Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation
Abstract
:1. Introduction
2. Device Design, Working Mechanism and Post-Processing Algorithm
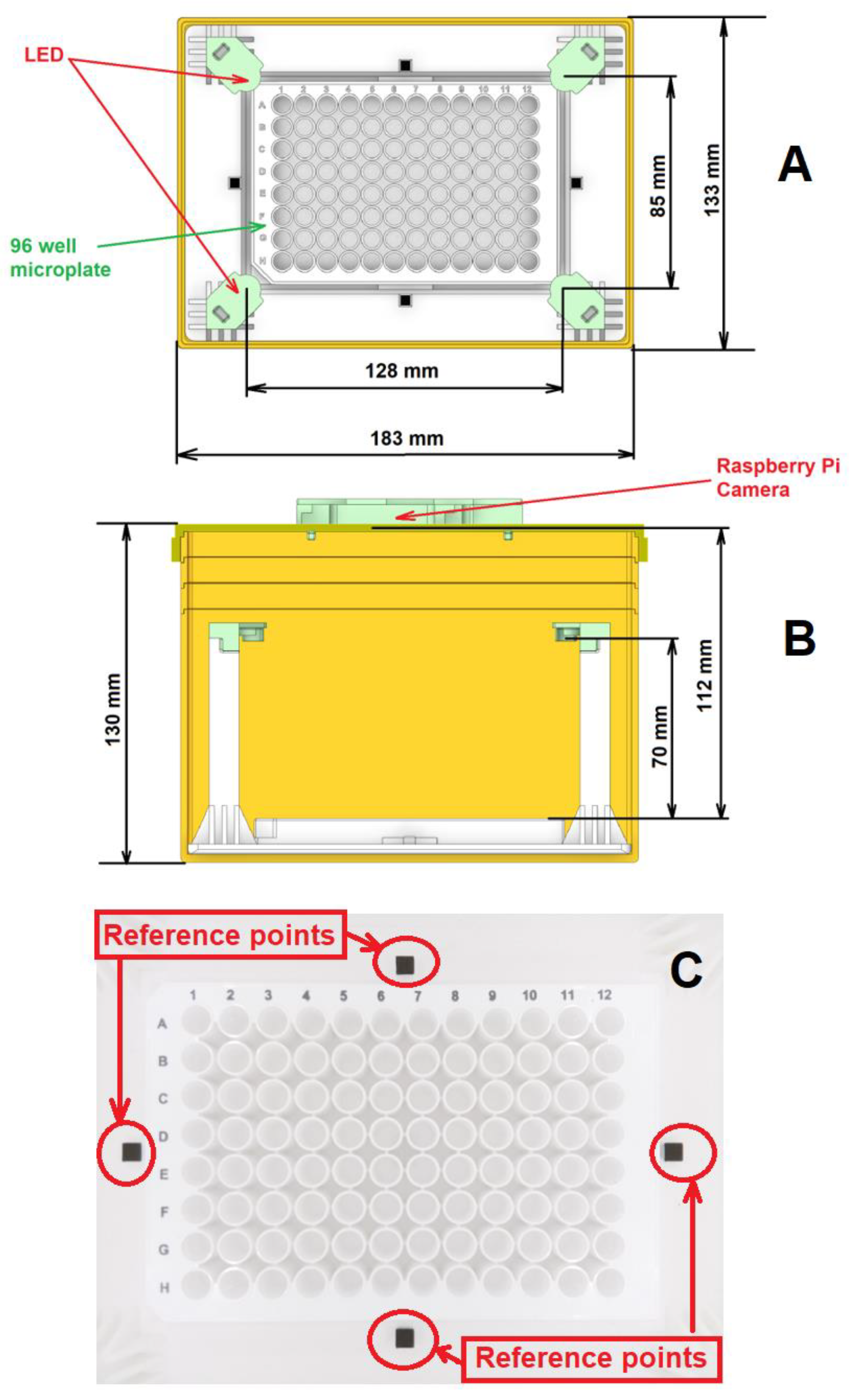
2.1. Device Design and Fabrication
2.1.1. The Camera and Microplate
2.1.2. The White Light Sources
2.1.3. Main Hardware Body
2.2. Reagent Kits and Reaction Mechanism
2.2.1. Creatinine Test Reagent and Sensing Mechanism
2.2.2. Glucose Test Reagent and Sensing Mechanism
2.3. Image Processing Algorithm
2.3.1. Alignment
2.3.2. Calibration
2.3.3. Segmentation and Intensity Measurement
2.3.4. Measurement
2.4. Sample Evaluation Procedure
2.4.1. Commercial Comparison
2.4.2. Clinical Comparison
3. Results and Discussion
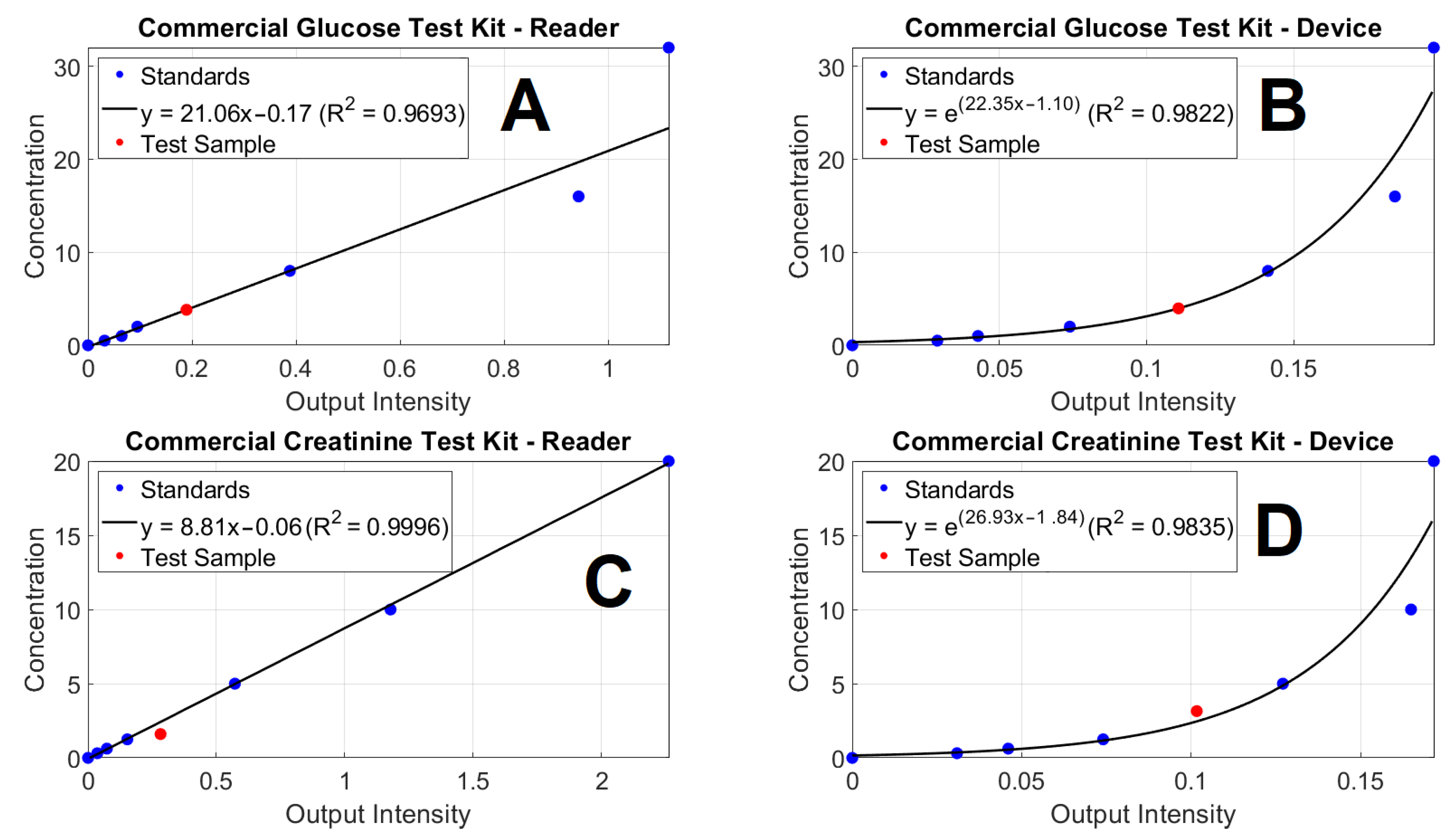
3.1. Evaluation of the Device in Compared with a Commercial Microplate Reader
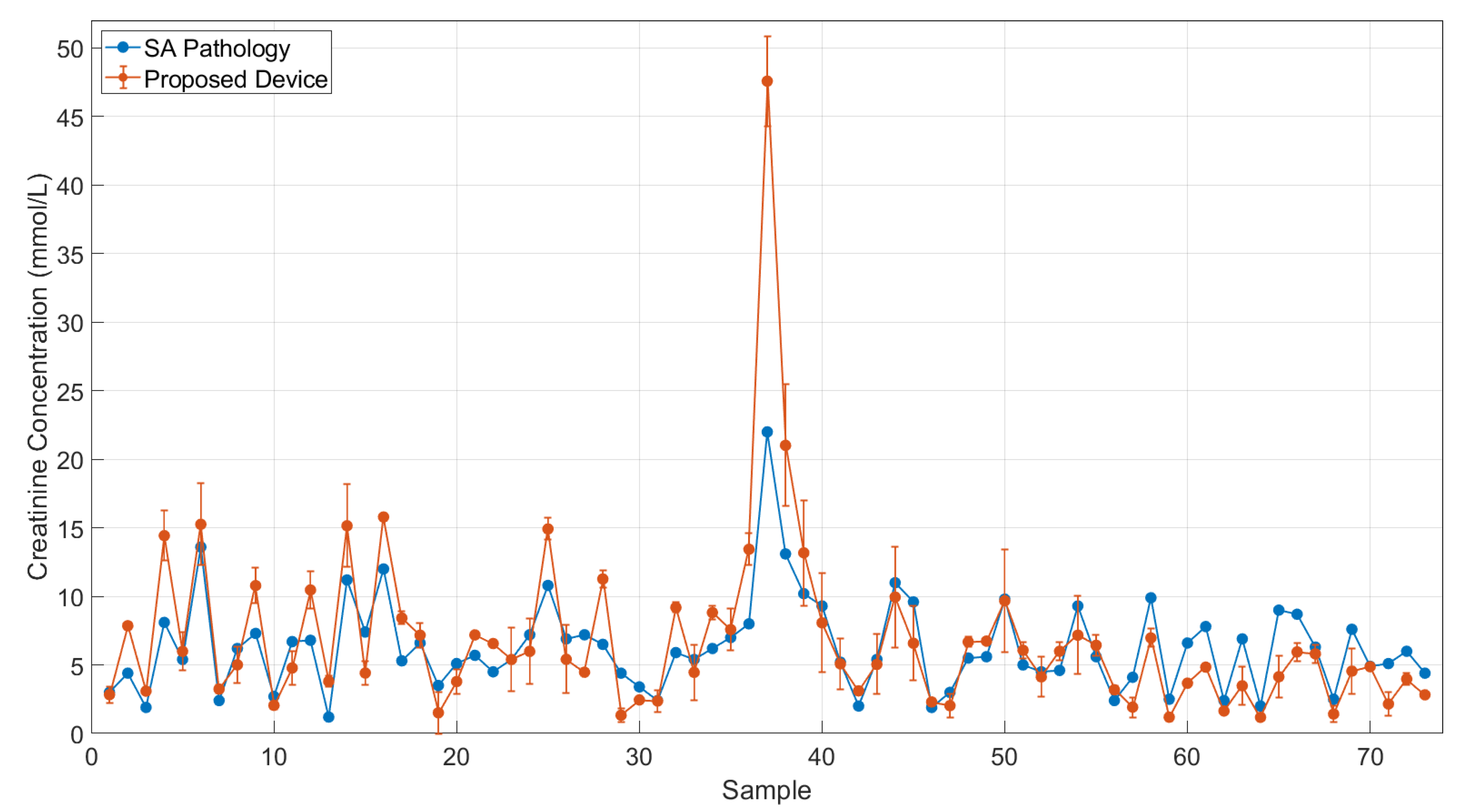
3.2. Clinical Evaluation
3.3. Dilution Factor on Creatinine Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sanjay, S.T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.; Lu, M.; Chen, G.; Tang, D. Urchin-like (gold core)@(platinum shell) nanohybrids: A highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens. Bioelectron. 2015, 70, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, C.M.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay—A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Vessels, M.; Rothenberg, M. Selecting the Detection System—Colorimetric, Fluorescent, Luminescent Methods for ELISA Assays; ELISA Technical Bulletin—No 5; Corning Life Sciences: Kennebunk, ME, USA, 2017; pp. 1–14. [Google Scholar]
- Pham, A.T.T.; Wallace, A.; Zhang, X.; Tohl, D.; Fu, H.; Chuah, C.; Reynolds, K.J.; Ramsey, C.; Tang, Y. Optical-Based Biosensors and Their Portable Healthcare Devices for Detecting and Monitoring Biomarkers in Body Fluids. Diagnostics 2021, 11, 1285. [Google Scholar] [CrossRef]
