Advanced Textile-Based Wearable Biosensors for Healthcare Monitoring
Abstract
:1. Introduction
2. Materials and Preparation Methods for Textile-Based Wearable Biosensors
2.1. Materials of Fabrication
2.2. Methods for Fabrication
2.2.1. Attachment and Embedding
2.2.2. Coating and Printing

2.2.3. Spinning Technology

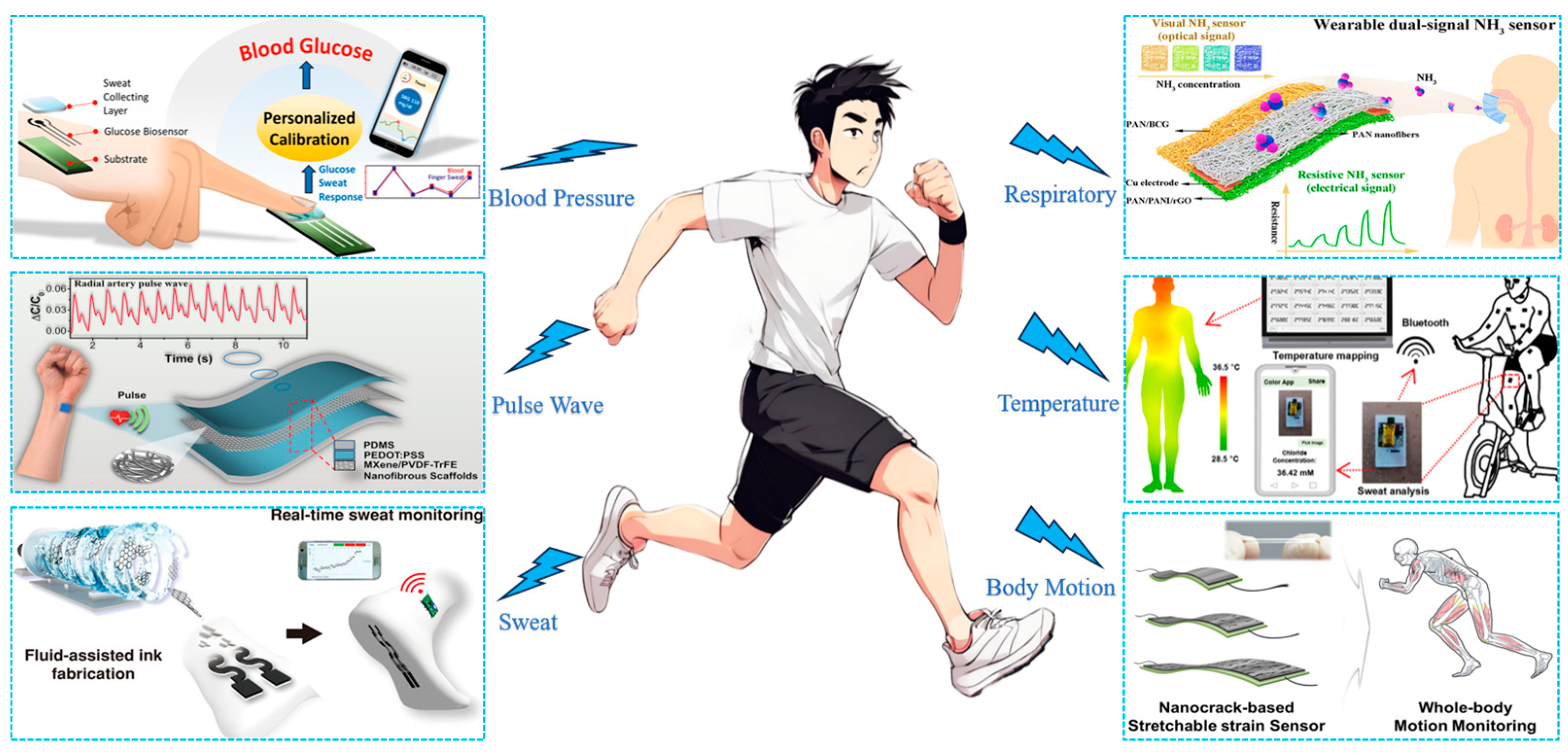
3. Textile-Based Wearable Biosensors and Their Applications
3.1. Vital Signs Testing
3.1.1. Tissue Pressure
| Analyte | Materials | Sensor Mechanism | Fabrication Method | Response Time | Gauge Factor | Sensitivity | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Breathing, Pulse Wave | Graphene | Piezoresistive | Impregnation Coating | - | −26 (15%) | - | 10,000 cycles | [150] |
| Pulse Wave | CNTs/PDMS | Piezoresistive | Shrinking Fabrication | <20 ms | - | 278.5 kPa–1 | - | [151] |
| Breathing, Pulse Wave | AuNW/textile | Piezoresistive | Impregnation Coating | <40 ms | - | 914.970 kPa–1 | 30,000 cycles | [152] |
| Motion, Respiration | PPy/polyester/spandex | Piezoelectric | Spray Coating | <15 ms | −0.46 (0–71%) | - | 500 times | [153] |
| Heartbeat, Motion | MWCNT/PVDF-HFP | Piezoelectric | Electrospinning | - | −0.7 (40%) | 0.25 kPa–1 | 10,000 cycles | [156] |
| Breathing, Pulse Wave | MXene/PVDF | Piezoelectric | Electrospinning | 3.1 ms | - | 0.0480 V/N | - | [137] |
| Blood Pressure | TiO2 nanofibrous network | Capacitive | Electrospinning | <16 ms | −2.1 (60%) | 4.4 kPa–1 | 50,000 cycles | [139] |
| Motion, Heartbeat | PVA/Ag NFs | Capacitive | Electrospinning | <10 ms | - | 0.283 kPa–1 | ≥20,000 cycles | [160] |
| Pulse, Respiratory | LMs@PDMS | Capacitive | Stencil Printing | - | 0.33 (0–50%) | 0.0021 kPa–1 | 1000 cycles | [161] |
| Heartbeat, Respiratory | Conductive/nylon yarns | Triboelectric | Weaving | 20 ms | - | 7.84 mV Pa−1 | >100,000 cycles | [162] |
| Pulse Wave, Motion | Nylon yarns/PTFE filament | Triboelectric | Twisting and Weaving | <15 ms | 7.2 (30%) | 1.33 V·kPa–1 | 4200 cycles | [163] |

3.1.2. Body Motion
| Materials | Fabrication Method | Strain | Gauge Factor | Stability | Ref. |
|---|---|---|---|---|---|
| Graphene | Coating | 10% | 124 | 1000 cycles | [173] |
| MXene/PU | Wet spinning | 50% | 238 | 1000 cycles | [164] |
| 152% | 12,900 | ||||
| PPy/Al | Impregnation coating | 25.3% | 15 | 600 cycles | [174] |
| CNTs/PDMS | Drop coating | 5%–30% | 10 | 5000 cycles | [175] |
| >30% | 200 | ||||
| RGO | Adhere | 0%–60% | 16.2 | >5000 cycles | [176] |
| >60% | 150 |

3.1.3. Temperature and Humidity
3.2. Sweat Analysis
| Detectives | Material | Response Time | Sensitivity | Detection Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| Glucose | Au NFs | 2~3 s | 63.9 μA/mM/cm2 | 0.1~5 mM | - | [201] |
| Glucose | Conductive Gold Coated Threads | 4~6 s | 126 ± 14 nA/mM | 0.01–100 mM | 301 ± 2 nM | [202] |
| Glucose | Cellulose nanofiber | 3~4 s | - | 0.1~3 mM | 0.1 mM | [203] |
| Lactic acid | Gold fibers | 1~3 s | 14.6 μA/mM cm2 | 0.1~5 mM | 14.6 μA/Mm/cm2 | [204] |
| Lactic acid | Nanofiber | 1~2 s | 70.3 nA Mm−1 | - | 0.38 mM | [205] |
| PH | Sensing Fiber | 0.15~0.25 h | - | PH 4.0~7.0 | PH 3.0 | [210] |
| PH | Thermoplastic Polyurethane | 1.5~2 h | 0.14–0.33 PH | PH 5.5~7.0 | PH 4.0 | [211] |
| PH | Cotton Fabric | 0.2~0.4 h | - | PH 1.0~14.0 | PH 1.0 | [212] |
| Cortisol | Nanometer | 0.15~0.25 h | 0.25 Ohm/ng mL−1 | 1 pg/mL~1 μg/mL | 1 pg/mL | [213] |
| Cortisol | Paper Fibers | 0.15~0.25 h | - | 25–50 g/dL | 21.5 g/dL | [216] |
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, X.; Stylios, G. A Hybrid Textile Electrode for Electrocardiogram (ECG) Measurement and Motion Tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- André Vitor Alves, A.; Lorenzzo De Angeli, C.; Antonio Dionizio De Albuquerque, N.; Daniel De Assunção, C.; Rodrigo Calado Nunes, E.S.; Almiro, J.; Machado, J. Myositis Ossificans Traumatica of Bilateral Medial and Lateral Pterygoid Muscles: A Case Report. Int. Arch. Oral Maxillofac. Surg. 2019, 3, 22. [Google Scholar] [CrossRef]
- Atalay, A.; Sanchez, V.; Atalay, O.; Vogt, D.M.; Haufe, F.; Wood, R.J.; Walsh, C.J. Batch Fabrication of Customizable Silicone-Textile Composite Capacitive Strain Sensors for Human Motion Tracking. Adv. Mater. Technol. 2017, 2, 1700136. [Google Scholar] [CrossRef]
- Babusiak, B.; Borik, S.; Balogova, L. Textile Electrodes in Capacitive Signal Sensing Applications. Measurement 2018, 114, 69–77. [Google Scholar] [CrossRef]
- Bhat, A.; Ambrose, J.W.; Yeow, R.C.-H. Ultralow-Latency Textile Sensors for Wearable Interfaces with a Human-in-Loop Sensing Approach. Soft Robot. 2023, 10, 431–442. [Google Scholar] [CrossRef]
- Bremer, K.; Weigand, F.; Zheng, Y.; Alwis, L.S.; Helbig, R.; Roth, B. Structural Health Monitoring Using Textile Reinforcement Structures with Integrated Optical Fiber Sensors. Sensors 2017, 17, 345. [Google Scholar] [CrossRef]
- Chen, L.; Lu, M.; Yang, H.; Salas Avila, J.R.; Shi, B.; Ren, L.; Wei, G.; Liu, X.; Yin, W. Textile-Based Capacitive Sensor for Physical Rehabilitation via Surface Topological Modification. ACS Nano 2020, 14, 8191–8201. [Google Scholar] [CrossRef]
- Cheng, J.; Sundholm, M.; Zhou, B.; Hirsch, M.; Lukowicz, P. Smart-Surface: Large Scale Textile Pressure Sensors Arrays for Activity Recognition. Pervasive Mob. Comput. 2016, 30, 97–112. [Google Scholar] [CrossRef]
- Jia, J.; Xu, C.; Pan, S.; Xia, S.; Wei, P.; Noh, H.Y.; Zhang, P.; Jiang, X. Conductive Thread-Based Textile Sensor for Continuous Perspiration Level Monitoring. Sensors 2018, 18, 3775. [Google Scholar] [CrossRef]
- Jin, C.; Bai, Z. MXene-Based Textile Sensors for Wearable Applications. ACS Sens. 2022, 7, 929–950. [Google Scholar] [CrossRef]
- Possanzini, L.; Tessarolo, M.; Mazzocchetti, L.; Campari, E.G.; Fraboni, B. Impact of Fabric Properties on Textile Pressure Sensors Performance. Sensors 2019, 19, 4686. [Google Scholar] [CrossRef] [PubMed]
- Hatamie, A.; Angizi, S.; Kumar, S.; Pandey, C.M.; Simchi, A.; Willander, M.; Malhotra, B.D. Textile Based Chemical and Physical Sensors for Healthcare Monitoring. J. Electrochem. Soc. 2020, 167, 037546. [Google Scholar] [CrossRef]
- Hofmann, P.; Walch, A.; Dinkelmann, A.; Selvarayan, S.K.; Gresser, G.T. Woven Piezoelectric Sensors as Part of the Textile Reinforcement of Fiber Reinforced Plastics. Compos. Part Appl. Sci. Manuf. 2019, 116, 79–86. [Google Scholar] [CrossRef]
