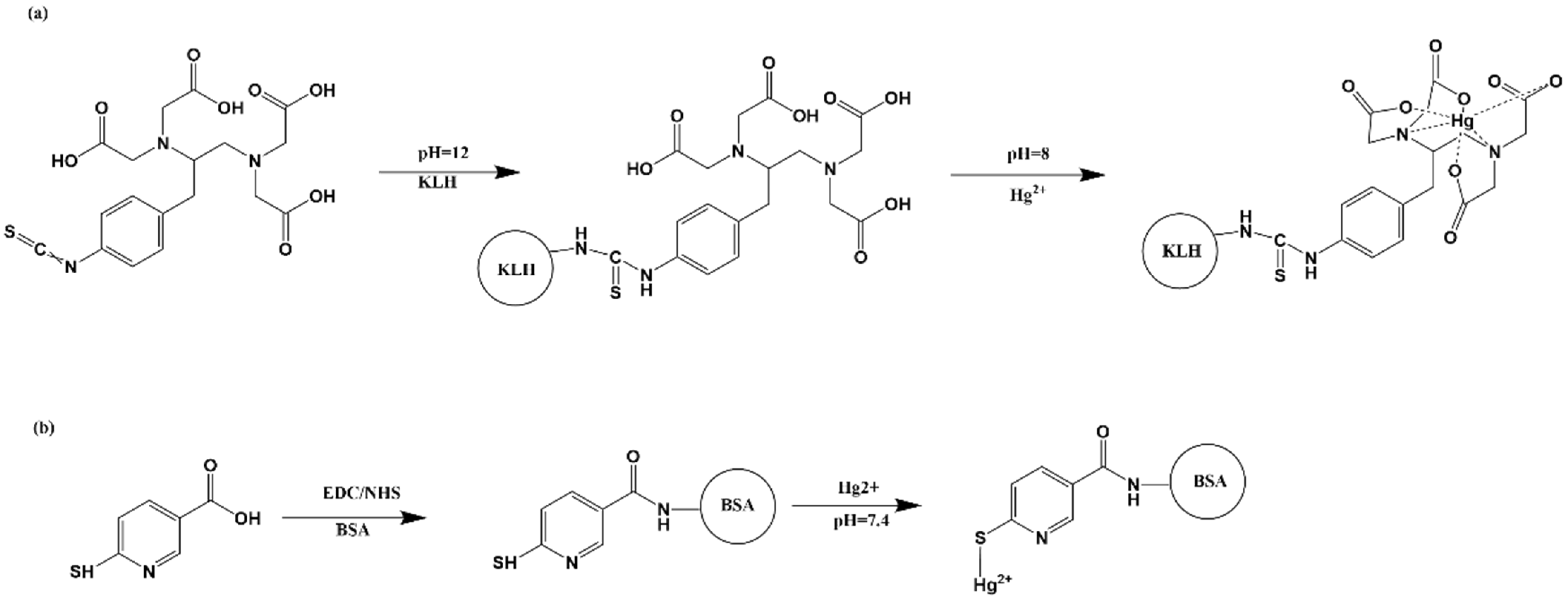
2.2. Synthesis of Immunogen and Coating Antigen
2.2.1. Synthesis of Immunogen
2.2.2. Synthesis of Coating Conjugate
2.3. Preparation of Anti-Mercury mAb
2.4. Characterization of mAb
2.5. Preparation of Colloidal Gold-mAb
2.6. Preparation of the ICA Strip
2.7. Evaluation of the ICA Strip
2.8. True Samples Characterized by ICP-MS
2.9. Sample Pretreatment
3. Results and Discussion
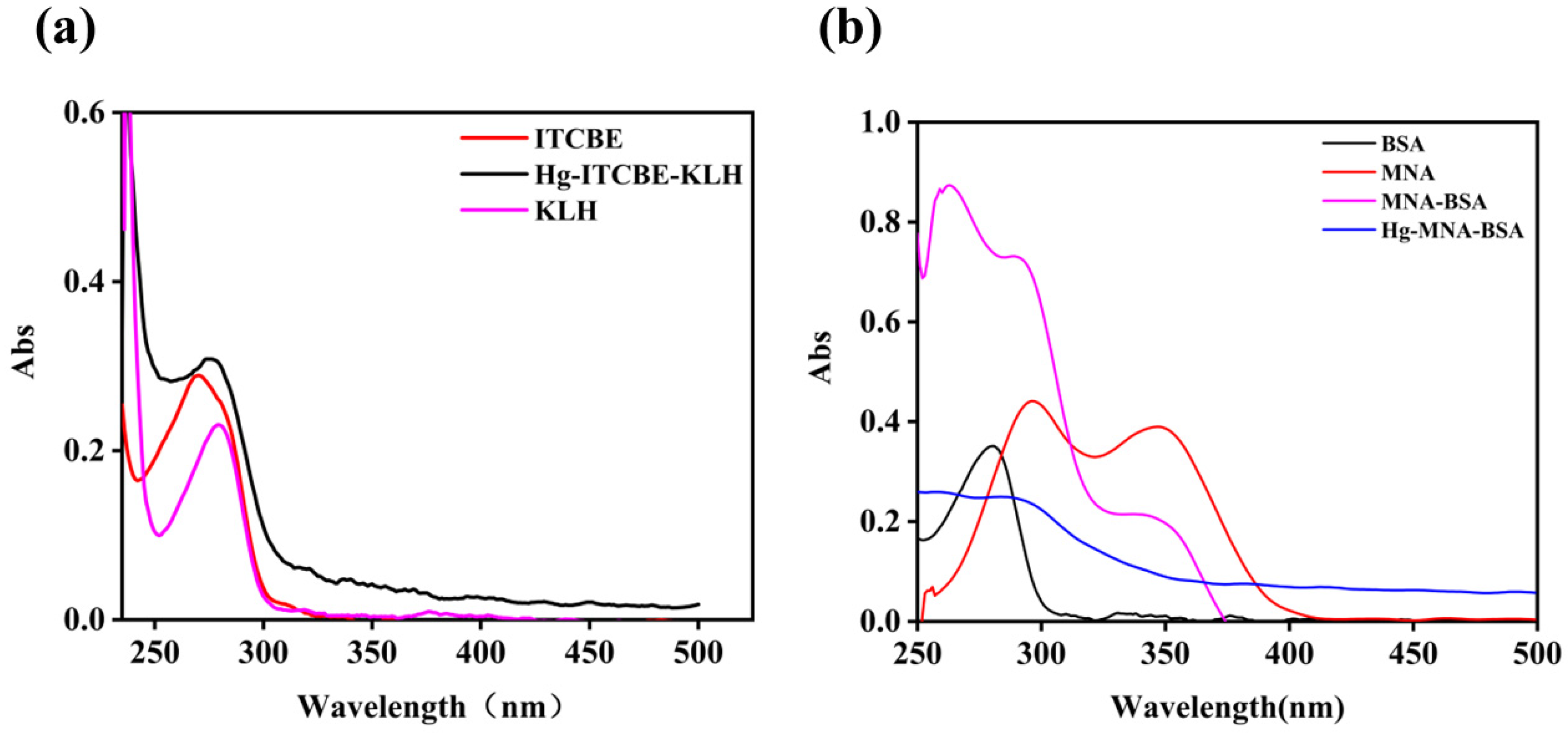
3.1. Antigen Characterization
3.2. Characterization of Anti-Mercury mAb
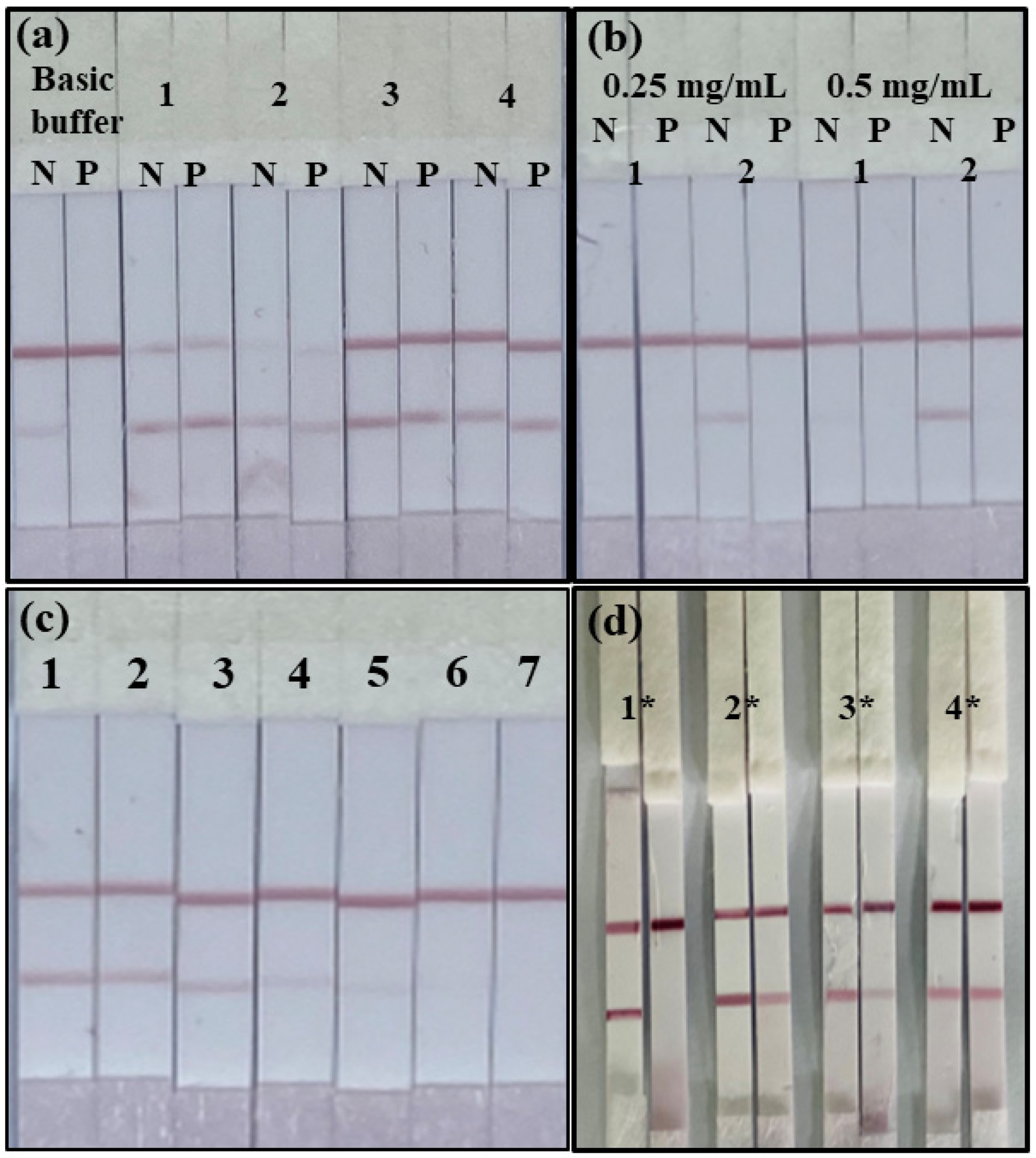
3.3. Performance of the ICA Strip
3.4. Matrix Evaluation of the ICA Strip
3.5. Analysis of Mercury in True Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [PubMed]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Santa Clara, CA, USA; Palo Alto: Santa Clara, CA, USA, 2016; Volume 67, pp. 489–512. [Google Scholar]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dabrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 19. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar]
- Sommar, J.; Zhu, W.; Shang, L.H.; Lin, C.J.; Feng, X.B. Seasonal variations in metallic mercury (Hg-0) vapor exchange over biannual wheat-corn rotation cropland in the North China Plain. Biogeosciences 2016, 13, 2029–2049. [Google Scholar]
- Chen, X.; Lyu, H.Y.; Zhang, J.; Bai, L.; Wang, J. National Food Safety Standards Related to Microbiological Contaminants in China: Recent Progress and Challenges. Foodborne Pathog. Dis. 2021, 18, 528–537. [Google Scholar]
- da Silva, D.L.F.; da Costa, M.A.P.; Silva, L.O.B.; dos Santos, W.N.L. Simultaneous determination of mercury and selenium in fish by CVG AFS. Food Chem. 2019, 273, 24–30. [Google Scholar]