- Cho, Y.-T.; Su, H.; Wu, W.-J.; Wu, D.-C.; Hou, M.-F.; Kuo, C.-H.; Shiea, J. Biomarker Characterization by MALDI-TOF/MS. Adv. Clin. Chem. 2015, 69, 209–254. [Google Scholar]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Bergua, J.F.; Alvarez-Diduk, R.; Idili, A.; Parolo, C.; Maymo, M.; Hu, L.; Merkoci, A. Low-cost, user-friendly, all-integrated smartphone-based microplate reader for optical-based biological and chemical analyses. Anal. Chem. 2022, 94, 1271–1285. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Ringuet, S.; Sassano, L.; Johnson, Z.I.J. A suite of microplate reader-based colorimetric methods to quantify ammonium, nitrate, orthophosphate and silicate concentrations for aquatic nutrient monitoring. Environ. Monit. 2011, 13, 370–376. [Google Scholar] [CrossRef]
- Feng, S.; Tseng, D.; Di Carlo, D.; Garner, O.B.; Ozcan, A. High-throughput and automated diagnosis of antimicrobial resistance using a cost-effective cellphone-based micro-plate reader. Sci. Rep. 2016, 6, 39203. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, Z.; Li, X.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Novel versatile smart phone based Microplate readers for on-site diagnoses. Biosens. Bioelectron. 2016, 81, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Naude, N.; Demissie, M.; Crivaro, A.; Kamoun, M.; Wang, P.; Li, L. Analytical validation of an ultra low-cost mobile phone microplate reader for infectious disease testing. Clin. Chim. Acta 2018, 482, 21–26. [Google Scholar] [CrossRef]
- Ogirala, T.; Eapen, A.; Salvante, K.G.; Rapaport, T.; Nepomnaschy, P.A.; Parameswaran, A.M. Smartphone-based colorimetric ELISA implementation for determination of women’s reproductive steroid hormone profiles. Med. Biol. Eng. Comput. 2017, 55, 1735–1741. [Google Scholar] [CrossRef]