- Islam, G.N.; Ali, A.; Collie, S. Textile Sensors for Wearable Applications: A Comprehensive Review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Recent Advances in Noninvasive Flexible and Wearable Wireless Biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef]
- Simić, M.; Stavrakis, A.K.; Sinha, A.; Premčevski, V.; Markoski, B.; Stojanović, G.M. Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors 2022, 12, 339. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. Role of Graphene in Biosensor and Protective Textile against Viruses. Med. Hypotheses 2020, 144, 110253. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Meng, J.; Wang, A.C.; Tan, P.; Zou, Y.; Yuan, Z.; Lu, J.; Pan, C.; Fan, Y.; et al. Reversible Conversion between Schottky and Ohmic Contacts for Highly Sensitive, Multifunctional Biosensors. Adv. Funct. Mater. 2020, 30, 1907999. [Google Scholar] [CrossRef]
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.-H.; Ishidai, H.; Hattori, R. Reflectance-Based Organic Pulse Meter Sensor for Wireless Monitoring of Photoplethysmogram Signal. Biosensors 2019, 9, 87. [Google Scholar] [CrossRef]
- Yang, A.; Yan, F. Flexible Electrochemical Biosensors for Health Monitoring. ACS Appl. Electron. Mater. 2021, 3, 53–67. [Google Scholar] [CrossRef]
- Tan, P.; Xi, Y.; Chao, S.; Jiang, D.; Liu, Z.; Fan, Y.; Li, Z. An Artificial Intelligence-Enhanced Blood Pressure Monitor Wristband Based on Piezoelectric Nanogenerator. Biosensors 2022, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhao, X.; Tat, T.; Xiao, X.; Chen, G.; Xu, J.; Chen, J. All-in-One Conformal Epidermal Patch for Multimodal Biosensing. Matter 2021, 4, 1102–1105. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Multimodal Wrist Biosensor for Wearable Cuff-Less Blood Pressure Monitoring System. Sci. Rep. 2019, 9, 7947. [Google Scholar] [CrossRef]
- Wiorek, A.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Epidermal Patch with Glucose Biosensor: PH and Temperature Correction toward More Accurate Sweat Analysis during Sport Practice. Anal. Chem. 2020, 92, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Zhou, X.; Gong, P.; Zhao, Y. U-Fiber-Based Biosensor for Temperature-Compensated Acetylcholine-Specific Measurement. Opt. Lett. 2023, 48, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Yokus, M.A.; Songkakul, T.; Pozdin, V.A.; Bozkurt, A.; Daniele, M.A. Wearable Multiplexed Biosensor System toward Continuous Monitoring of Metabolites. Biosens. Bioelectron. 2020, 153, 112038. [Google Scholar] [CrossRef]
- Jauregui-Vazquez, D.; Lozano-Sotomayor, P.; Mejía-Benavides, J.E.; Díaz-Cervantes, E. Binding Analysis of Functionalized Multimode Optical-Fiber Sandwich-like Structure with Organic Polymer and Its Sensing Application for Humidity and Breath Monitoring. Biosensors 2021, 11, 324. [Google Scholar] [CrossRef]
- Chen, X.; Ma, K.; Ou, J.; Mo, D.; Lian, H.; Li, X.; Cui, Z.; Luo, Y. Fast-Response Non-Contact Flexible Humidity Sensor Based on Direct-Writing Printing for Respiration Monitoring. Biosensors 2023, 13, 792. [Google Scholar] [CrossRef]
- Myung, D.; Hussain, S.; Park, S.-Y. Photonic Calcium and Humidity Array Sensor Prepared with Reactive Cholesteric Liquid Crystal Mesogens. Sens. Actuators B Chem. 2019, 298, 126894. [Google Scholar] [CrossRef]
- Ye, X.; Shi, B.; Li, M.; Fan, Q.; Qi, X.; Liu, X.; Zhao, S.; Jiang, L.; Zhang, X.; Fu, K. All-Textile Sensors for Boxing Punch Force and Velocity Detection. Nano Energy 2022, 97, 107114. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Reddy, V.S.; Ji, D.; Ramakrishna, S.; Xu, L. Flexible Textile-Based Sweat Sensors for Wearable Applications. Biosensors 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhu, C.; Wan, J.; Li, Y.; Hong, X. Review of Graphene-Based Textile Strain Sensors, with Emphasis on Structure Activity Relationship. Polymers 2021, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Li, Y.; Wang, P. Textile-Based Flexible Pressure Sensors: A Review. Polym. Rev. 2022, 62, 65–94. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.L.; Xia, Y.; Zhang, P.F.; Kirk, T.V.; Chen, X.D. Textile-Only Capacitive Sensors for Facile Fabric Integration without Compromise of Wearability. Adv. Mater. Technol. 2019, 4, 1900485. [Google Scholar] [CrossRef]
- Yan, S.; Dinh, D.K.; Shang, G.; Wang, S.; Zhao, W.; Liu, X.; Robinson, R.; Lombardi, J.P., III; He, N.; Lu, S. Nano-Filamented Textile Sensor Platform with High Structure Sensitivity. ACS Appl. Mater. Interfaces 2022, 14, 15391–15400. [Google Scholar] [CrossRef]
- Nie, B.; Huang, R.; Yao, T.; Zhang, Y.; Miao, Y.; Liu, C.; Liu, J.; Chen, X. Textile-Based Wireless Pressure Sensor Array for Human-Interactive Sensing. Adv. Funct. Mater. 2019, 29, 1808786. [Google Scholar] [CrossRef]
- Paiva, A.; Ferreira, F.; Catarino, A.; Carvalho, M.; Carvalho, H. Design of Smart Garments for Sports and Rehabilitation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012083. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gandla, S.; Gupta, D. Highly Sensitive, Rugged, and Wearable Fabric Strain Sensor Based on Graphene Clad Polyester Knitted Elastic Band for Human Motion Monitoring. Adv. Mater. Interfaces 2019, 6, 1900409. [Google Scholar] [CrossRef]
- Rauf, S.; Vijjapu, M.T.; Andrés, M.A.; Gascón, I.; Roubeau, O.; Eddaoudi, M.; Salama, K.N. Highly Selective Metal–Organic Framework Textile Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 29999–30006. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef] [PubMed]
- Kil, M.S.; Kim, S.J.; Park, H.J.; Yoon, J.H.; Jeong, J.-M.; Choi, B.G. Highly Stretchable Sensor Based on Fluid Dynamics-Assisted Graphene Inks for Real-Time Monitoring of Sweat. ACS Appl. Mater. Interfaces 2022, 14, 48072–48080. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Liu, Y.; Li, B.; Li, H.; Zhang, X.; Lv, C.; Dong, H. Wearable Dual-Signal NH3 Sensor with High Sensitivity for Non-Invasive Diagnosis of Chronic Kidney Disease. Langmuir 2023, 39, 3420–3430. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Kim, J.K.; Choi, J. Multifunctional Wearable System for Mapping Body Temperature and Analyzing Sweat. ACS Sens. 2023, 8, 1980–1988. [Google Scholar] [CrossRef]