- Vacchina, V.; Epova, E.N.; Berail, S.; Medina, B.; Donard, O.F.X.; Seby, F. Tin and mercury and their speciation (organotin compounds and methylmercury) in worldwide red wine samples determined by ICP-MS and GC-ICP-MS. Food Addit. Contam. Part B-Surveill. 2020, 13, 88–98. [Google Scholar]
- Saleh, T.A.; Fadillah, G.; Ciptawati, E.; Khaled, M. Analytical methods for mercury speciation, detection, and measurement in water, oil, and gas. Trac-Trends Anal. Chem. 2020, 132, 116016. [Google Scholar]
- Wang, Y.H.; Ding, M.Y.; Ma, H.Q.; Wu, J.; Zhao, H.W.; Wan, Y.L. Development of a specific monoclonal antibody-based icELISA for detection of arecoline in traditional Chinese medicines and fresh areca nuts. Food Agric. Immunol. 2022, 33, 113–126. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Shao, Q.; Yin, Z.; Wang, L.; Fu, Z. A novel chemiluminescent immunochromatographic assay strip for rapid detection of mercury ions. Anal. Methods 2017, 9, 2401–2406. [Google Scholar]
- Wu, Z.; Shen, H.; Hu, J.; Fu, Q.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [PubMed]
- Zhang, K.Z.; Cai, H.D.; Lu, M.; Wei, D.X.; Yin, J.Q.; Ding, N.S.; Lai, W.H.; Peng, J. Quantum dot nanobead immunochromatographic assay based on bispecific monoclonal antibody for the simultaneous detection of aflatoxin B-1 and amantadine. Food Agric. Immunol. 2022, 33, 403–418. [Google Scholar]
- She, P.; Chu, Y.; Liu, C.; Guo, X.; Zhao, K.; Li, J.; Du, H.; Zhang, X.; Wang, H.; Deng, A. A competitive immunoassay for ultrasensitive detection of Hg(2+) in water, human serum and urine samples using immunochromatographic test based on surface-enhanced Raman scattering. Anal. Chim. Acta 2016, 906, 139–147. [Google Scholar] [PubMed]
- Song, S.S.; Chen, Z.W.; Liu, J.; Xu, L.G.; Kuang, H.; Zhu, J.P.; Liu, L.Q. Development of an immunocolloidal strip for rapid detection of picoxystrobin. Food Agric. Immunol. 2020, 31, 711–722. [Google Scholar]