- Shabut, A.M.; Hoque Tania, M.; Lwin, K.T.; Evans, B.A.; Yusof, N.A.; Abu-Hassan, K.J.; Hossain, M.A. An intelligent mobile-enabled expert system for tuberculosis disease diagnosis in real time. Expert Syst. Appl. 2018, 114, 65–77. [Google Scholar] [CrossRef]
- Zhdanov, A.; Keefe, J.; Franco-Waite, L.; Konnaiyan, K.R.; Pyayt, A. Mobile phone based ELISA (MELISA). Biosens. Bioelectron. 2018, 103, 138–142. [Google Scholar] [CrossRef]
- Agu, E.; Pedersen, P.; Strong, D.; Tulu, B.; He, Q.; Wang, L.; Li, Y. The smartphone as a medical device: Assessing enablers, benefits and challenges. IEEE Int. Conf. Sens. Commun. Netw. 2013, 2013, 76–80. [Google Scholar]
- Zhang, X.; Yao, B.; Hu, Q.; Hong, Y.; Wallace, A.; Reynolds, K.; Ramsey, C.; Maeder, A.; Reed, R.; Tang, Y. Detection of biomarkers in body fluids using bioprobes based on aggregation-induced emission fluorogens. Mater. Chem. Front. 2020, 4, 2548–2570. [Google Scholar] [CrossRef]
- Pham, A.T.T.; Tohl, D.; Wallace, A.; Hu, Q.; Li, J.; Reynolds, K.J.; Tang, Y. Developing a fluorescent sensing based portable medical open-platform—A case study for albuminuria measurement in chronic kidney disease screening and monitoring. Sens. Biosens. Res. 2022, 37, 100504. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Derindag, G.; Akgul, H.M.; Kiziltunc, A.; Ozkan, H.I.; Kiziltunc Ozmen, H.; Akgul, N. Evaluation of saliva glutathione, glutathione peroxidase, and malondialdehyde levels inhead-neck radiotherapy patients. Turk. J. Med. Sci. 2021, 51, 644–649. [Google Scholar] [CrossRef]
- Lewińska, I.; Speichert, M.; Granica, M.; Tymecki, Ł. Colorimetric point-of-care paper-based sensors for urinary creatinine with smartphone readout. Sens. Actuators B Chem. 2021, 340, 129915. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific detection of D-glucose by a tetraphenylethene-based fluorescent sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef]