- Jeon, H.; Hong, S.K.; Kim, M.S.; Cho, S.J.; Lim, G. Omni-Purpose Stretchable Strain Sensor Based on a Highly Dense Nanocracking Structure for Whole-Body Motion Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 41712–41721. [Google Scholar] [CrossRef]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Possanzini, L.; Scavetta, E.; Fraboni, B. Textile Chemical Sensors Based on Conductive Polymers for the Analysis of Sweat. Polymers 2021, 13, 894. [Google Scholar] [CrossRef]
- Kim, G.; Vu, C.C.; Kim, J. Single-Layer Pressure Textile Sensors with Woven Conductive Yarn Circuit. Appl. Sci. 2020, 10, 2877. [Google Scholar] [CrossRef]
- Lugoda, P.; Hughes-Riley, T.; Morris, R.; Dias, T. A Wearable Textile Thermograph. Sensors 2018, 18, 2369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, R.; Xu, X.; Iqbal, S.; Hu, J. Fibers/Textiles-Based Flexible Sweat Sensors: A Review. ACS Mater. Lett. 2023, 5, 1420–1440. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Wang, F.; Kong, D. Stretchable and Superwettable Colorimetric Sensing Patch for Epidermal Collection and Analysis of Sweat. ACS Sens. 2021, 6, 2261–2269. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-Powered and Wearable Biosensors for Healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Liang, X.; Zhang, M.; Liang, H.; Zhang, Y. Silk Materials for Intelligent Fibers and Textiles: Potential, Progress and Future Perspective. Acta Phys. Chim. Sin. 2022, 38, 2103034. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized Silk Fabric for Ultrastretchable, Highly Sensitive, and Wearable Strain Sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef] [PubMed]
- Balla, V.K.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive Manufacturing of Natural Fiber Reinforced Polymer Composites: Processing and Prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Guan, F.; Xie, Y.; Wu, H.; Meng, Y.; Shi, Y.; Gao, M.; Zhang, Z.; Chen, S.; Chen, Y.; Wang, H.; et al. Silver Nanowire–Bacterial Cellulose Composite Fiber-Based Sensor for Highly Sensitive Detection of Pressure and Proximity. ACS Nano 2020, 14, 15428–15439. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Thomas, V.M.; Yoo, C.G.; Ozcan, S.; Deng, Y.; Nelson, K.; Ragauskas, A.J. Recent Advancements of Plant-Based Natural Fiber–Reinforced Composites and Their Applications. Compos. Part B Eng. 2020, 200, 108254. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Nanoscale LnMOF-Functionalized Nonwoven Fibers Protected by a Polydimethylsiloxane Coating Layer as a Highly Sensitive Ratiometric Oxygen Sensor. J. Mater. Chem. C 2016, 4, 8514–8521. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L.; Gopalakrishna, K.; Yogesha, B. Applications of Natural Fibers and Its Composites: An Overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Enabled Wearable Sensors: Material Synthesis, Sensor Design, and Personal Health Monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef]
- Demir, B.; Henderson, L.C.; Walsh, T.R. Design Rules for Enhanced Interfacial Shear Response in Functionalized Carbon Fiber Epoxy Composites. ACS Appl. Mater. Interfaces 2017, 9, 11846–11857. [Google Scholar] [CrossRef] [PubMed]
- Ruth, S.R.A.; Feig, V.R.; Tran, H.; Bao, Z. Microengineering Pressure Sensor Active Layers for Improved Performance. Adv. Funct. Mater. 2020, 30, 2003491. [Google Scholar] [CrossRef]
- Lin, L.; Lopez, R.; Ramon, G.Z.; Coronell, O. Investigating the Void Structure of the Polyamide Active Layers of Thin-Film Composite Membranes. J. Membr. Sci. 2016, 497, 365–376. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Interfacial Composition, Structure, and Properties of Ionic Liquids and Conductive Polymers for the Construction of Chemical Sensors and Biosensors: A Perspective. Curr. Opin. Electrochem. 2020, 23, 47–56. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; AlSaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic Liquid-Carbon Nanomaterial Hybrids for Electrochemical Sensor Applications: A Review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial Properties and Toxicity from Metallic Nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Chou Chau, Y.-F.; Chen, K.-H.; Chiang, H.-P.; Lim, C.M.; Huang, H.J.; Lai, C.-H.; Kumara, N.T.R.N. Fabrication and Characterization of a Metallic–Dielectric Nanorod Array by Nanosphere Lithography for Plasmonic Sensing Application. Nanomaterials 2019, 9, 1691. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon Nanomaterials-Based Electrochemical Aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Zhou, K.; Dai, K.; Liu, C.; Shen, C. Flexible Conductive Polymer Composites for Smart Wearable Strain Sensors. SmartMat 2020, 1, e1010. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors Based on Conductive Polymers and Their Composites: A Review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Zhang, S.; Yin, R.; Liu, X.; He, Y.; Dai, K.; Shan, C.; Guo, J.; Liu, C. Electrically Conductive Polymer Composites for Smart Flexible Strain Sensors: A Critical Review. J. Mater. Chem. C 2018, 6, 12121–12141. [Google Scholar] [CrossRef]
- Muginova, S.V.; Myasnikova, D.A.; Kazarian, S.G.; Shekhovtsova, T.N. Applications of Ionic Liquids for the Development of Optical Chemical Sensors and Biosensors. Anal. Sci. 2017, 33, 261–265. [Google Scholar] [CrossRef]