- Zhang, Y.; Li, X.; Liu, G.; Wang, Z.; Kong, T.; Tang, J.; Zhag, P.; Yang, W.; Li, D.; Liu, L.; et al. Development of ELISA for detection of mercury based on specific monoclonal antibodies against mercury-chelate. Biol. Trace Elem Res. 2011, 144, 854–864. [Google Scholar]
- Wang, Y.; Yang, H.; Pschenitza, M.; Niessner, R.; Li, Y.; Knopp, D.; Deng, A. Highly sensitive and specific determination of mercury(II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Anal. Bioanal. Chem. 2012, 403, 2519–2528. [Google Scholar]
- Guo, L.L.; Wu, X.L.; Liu, L.Q.; Kuang, H.; Xu, C.L. Gold Immunochromatographic Assay for Rapid On-Site Detection of Lincosamide Residues in Milk, Egg, Beef, and Honey Samples. Biotechnol. J. 2020, 15, 1900174. [Google Scholar]
- Xu, X.; Liu, L.; Wu, X.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic strips for fast screening of the nicarbazin marker in chicken breast and liver samples based on monoclonal antibodies. Anal. Methods 2020, 12, 2143–2151. [Google Scholar]
- Zeng, L.; Xu, X.; Song, S.; Xu, L.; Liu, L.; Xiao, J.; Xu, C.; Kuang, H. Synthesis of haptens and gold-based immunochromatographic paper sensor for vitamin B6 in energy drinks and dietary supplements. Nano Res. 2021, 15, 2479–2488. [Google Scholar]
- Zeng, L.; Xu, X.; Ding, H.; Song, S.; Xu, L.; Xu, C.; Kuang, H. A gold nanoparticle based colorimetric sensor for the rapid detection of Yersinia enterocolitica serotype O:8 in food samples. J. Mater. Chem. B 2022, 10, 909–914. [Google Scholar]