- Chen, T.; Xie, N.; Viglianti, L.; Zhou, Y.; Tan, H.; Tang, B.Z.; Tang, Y. Quantitative urinalysis using aggregation-induced emission bioprobes for monitoring chronic kidney disease. Faraday Dis. 2017, 196, 351–362. [Google Scholar] [CrossRef]
- Kim, S.C.; Jalal, U.M.; Im, S.B.; Ko, S.; Shim, J.S. A smartphone-based optical platform for colorimetric analysis of microfluidic device. Sens. Actuators B Chem 2017, 239, 52–59. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. A 3D Printed Lightbox for Enhancing Nitrate Detection in the Field Using Microfluidic Paper-Based Devices. Presented Innov. Microfluid. 2022, 3, 21. [Google Scholar]
- Choudhury, A.K.R. Colour Measurement Instruments; Woodhead Publishing Limited: Philadelphia, PA, USA, 2014; pp. 221–269. [Google Scholar]
- Delanghe, J.R.; Speeckaert, M.M. Creatinine determination according to Jaffe-what does it stand for? Clin. Kidney J. 2011, 4, 83–86. [Google Scholar] [CrossRef]
- Tohl, D.; Pham AT, T.; Li, J.; Tang, Y. Point-of-care image-based quantitative urinalysis with commercial reagent strips: Design and clinical evaluation. Biosens. Bioelectron. 2022; Submitted. [Google Scholar]
- Tohl, D.; Teferra, M.N.; Wallace, A.; Pham, A.T.T.; Tang, Y. Re-Referencing and Calibration for Robust Ratiometric Light Intensity Measurement. IEEE Trans. Instrum. Meas. 2022, 71, 4505208. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Ahn, Y.J.; Kim, M.; Lee, G.-J. In situ detection of hydrogen sulfide in 3D-cultured, live prostate cancer cells using a paper-integrated analytical device. Chemosensors 2022, 10, 27. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based microfluidic device for improved detection of nitrate in water. Sensors 2020, 21, 102. [Google Scholar] [CrossRef]