- Khan, A.; Gunawan, C.A.; Zhao, C. Oxygen Reduction Reaction in Ionic Liquids: Fundamentals and Applications in Energy and Sensors. ACS Sustain. Chem. Eng. 2017, 5, 3698–3715. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Wang, F.-X.; Li, J.-J.; Peng, H.-D.; Yan, J.-H.; Pan, G.-B. Wearable Wide-Range Strain Sensors Based on Ionic Liquids and Monitoring of Human Activities. Sensors 2017, 17, 2621. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, Q.; Sun, Y.; Wang, L.; Sun, Q.; Wang, L. High-Performance NO2 Sensors Based on Ultrathin Heterogeneous Interface Layers. Adv. Mater. Interfaces 2020, 7, 1901579. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Chen, S.-C.; Zhao, N. Emerging Technologies of Flexible Pressure Sensors: Materials, Modeling, Devices, and Manufacturing. Adv. Funct. Mater. 2019, 29, 1808509. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical Sensors Based on Magnetic Molecularly Imprinted Polymers: A Review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.-T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.; Smoukov, S.K. Electroactive Polymers for Sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-Based Sensors: A Review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Yuan, X.; Wei, Y.; Chen, S.; Wang, P.; Liu, L. Bio-Based Graphene/Sodium Alginate Aerogels for Strain Sensors. RSC Adv. 2016, 6, 64056–64064. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Wang, X.; He, S.; Yao, F.; Li, J. A Transparent, Ultrastretchable and Fully Recyclable Gelatin Organohydrogel Based Electronic Sensor with Broad Operating Temperature. J. Mater. Chem. A 2020, 8, 4447–4456. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between Gelatin and Sodium Alginate: UV and FTIR Studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric Sensor Array Based on Gold Nanoparticles: Design Principles and Recent Advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, Mechanisms, and Application of Carbon Quantum Dots in Sensors: A Review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Zahid, M.; Anwer Rathore, H.; Tayyab, H.; Ahmad Rehan, Z.; Abdul Rashid, I.; Lodhi, M.; Zubair, U.; Shahid, I. Recent Developments in Textile Based Polymeric Smart Sensor for Human Health Monitoring: A Review. Arab. J. Chem. 2022, 15, 103480. [Google Scholar] [CrossRef]
- Oh, J.H.; Hong, S.Y.; Park, H.; Jin, S.W.; Jeong, Y.R.; Oh, S.Y.; Yun, J.; Lee, H.; Kim, J.W.; Ha, J.S. Fabrication of High-Sensitivity Skin-Attachable Temperature Sensors with Bioinspired Microstructured Adhesive. ACS Appl. Mater. Interfaces 2018, 10, 7263–7270. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Franklin, S.; Hassani, F.A.; Yokota, T.; Nayeem, M.O.G.; Wang, Y.; Leib, R.; Cheng, G.; Franklin, D.W.; Someya, T. Nanomesh Pressure Sensor for Monitoring Finger Manipulation without Sensory Interference. Science 2020, 370, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Ravali, K.V.; Khan, P.F.; Nisha, M.S. Structural Health Monitoring of Glass Fiber Reinforced Polymer Using Nanofiber Sensor.
- Jeong, W.; Song, J.; Bae, J.; Nandanapalli, K.R.; Lee, S. Breathable Nanomesh Humidity Sensor for Real-Time Skin Humidity Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 44758–44763. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.R.; Lee, C.K.; Parker-Gilham, J.; de Anda, J.; Xia, A.; Zhao, K.; Murakami, K.; Tseng, B.S.; Hoffman, L.R.; Jin, F. Heterogeneity in Surface Sensing Suggests a Division of Labor in Pseudomonas Aeruginosa Populations. Elife 2019, 8, e45084. [Google Scholar] [CrossRef]
- Vu, C.C.; Kim, J. Highly Elastic Capacitive Pressure Sensor Based on Smart Textiles for Full-Range Human Motion Monitoring. Sens. Actuators Phys. 2020, 314, 112029. [Google Scholar] [CrossRef]
- Romano, C.; Nicolò, A.; Innocenti, L.; Bravi, M.; Miccinilli, S.; Sterzi, S.; Sacchetti, M.; Schena, E.; Massaroni, C. Respiratory Rate Estimation during Walking and Running Using Breathing Sounds Recorded with a Microphone. Biosensors 2023, 13, 637. [Google Scholar] [CrossRef]
- Lu, S.; Chen, D.; Liu, C.; Jiang, Y.; Wang, M. A 3-D Finger Motion Measurement System via Soft Strain Sensors for Hand Rehabilitation. Sens. Actuators Phys. 2019, 285, 700–711. [Google Scholar] [CrossRef]
- Lan, T.; He, Q.; Lan, Y.; Guo, T.; Sun, Y. Operational Environment Analysis of HVDC Converter Station for Development of Energy Harvester: Electromagnetic Field and Ambient Power Source. In Proceedings of the 2021 4th International Conference on Energy, Electrical and Power Engineering (CEEPE), Chongqing, China, 25 April 2021; pp. 925–929. [Google Scholar]
- Xiong, J.; Chen, J.; Lee, P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2021, 33, 2002640. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ching, C.T.-S.; Wang, H.-M.D.; Liao, L.-D. Emerging Wearable Biosensor Technologies for Stress Monitoring and Their Real-World Applications. Biosensors 2022, 12, 1097. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhang, J.; Zhang, W.; Chen, X.; Wei, D.; Fang, P.; Shen, Y. Highly Selective NO2 Sensor Based on P-Type Nanocrystalline NiO Thin Films Prepared by Sol–Gel Dip Coating. Ceram. Int. 2018, 44, 753–759. [Google Scholar] [CrossRef]
- Zhao, J.; Huo, L.-H.; Gao, S.; Zhao, H.; Zhao, J.-G. Alcohols and Acetone Sensing Properties of SnO2 Thin Films Deposited by Dip-Coating. Sens. Actuators B Chem. 2006, 115, 460–464. [Google Scholar] [CrossRef]
- Ge, C.; Xie, C.; Cai, S. Preparation and Gas-Sensing Properties of Ce-Doped ZnO Thin-Film Sensors by Dip-Coating. Mater. Sci. Eng. B 2007, 137, 53–58. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yang, S.; Shi, X.; Zhang, D.; Shan, C.; Mi, L.; Liu, C.; Shen, C.; Guo, Z. Ultrasensitive and Highly Compressible Piezoresistive Sensor Based on Polyurethane Sponge Coated with a Cracked Cellulose Nanofibril/Silver Nanowire Layer. ACS Appl. Mater. Interfaces 2019, 11, 10922–10932. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Omami, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghani, S. Reduction of Measurement Noise in a Continuous Glucose Monitor by Coating the Sensor with a Zwitterionic Polymer. Nat. Biomed. Eng. 2018, 2, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Seyedin, S.; Zhang, P.; Naebe, M.; Qin, S.; Chen, J.; Wang, X.; Razal, J.M. Textile Strain Sensors: A Review of the Fabrication Technologies, Performance Evaluation and Applications. Mater. Horiz. 2019, 6, 219–249. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, P.; Ren, M.; Zheng, G.; Dai, K.; Mi, L.; Liu, C.; Shen, C. Significant Stretchability Enhancement of a Crack-Based Strain Sensor Combined with High Sensitivity and Superior Durability for Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 7405–7414. [Google Scholar] [CrossRef]
- Maity, D.; Rajavel, K.; Kumar, R.T.R. Polyvinyl Alcohol Wrapped Multiwall Carbon Nanotube (MWCNTs) Network on Fabrics for Wearable Room Temperature Ethanol Sensor. Sens. Actuators B Chem. 2018, 261, 297–306. [Google Scholar] [CrossRef]