- Zou, S.; Cui, G.; Liu, L.; Song, S.; Kuang, H. Development of ic-ELISA and an immunochromatographic strip assay for the detection of methylmercury. Food Agric. Immunol. 2017, 28, 699–710. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Wu, A.; Song, S.; Kuang, H.; Liu, L.; Wang, Z.; Xu, L.; Sun, M.; Xu, C. An immunochromatographic assay for the rapid detection of oxadixyl in cucumber, tomato and wine samples. Food Chem. 2022, 379, 132131. [Google Scholar] [PubMed]
- Liu, J.; Xu, X.X.; Wu, A.H.; Wang, Z.Y.; Song, S.S.; Kuang, H.; Liu, L.Q.; Xu, C.L. Preparing monoclonal antibodies and developing immunochromatographic assay strips for the determination of propamocarb levels. Food Chem. 2022, 370, 131284. [Google Scholar] [PubMed]
- Zeng, L.; Guo, L.; Wang, Z.; Xu, X.; Ding, H.; Song, S.; Xu, L.; Kuang, H.; Xu, C. Gold nanoparticle-based immunochromatographic assay for detection Pseudomonas aeruginosa in water and food samples. Food Chem. X 2021, 9, 100117. [Google Scholar]
- Jiang, H.T.; Xu, X.X.; Song, S.S.; Wu, A.H.; Liu, L.Q.; Kuang, H.; Xu, C.L. A monoclonal antibody-based colloidal gold immunochromatographic strip for the analysis of novobiocin in beef and chicken. Food Addit. Contam. Part A 2022, 39, 1053–1064. [Google Scholar]
- Zheng, S.; Wu, T.; Li, J.X.; Jin, Q.; Xiao, R.; Wang, S.Q.; Wang, C.W. Difunctional immunochromatographic assay based on magnetic quantum dot for ultrasensitive and simultaneous detection of multiple mycotoxins in foods. Sens. Actuators B Chem. 2022, 359, 131528. [Google Scholar]
- Lin, L.; Song, S.S.; Wu, X.L.; Liu, L.Q.; Kuang, H. A colloidal gold immunochromatography test strip based on a monoclonal antibody for the rapid detection of triadimefon and triadimenol in foods. Food Agric. Immunol. 2020, 31, 475–488. [Google Scholar]
- Safaei, S.; Kazemian, H.; Junk, P.C. Dual functional MOF as a selective fluorescent naked-eye detector and effective sorbent for mercury ion. J. Solid State Chem. 2021, 300, 122267. [Google Scholar]