- Ferreira, F.; Mesquita, R.B.R.; Rangel, A. Novel microfluidic paper-based analytical devices (μPADs) for the determination of nitrate and nitrite in human saliva. Talanta 2020, 219, 121183. [Google Scholar] [CrossRef] [PubMed]
- Pagnutti, M.; Ryan, R.E.; Cazenavette, G.; Gold, M.; Harlan, R.; Leggett, E.; Pagnutti, J. Laying the foundation to use Raspberry Pi 3 V2 camera module imagery for scientific and engineering purposes. J. Elect. Imaging 2017, 26, 013014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.J.; Hoshino, K. Chapter 5 Optical transducers: Optical molecular sensing and spectroscopy. Mol. Sens. Nanodevices 2019, 2019, 231–309. [Google Scholar]


| Dilution Factor | 1st Sample | 2nd Sample | Average | SD of Two Samples’ Results |
|---|---|---|---|---|
| 10 | 2.83 | 5.31 | 4.07 | 1.75 |
| 50 | 6.00 | 6.30 | 6.15 | 0.21 |
| 100 | 7.14 | 7.41 | 7.28 | 0.19 |
| 150 | 7.28 | 7.64 | 7.46 | 0.25 |
| 200 | 5.28 | 6.68 | 6.68 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, A.T.T.; Tohl, D.; Hu, Q.; Li, J.; Reynolds, K.J.; Tang, Y. Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation. Biosensors 2022, 12, 723. https://doi.org/10.3390/bios12090723
Pham ATT, Tohl D, Hu Q, Li J, Reynolds KJ, Tang Y. Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation. Biosensors. 2022; 12(9):723. https://doi.org/10.3390/bios12090723
Chicago/Turabian StylePham, Anh Tran Tam, Damian Tohl, Qi Hu, Jordan Li, Karen J. Reynolds, and Youhong Tang. 2022. "Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation" Biosensors 12, no. 9: 723. https://doi.org/10.3390/bios12090723
APA StylePham, A. T. T., Tohl, D., Hu, Q., Li, J., Reynolds, K. J., & Tang, Y. (2022). Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation. Biosensors, 12(9), 723. https://doi.org/10.3390/bios12090723