- Kim, T.; Park, C.; Samuel, E.P.; An, S.; Aldalbahi, A.; Alotaibi, F.; Yarin, A.L.; Yoon, S.S. Supersonically Sprayed Washable, Wearable, Stretchable, Hydrophobic, and Antibacterial RGO/AgNW Fabric for Multifunctional Sensors and Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 10013–10025. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, J.; Tolle, C.R.; Zhu, Z. Flexible and Compressible PEDOT: PSS@ Melamine Conductive Sponge Prepared via One-Step Dip Coating as Piezoresistive Pressure Sensor for Human Motion Detection. ACS Appl. Mater. Interfaces 2018, 10, 16077–16086. [Google Scholar] [CrossRef]
- Sánchez, M.; Rincón, M.E. Sensor Response of Sol–Gel Multiwalled Carbon Nanotubes-TiO2 Composites Deposited by Screen-Printing and Dip-Coating Techniques. Sens. Actuators B Chem. 2009, 140, 17–23. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E.; et al. Conductive Fiber-Based Ultrasensitive Textile Pressure Sensor for Wearable Electronics. Adv. Mater. 2015, 27, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Wang, F.; Su, W.; Yang, L.; Lv, J.; Fu, G.; Li, X.; Liu, Q.; Sun, T. Surface Plasmon Resonance (SPR) Infrared Sensor Based on D-Shape Photonic Crystal Fibers with ITO Coatings. Opt. Commun. 2020, 464, 125496. [Google Scholar] [CrossRef]
- Rehman, A.; Houshyar, S.; Reineck, P.; Padhye, R.; Wang, X. Multifunctional Smart Fabrics through Nanodiamond-Polyaniline Nanocomposites. ACS Appl. Polym. Mater. 2020, 2, 4848–4855. [Google Scholar] [CrossRef]
- Li, W.; Teng, C.; Sun, Y.; Cai, L.; Xu, J.-L.; Sun, M.; Li, X.; Yang, X.; Xiang, L.; Xie, D. Sprayed, Scalable, Wearable, and Portable NO2 Sensor Array Using Fully Flexible AgNPs-All-Carbon Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 34485–34493. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, G.; Liu, F.; Qiao, Y.; Li, C.M.; Lu, Z. A Flexible Humidity Sensor Based on Silk Fabrics for Human Respiration Monitoring. J. Mater. Chem. C 2018, 6, 4549–4554. [Google Scholar] [CrossRef]
- Maity, D.; Rajavel, K.; Rajendra Kumar, R.T. MWCNT Enabled Smart Textiles Based Flexible and Wearable Sensor for Human Motion and Humidity Monitoring. Cellulose 2021, 28, 2505–2520. [Google Scholar] [CrossRef]
- Sadi, M.S.; Yang, M.; Luo, L.; Cheng, D.; Cai, G.; Wang, X. Direct Screen Printing of Single-Faced Conductive Cotton Fabrics for Strain Sensing, Electrical Heating and Color Changing. Cellulose 2019, 26, 6179–6188. [Google Scholar] [CrossRef]
- Singh, S.; Wang, J.; Cinti, S. An Overview on Recent Progress in Screen-Printed Electroanalytical (Bio) Sensors. ECS Sens. Plus 2022, 1, 023401. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhang, L.; Xu, J.; Xiao, X.; Zhang, X. Inkjet-Printed Flexible Sensors: From Function Materials, Manufacture Process, and Applications Perspective. Mater. Today Commun. 2022, 31, 103263. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent Advances in Nanomaterial-Enabled Screen-Printed Electrochemical Sensors for Heavy Metal Detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable Biofuel Cells as Wearable Textile-Based Self-Powered Sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.-W.; Shi, H.; Wan, H.; Xu, Z.; Tan, X.; Wang, C. Inkjet-Printed Soft Resistive Pressure Sensor Patch for Wearable Electronics Applications. Adv. Mater. Technol. 2020, 5, 1900717. [Google Scholar] [CrossRef]
- Hartwig, M.; Zichner, R.; Joseph, Y. Inkjet-Printed Wireless Chemiresistive Sensors—A Review. Chemosensors 2018, 6, 66. [Google Scholar] [CrossRef]
- Kim, I.; Shahariar, H.; Ingram, W.F.; Zhou, Y.; Jur, J.S. Inkjet Process for Conductive Patterning on Textiles: Maintaining Inherent Stretchability and Breathability in Knit Structures. Adv. Funct. Mater. 2019, 29, 1807573. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kwon, S.-N.; Na, S.-I. Stretchable and Electrically Conductive Polyurethane-Silver/Graphene Composite Fibers Prepared by Wet-Spinning Process. Compos. Part B Eng. 2019, 167, 573–581. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, M.; Kang, X.; Zhu, C.; Ge, M. Continuous Wet-Spinning of Flexible and Water-Stable Conductive PEDOT: PSS/PVA Composite Fibers for Wearable Sensors. Compos. Commun. 2020, 17, 134–140. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Fu, J.; Liu, R.; He, H.; Ma, J.; Yu, M.; Ramakrishna, S.; Long, Y. Electrospinning of Ultrafine Conducting Polymer Composite Nanofibers with Diameter Less than 70 Nm as High Sensitive Gas Sensor. Materials 2018, 11, 1744. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Xin, Y.; Lubineau, G. Coaxial Thermoplastic Elastomer-Wrapped Carbon Nanotube Fibers for Deformable and Wearable Strain Sensors. Adv. Funct. Mater. 2018, 28, 1705591. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly Stretchable Core–Sheath Fibers via Wet-Spinning for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Yang, B.; Tan, J. Lightweight, Robust, Conductive Composite Fibers Based on MXene@ Aramid Nanofibers as Sensors for Smart Fabrics. ACS Appl. Mater. Interfaces 2021, 13, 41933–41945. [Google Scholar] [CrossRef]
- Liu, W.; Xue, C.; Long, X.; Ren, Y.; Chen, Z.; Zhang, W. Highly Flexible and Multifunctional CNTs/TPU Fiber Strain Sensor Formed in One-Step via Wet Spinning. J. Alloys Compd. 2023, 948, 169641. [Google Scholar] [CrossRef]
- Wei, J.S.; Ma, S.Y.; Cai, Y.H.; Xu, C.Y.; Liu, J.M.; Jiang, H.T. A High-Performance Ethylene Glycol Sensor Based on Fibrous ErFeO3 Prepared by Electrostatic Spinning. Ceram. Int. 2023, 49, 32611–32618. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Zhao, X.; Tian, Y.; Duan, Y. Electrospun Nanofiber-Based Indicatorpaper Sensing Platform for Fluorescence and Visualization Detection of Norfloxacin. Biosens. Bioelectron. 2023, 2023, 115562. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Yuan, W. Smart Nanocomposite Nonwoven Wearable Fabrics Embedding Phase Change Materials for Highly Efficient Energy Conversion–Storage and Use as a Stretchable Conductor. ACS Appl. Mater. Interfaces 2021, 13, 4508–4518. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, L.; Ma, Y.; Huang, H.; He, H.; Ji, H.; Wang, Z.; Qiu, J. Highly Sensitive, Reliable and Flexible Pressure Sensor Based on Piezoelectric PVDF Hybrid Film Using MXene Nanosheet Reinforcement. J. Alloys Compd. 2021, 886, 161069. [Google Scholar] [CrossRef]