- Wu, X.Y.; Cobbina, S.J.; Mao, G.H.; Xu, H.; Zhang, Z.; Yang, L.Q. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury pollution in Asia: A review of the contaminated sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar]
- Basu, N.; Abass, K.; Dietz, R.; Krummel, E.; Rautio, A.; Weihe, P. The impact of mercury contamination on human health in the Arctic: A state of the science review. Sci. Total Environ. 2022, 831, 154793. [Google Scholar] [PubMed]
- Kannappan, S.; Ramisetty, B.C.M. Engineered Whole-Cell-Based Biosensors: Sensing Environmental Heavy Metal Pollutants in Water-a Review. Appl. Biochem. Biotechnol. 2022, 194, 1814–1840. [Google Scholar] [PubMed]
- Xing, Y.P.; Xue, B.Y.; Qi, P.S.; Chen, G.Y.; Zhou, X.H. A rapid and sensitive fluorescence biosensor for Hg2+ detection in environmental samples. Sens. Actuator Rep. 2022, 4, 100101. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Wei, J.C.; Gu, L.Q.; Zou, L.; Wang, T.; Chen, L.; Li, Y.Q.; Yang, Y.; Li, P. DNAzyme-based biosensors for mercury (II) detection: Rational construction, advances and perspectives. J. Hazard. Mater. 2022, 431, 128606. [Google Scholar]


| Metals | IC50(ng·mL−1) | CR (%) |
|---|---|---|
| Hg2+ (Mercury II) | 0.606 | 100 |
| MeHg (Methyl mercury) | 8.9 | 9.05 |
| Hg+ (Mercury I) | 25 | 3.22 |
| Pb2+ (Lead) | >10,000 | <0.001 |
| Cd2+ (Cadmium) | >10,000 | <0.001 |
| Cr3+ (chromium) | >10,000 | <0.001 |
| Ni2+ (Nickel) | >10,000 | <0.001 |
| Mg2+ (Magnesium) | >10,000 | <0.001 |
| Ca2+ (Calcium) | >10,000 | <0.001 |
| Cu2+ (Copper) | >10,000 | <0.001 |
| Mn2+ (Manganese) | >10,000 | <0.001 |
| Samples | Spiked Level (ng/mL) | ic-ELISA | ICP-MS | ICA Strip | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Recovery ± SD (%) | CV (%) | Mean ± SD (ng/mL) | Recovery ± SD (%) | CV (%) | |||||||