- Lim, W.Y.; Goh, C.-H.; Yap, K.Z.; Ramakrishnan, N. One-Step Fabrication of Paper-Based Inkjet-Printed Graphene for Breath Monitor Sensors. Biosensors 2023, 13, 209. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, J.; Jin, Y.; Zhao, Y.; Huang, S.; Guo, C.F. A Highly Sensitive, Reliable, and High-Temperature-Resistant Flexible Pressure Sensor Based on Ceramic Nanofibers. Adv. Sci. 2020, 7, 2000258. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, J.; Liu, H.; Zhang, L. Silk Nanofibrous Iontronic Sensors for Accurate Blood Pressure Monitoring. Chem. Eng. J. 2023, 453, 139815. [Google Scholar] [CrossRef]
- Tan, S.; Islam, M.R.; Li, H.; Fernando, A.; Afroj, S.; Karim, N. Highly Scalable, Sensitive and Ultraflexible Graphene-Based Wearable E-Textiles Sensor for Bio-Signal Detection. Adv. Sens. Res. 2022, 1, 2200010. [Google Scholar] [CrossRef]
- Singh, S.U.; Chatterjee, S.; Lone, S.A.; Ho, H.-H.; Kaswan, K.; Peringeth, K.; Khan, A.; Chiang, Y.-W.; Lee, S.; Lin, Z.-H. Advanced Wearable Biosensors for the Detection of Body Fluids and Exhaled Breath by Graphene. Microchim. Acta 2022, 189, 236. [Google Scholar] [CrossRef]
- Lou, M.; Abdalla, I.; Zhu, M.; Yu, J.; Li, Z.; Ding, B. Hierarchically Rough Structured and Self-Powered Pressure Sensor Textile for Motion Sensing and Pulse Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhang, M. Highly Sensitive and Stretchable Piezoelectric Strain Sensor Enabled Wearable Devices for Real-Time Monitoring of Respiratory and Heartbeat Simultaneously. Nanotechnol. Precis. Eng. 2022, 5, 013002. [Google Scholar] [CrossRef]
- Kim, J.; Chou, E.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv. Healthc. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef]
- Jia, M.; Yi, C.; Han, Y.; Wang, L.; Li, X.; Xu, G.; He, K.; Li, N.; Hou, Y.; Wang, Z.; et al. Hierarchical Network Enabled Flexible Textile Pressure Sensor with Ultrabroad Response Range and High-Temperature Resistance. Adv. Sci. 2022, 9, 2105738. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, Y.; Cui, C.; Li, A.; Wang, W.; Guo, R.; Qin, W.; Ren, E.; Xiao, H.; Zhou, M. Flexible Waterborne Polyurethane/Cellulose Nanocrystal Composite Aerogels by Integrating Graphene and Carbon Nanotubes for a Highly Sensitive Pressure Sensor. ACS Sustain. Chem. Eng. 2021, 9, 14029–14039. [Google Scholar] [CrossRef]
- Zhong, W.; Ming, X.; Jiang, H.; Ke, Y.; Ding, X.; Li, M.; Jia, K.; Wang, D. Full-Textile Human Motion Detection Systems Integrated by Facile Weaving with Hierarchical Core–Shell Piezoresistive Yarns. ACS Appl. Mater. Interfaces 2021, 13, 52901–52911. [Google Scholar] [CrossRef]
- Deb, M.; Chen, M.-Y.; Chang, P.-Y.; Li, P.-H.; Chan, M.-J.; Tian, Y.-C.; Yeh, P.-H.; Soppera, O.; Zan, H.-W. SnO2-Based Ultra-Flexible Humidity/Respiratory Sensor for Analysis of Human Breath. Biosensors 2023, 13, 81. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Y.; Han, X.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.-L. Graphene Textile Strain Sensor with Negative Resistance Variation for Human Motion Detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, J.; Chu, M.; Khine, M. Flexible Piezoresistive Pressure Sensor Using Wrinkled Carbon Nanotube Thin Films for Human Physiological Signals. Adv. Mater. Technol. 2018, 3, 1700158. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Dong, Y.; Gong, J.; Li, Z.; Zhang, J. Washable Patches with Gold Nanowires/Textiles in Wearable Sensors for Health Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 18884–18900. [Google Scholar] [CrossRef]
- Chen, X.; Li, B.; Qiao, Y.; Lu, Z. Preparing Polypyrrole-Coated Stretchable Textile via Low-Temperature Interfacial Polymerization for Highly Sensitive Strain Sensor. Micromachines 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Li, J.; Li, Z.-J.; Zhang, Y.-P.; Zhang, X.; Wang, Z.-J.; Han, W.-P.; Sun, B.; Long, Y.-Z.; Zhang, H.-D. Hierarchical PVDF-HFP/ZnO Composite Nanofiber–Based Highly Sensitive Piezoelectric Sensor for Wireless Workout Monitoring. Adv. Compos. Hybrid Mater. 2022, 5, 766–775. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Hasanzadeh, M.; Simchi, A. Self-Powered Wearable Piezoelectric Sensors Based on Polymer Nanofiber–Metal–Organic Framework Nanoparticle Composites for Arterial Pulse Monitoring. ACS Appl. Nano Mater. 2020, 3, 8742–8752. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Ding, Q.; Jin, X.; Ke, Q.; Li, Z.; Wang, D.; Huang, C. Facile Strategy for Fabrication of Flexible, Breathable, and Washable Piezoelectric Sensors via Welding of Nanofibers with Multiwalled Carbon Nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2019, 11, 38023–38030. [Google Scholar] [CrossRef]
- Li, T.; Qiao, F.; Huang, P.; Su, Y.; Wang, L.; Li, X.; Li, H.; Tan, Y.; Zhou, Z. Flexible Optical Fiber-Based Smart Textile Sensor for Human–Machine Interaction. IEEE Sens. J. 2022, 22, 19336–19345. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Sun, Y.-Q.; Xiao, X.; Wang, H.-Q.; Zhang, M.-H.; Wang, F.-Z.; Lai, J.-C.; Zhang, D.-S.; Pan, L.-J.; Li, C.-H. An Ultra-Tough and Ultra-Sensitive Ionogel Pressure/Temperature Sensor Enabled by Hierarchical Design of Both Materials and Devices. J. Mater. Chem. A 2023, 11, 8359–8367. [Google Scholar] [CrossRef]
- Ji, G.; Chen, Z.; Li, H.; Awuye, D.E.; Guan, M.; Zhu, Y. Electrospinning-Based Biosensors for Health Monitoring. Biosensors 2022, 12, 876. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Patil, A.; Hou, C.; Zhu, S.; Fan, X.; Lin, H.; Yu, W.; Guo, W.; Liu, X.Y. All-Textile Electronic Skin Enabled by Highly Elastic Spacer Fabric and Conductive Fibers. ACS Appl. Mater. Interfaces 2019, 11, 33336–33346. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Mao, G.; Li, Y.; Pravarthana, D.; Asghar, W.; Liu, Y.; Qu, S.; Shang, J.; Li, R. A Wearable Capacitive Sensor Based on Ring/Disk-Shaped Electrode and Porous Dielectric for Noncontact Healthcare Monitoring. Glob. Chall. 2020, 4, 1900079. [Google Scholar] [CrossRef]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30, 1910504. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Yu, Q.; Zhang, H.; Wu, X.; Yao, M.; Liu, W.; Yao, F.; Li, J. Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-Knitted Washable Sensor Array Textile for Precise Epidermal Physiological Signal Monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Abdalla, I.; Zhu, M.; Wei, X.; Yu, J.; Li, Z.; Ding, B. Highly Wearable, Breathable, and Washable Sensing Textile for Human Motion and Pulse Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 19965–19973. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dai, M.; Dong, T.; Liu, T. A Textile Sensor for Long Durations of Human Motion Capture. Sensors 2019, 19, 2369. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Chen, X.; Zhao, Y.; Zhang, H.; Wen, N.; Fan, Z.; Pan, L. Facile Fabrication of High-Performance Pen Ink-Decorated Textile Strain Sensors for Human Motion Detection. ACS Appl. Mater. Interfaces 2020, 12, 19874–19881. [Google Scholar] [CrossRef]
- Khundaqji, H.; Hing, W.; Furness, J.; Climstein, M. Smart Shirts for Monitoring Physiological Parameters: Scoping Review. JMIR MHealth UHealth 2020, 8, e18092. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, L.; Yang, X.; Chen, X.; Zhang, Z.; Zhao, T.; Li, X.; Zhang, J. A Flexible Pressure Sensor Based on an MXene–Textile Network Structure. J. Mater. Chem. C 2019, 7, 1022–1027. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Hua, T.; Xu, B.; Jiang, S. Wearable Strain Sensing Textile Based on One-Dimensional Stretchable and Weavable Yarn Sensors. Nano Res. 2018, 11, 5799–5811. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Sun, X.; Liang, C.; Han, Y.; Wu, X.; Wang, Z. All Textile-Based Robust Pressure Sensors for Smart Garments. Chem. Eng. J. 2023, 454, 140302. [Google Scholar] [CrossRef]
- Cai, G.; Yang, M.; Xu, Z.; Liu, J.; Tang, B.; Wang, X. Flexible and Wearable Strain Sensing Fabrics. Chem. Eng. J. 2017, 325, 396–403. [Google Scholar] [CrossRef]
- Wang, S.; Ning, H.; Hu, N.; Liu, Y.; Liu, F.; Zou, R.; Huang, K.; Wu, X.; Weng, S. Alamusi Environmentally-Friendly and Multifunctional Graphene-Silk Fabric Strain Sensor for Human-Motion Detection. Adv. Mater. Interfaces 2020, 7, 1901507. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, G.; Ye, D.; Bai, Z.; Deng, Z.; Xu, J. A Self-Powered Laminated Fabric Sensor for Human Motion Detection and Heart-Rate Monitoring Based on PPy/Al Schottky Contact. J. Sandw. Struct. Mater. 2022, 24, 503–516. [Google Scholar] [CrossRef]
- Martinez-Estrada, M.; Moradi, B.; Fernández-Garcia, R.; Gil, I. Impact of Manufacturing Variability and Washing on Embroidery Textile Sensors. Sensors 2018, 18, 3824. [Google Scholar] [CrossRef]
- Du, Y.; Sun, L.; Yang, L.; Xu, W. A Wearable Flexible Tactile Sensor with Textile Microstructure for Wirelessly Recognizing Human Activity. Adv. Sens. Res. 2023, 2, 2200051. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Li, Y.; Shi, G. High-Performance Strain Sensors with Fish-Scale-Like Graphene-Sensing Layers for Full-Range Detection of Human Motions. ACS Nano 2016, 10, 7901–7906. [Google Scholar] [CrossRef]
- Afroj, S.; Karim, N.; Wang, Z.; Tan, S.; He, P.; Holwill, M.; Ghazaryan, D.; Fernando, A.; Novoselov, K.S. Engineering Graphene Flakes for Wearable Textile Sensors via Highly Scalable and Ultrafast Yarn Dyeing Technique. ACS Nano 2019, 13, 3847–3857. [Google Scholar] [CrossRef]
- Luo, J.; Gao, S.; Luo, H.; Wang, L.; Huang, X.; Guo, Z.; Lai, X.; Lin, L.; Li, R.K.Y.; Gao, J. Superhydrophobic and Breathable Smart MXene-Based Textile for Multifunctional Wearable Sensing Electronics. Chem. Eng. J. 2021, 406, 126898. [Google Scholar] [CrossRef]
- Hsiao, F.-R.; Liao, Y.-C. Printed Micro-Sensors for Simultaneous Temperature and Humidity Detection. IEEE Sens. J. 2018, 18, 6788–6793. [Google Scholar] [CrossRef]
- Geng, Y.; Cao, R.; Innocent, M.T.; Hu, Z.; Zhu, L.; Wang, L.; Xiang, H.; Zhu, M. A High-Sensitive Wearable Sensor Based on Conductive Polymer Composites for Body Temperature Monitoring. Compos. Part Appl. Sci. Manuf. 2022, 163, 107269. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Wang, Y.; Zhao, Y.; Qin, Y.; Zhu, Z.; Yu, Z.; Wang, J. An Ultralight Self-Powered Fire Alarm e-Textile Based on Conductive Aerogel Fiber with Repeatable Temperature Monitoring Performance Used in Firefighting Clothing. ACS Nano 2022, 16, 2953–2967. [Google Scholar] [CrossRef]
- Jang, G.N.; Hong, S.Y.; Park, H.; Lee, Y.H.; Park, H.; Lee, H.; Jeong, Y.R.; Jin, S.W.; Keum, K.; Ha, J.S. Highly Sensitive Pressure and Temperature Sensors Fabricated with Poly(3-Hexylthiophene-2,5-Diyl)-Coated Elastic Carbon Foam for Bio-Signal Monitoring. Chem. Eng. J. 2021, 423, 130197. [Google Scholar] [CrossRef]
- Li, F.; Xue, H.; Lin, X.; Zhao, H.; Zhang, T. Wearable Temperature Sensor with High Resolution for Skin Temperature Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 43844–43852. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Bae, J.; Lee, J.; Ahn, J.; Hwang, W.-T.; Ko, J.; Kim, I.-D. Porous Nanofiber Membrane: Rational Platform for Highly Sensitive Thermochromic Sensor. Adv. Funct. Mater. 2022, 32, 2200463. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, M.; Jian, M.; Zhang, Y. An All-Silk-Derived Dual-Mode E-Skin for Simultaneous Temperature–Pressure Detection. ACS Appl. Mater. Interfaces 2017, 9, 39484–39492. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, J.-H.; Hwang, J.-Y.; Shin, U.S. Wearable CNTs-Based Humidity Sensors with High Sensitivity and Flexibility for Real-Time Multiple Respiratory Monitoring. NANO Converg. 2022, 9, 35. [Google Scholar] [CrossRef]
- Zhou, G.; Byun, J.-H.; Oh, Y.; Jung, B.-M.; Cha, H.-J.; Seong, D.-G.; Um, M.-K.; Hyun, S.; Chou, T.-W. Highly Sensitive Wearable Textile-Based Humidity Sensor Made of High-Strength, Single-Walled Carbon Nanotube/Poly(Vinyl Alcohol) Filaments. ACS Appl. Mater. Interfaces 2017, 9, 4788–4797. [Google Scholar] [CrossRef]
- Gaubert, V.; Gidik, H.; Koncar, V. Boxer Underwear Incorporating Textile Moisture Sensor to Prevent Nocturnal Enuresis. Sensors 2020, 20, 3546. [Google Scholar] [CrossRef] [PubMed]
- McColl, D.; Cartlidge, B.; Connolly, P. Real-Time Monitoring of Moisture Levels in Wound Dressings in Vitro: An Experimental Study. Int. J. Surg. 2007, 5, 316–322. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Vena, A.; Garcia, R.; Abboud, P.; Habchi, R.; Sorli, B. Highly Porous and Flexible Capacitive Humidity Sensor Based on Self-Assembled Graphene Oxide Sheets on a Paper Substrate. Sens. Actuators B Chem. 2019, 298, 126892. [Google Scholar] [CrossRef]