| Rice | 0 | ND a | NC b | NC b | ND a | NC b | NC b | − | − | − | − | + |
| 10 | 9.86 ± 1.23 | 98.6 ± 4.4 | 4.4 | 9.93 ± 1.34 | 99.3 ± 4.66 | 4.6 | − | − | ± | + | + | |
| 20 | 18.89 ± 4.23 | 94.5 ± 10.16 | 10.7 | 22.91 ± 4.13 | 114.5 ± 6.33 | 5.5 | ± | ± | + | + | − | |
| 100 | 112.7 ± 9.81 | 112.7 ± 13.59 | 12 | 93.6 ± 8.12 | 93.6 ± 13.12 | 14 | + | + | + | + | ± | |
| 200 | 226.8 ± 13.44 | 113.4 ± 8.93 | 7.8 | 232.9 ± 9.87 | 116.45 ± 10.9 | 9.3 | + | + | + | + | + | |
| Sample | ICP-MS | Visual | |||
|---|---|---|---|---|---|
| 1* Rice | 2287.5 ± 50.23 | + a | + a | + a | + a |
| 2* Rice | 507 ± 17.66 | ± b | + a | + a | + a |
| 3* Rice | 58.5 ± 8.17 | ± b | ± b | ± b | + a |
| 4* Rice | 5.4 ± 0.83 | − c | − c | − c | ± b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Xu, X.; Song, S.; Xu, L.; Liu, L.; Xu, C.; Kuang, H. An Immunochromatographic Assay for the Rapid and Qualitative Detection of Mercury in Rice. Biosensors 2022, 12, 694. https://doi.org/10.3390/bios12090694
Lv S, Xu X, Song S, Xu L, Liu L, Xu C, Kuang H. An Immunochromatographic Assay for the Rapid and Qualitative Detection of Mercury in Rice. Biosensors. 2022; 12(9):694. https://doi.org/10.3390/bios12090694
Chicago/Turabian StyleLv, Shuai, Xinxin Xu, Shanshan Song, Liguang Xu, Liqiang Liu, Chuanlai Xu, and Hua Kuang. 2022. "An Immunochromatographic Assay for the Rapid and Qualitative Detection of Mercury in Rice" Biosensors 12, no. 9: 694. https://doi.org/10.3390/bios12090694
APA StyleLv, S., Xu, X., Song, S., Xu, L., Liu, L., Xu, C., & Kuang, H. (2022). An Immunochromatographic Assay for the Rapid and Qualitative Detection of Mercury in Rice. Biosensors, 12(9), 694. https://doi.org/10.3390/bios12090694