- Jia, C.; Huang, J.; Fu, L.; Xiang, Y.; Chen, Y.; Yue, H.; Zhao, Q.; Gu, W.; Wu, Y.; Zhang, J. A Novel Differential Capacitive Humidity Sensor on SIW Re-Entrant Cavity Microwave Resonators With PEDOT:PSS Film. IEEE Sens. J. 2022, 22, 6576–6585. [Google Scholar] [CrossRef]
- Cho, H.; Lee, C.; Lee, C.; Lee, S.; Kim, S. Robust, Ultrathin, and Highly Sensitive Reduced Graphene Oxide/Silk Fibroin Wearable Sensors Responded to Temperature and Humidity for Physiological Detection. Biomacromolecules 2023, 24, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High Sensitivity Portable Capacitive Humidity Sensor Based on In2O3 Nanocubes-Decorated GO Nanosheets and Its Wearable Application in Respiration Detection. Sens. Actuators B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Ghaffari, R.; Yang, D.S.; Kim, J.; Mansour, A.; Wright, J.A.; Model, J.B.; Wright, D.E.; Rogers, J.A.; Ray, T.R. State of Sweat: Emerging Wearable Systems for Real-Time, Noninvasive Sweat Sensing and Analytics. ACS Sens. 2021, 6, 2787–2801. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qiao, L.; Chen, Z.; Liu, B.; Gao, L.; Zhang, L. Wearable Sensor for Continuous Sweat Biomarker Monitoring. Chemosensors 2022, 10, 273. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced Wearable Microfluidic Sensors for Healthcare Monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable Sweat Sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Heo, J.S.; Hossain, M.F.; Kim, I. Challenges in Design and Fabrication of Flexible/Stretchable Carbon- and Textile-Based Wearable Sensors for Health Monitoring: A Critical Review. Sensors 2020, 20, 3927. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, Y.; Xu, M.; Luo, J. Flexible Biosensor Based on Signal Amplification of Gold Nanoparticles-Composite Flower Clusters for Glucose Detection in Sweat. Colloids Surf. Physicochem. Eng. Asp. 2023, 661, 130908. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, T.; Li, K.; Long, D.; Zhao, J.; Zhu, F.; Gong, W. A Flexible Nonenzymatic Sweat Glucose Sensor Based on Au Nanoflowers Coated Carbon Cloth. Sens. Actuators B Chem. 2023, 388, 133798. [Google Scholar] [CrossRef]
- Piper, A.; Öberg Månsson, I.; Khaliliazar, S.; Landin, R.; Hamedi, M.M. A Disposable, Wearable, Flexible, Stitched Textile Electrochemical Biosensing Platform. Biosens. Bioelectron. 2021, 194, 113604. [Google Scholar] [CrossRef]
- Promphet, N.; Hinestroza, J.P.; Rattanawaleedirojn, P.; Soatthiyanon, N.; Siralertmukul, K.; Potiyaraj, P.; Rodthongkum, N. Cotton Thread-Based Wearable Sensor for Non-Invasive Simultaneous Diagnosis of Diabetes and Kidney Failure. Sens. Actuators B Chem. 2020, 321, 128549. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable Gold Fiber-Based Wearable Textile Electrochemical Biosensor for Lactate Monitoring in Sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, J.; Liu, J.; Li, Y. Wearable, Nanofiber-Based Microfluidic Systems with Integrated Electrochemical and Colorimetric Sensing Arrays for Multiplex Sweat Analysis. Chem. Eng. J. 2023, 454, 140248. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Li, H.; Meng, J.; Liu, Q.; Guo, X.; Qiu, Y.; Zhang, J.; Li, C. Ce(III)-Modulation over Non-Enzymatic Pt/CeO2/GO Biosensor with Outstanding Sensitivity and Stability for Lactic Acid Detection. J. Rare Earths 2023, 41, 1437–1447. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Greif, G.; Šturdík, E. A Rapid Method for Determination of L-Lactic Acid in Real Samples by Amperometric Biosensor Utilizing Nanocomposite. Food Control 2012, 23, 238–244. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent Advances in Biosensors for Real Time Monitoring of PH, Temperature, and Oxygen in Chronic Wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. One-Step Laser Synthesis Platinum Nanostructured 3D Porous Graphene: A Flexible Dual-Functional Electrochemical Biosensor for Glucose and PH Detection in Human Perspiration. Talanta 2023, 257, 124362. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, Y.; Pan, J.; Li, S.; Sun, X.; Zhang, B.; Peng, H. Weaving Sensing Fibers into Electrochemical Fabric for Real-Time Health Monitoring. Adv. Funct. Mater. 2018, 28, 1804456. [Google Scholar] [CrossRef]
- Chung, M.; Skinner, W.H.; Robert, C.; Campbell, C.J.; Rossi, R.M.; Koutsos, V.; Radacsi, N. Fabrication of a Wearable Flexible Sweat PH Sensor Based on SERS-Active Au/TPU Electrospun Nanofibers. ACS Appl. Mater. Interfaces 2021, 13, 51504–51518. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-Invasive Textile Based Colorimetric Sensor for the Simultaneous Detection of Sweat PH and Lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef]
- Lee, H.-B.; Meeseepong, M.; Trung, T.Q.; Kim, B.-Y.; Lee, N.-E. A Wearable Lab-on-a-Patch Platform with Stretchable Nanostructured Biosensor for Non-Invasive Immunodetection of Biomarker in Sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Zahraee, H.; Izadpanah Kazemi, M.; Habibi, Z.S.; Taghdisi, S.M.; Abnous, K.; Khoshbin, Z.; Chen, C.-H. Recent Advances in Aptamer-Based Platforms for Cortisol Hormone Monitoring. Talanta 2024, 266, 125010. [Google Scholar] [CrossRef]
- Olgaç, N.; Karakuş, E.; Şahin, Y.; Liv, L. Electrochemical Biosensing of Cortisol in a Hormone Tablet and Artificial Bodily Fluids. Diam. Relat. Mater. 2023, 132, 109622. [Google Scholar] [CrossRef]
- Apilux, A.; Rengpipat, S.; Suwanjang, W.; Chailapakul, O. Paper-Based Immunosensor with Competitive Assay for Cortisol Detection. J. Pharm. Biomed. Anal. 2020, 178, 112925. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, H.; Lu, Y.; Zhou, M.; Jiang, S.; Du, X.; Guo, C. Advanced Textile-Based Wearable Biosensors for Healthcare Monitoring. Biosensors 2023, 13, 909. https://doi.org/10.3390/bios13100909
Li S, Li H, Lu Y, Zhou M, Jiang S, Du X, Guo C. Advanced Textile-Based Wearable Biosensors for Healthcare Monitoring. Biosensors. 2023; 13(10):909. https://doi.org/10.3390/bios13100909
Chicago/Turabian StyleLi, Sheng, Huan Li, Yongcai Lu, Minhao Zhou, Sai Jiang, Xiaosong Du, and Chang Guo. 2023. "Advanced Textile-Based Wearable Biosensors for Healthcare Monitoring" Biosensors 13, no. 10: 909. https://doi.org/10.3390/bios13100909





