Sensitivity Equalization and Dynamic Range Expansion with Multiple Optofluidic Microbubble Resonator Sensors
Abstract
:1. Introduction
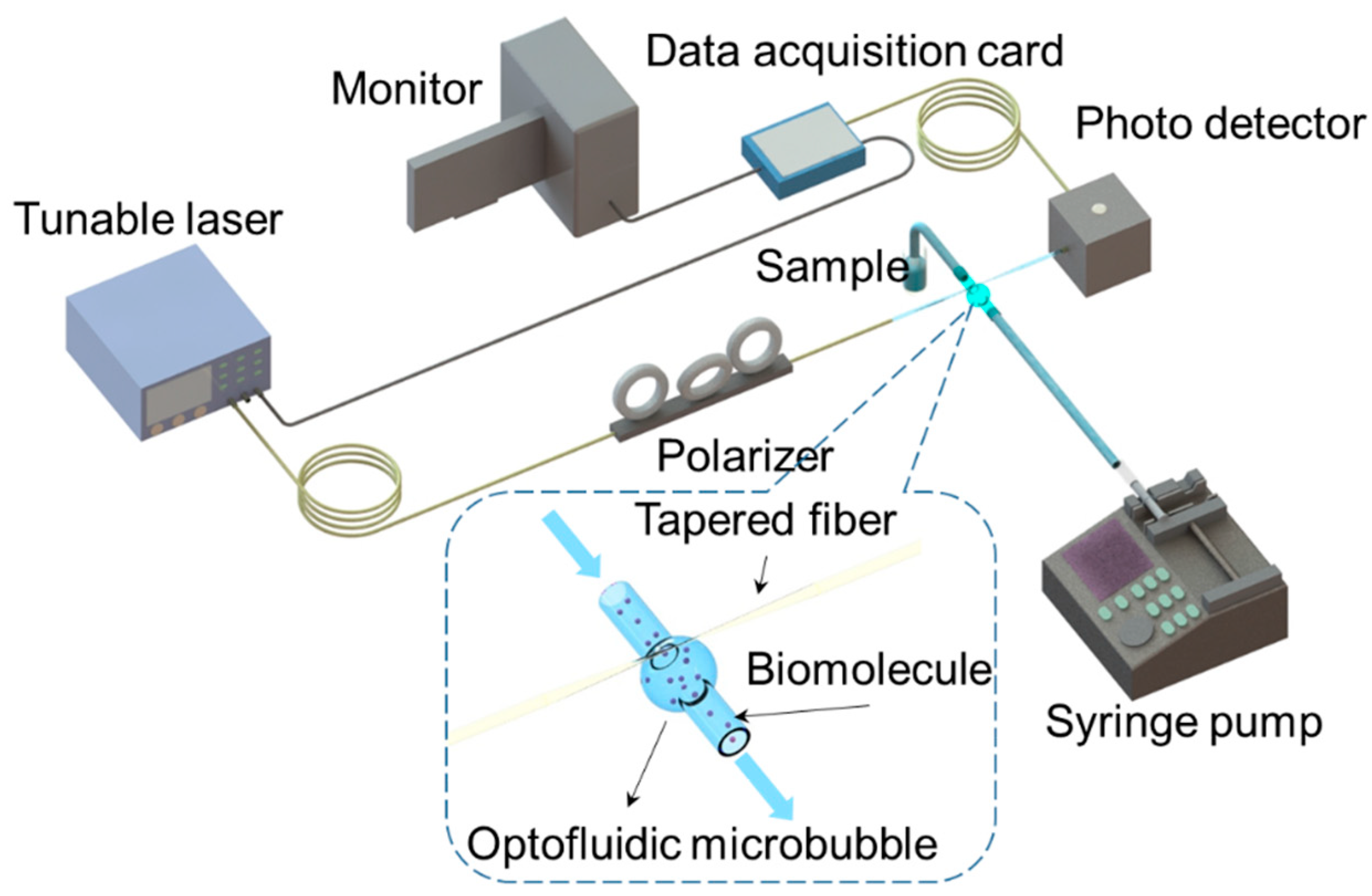
2. Principle and Experimental Setup
2.1. Equalization of Multi-OMBR Sensing Signal
2.2. Materials
2.3. Experimental Setup
2.4. Surface Treatment for Specific Detection of HIV-1 p24 Antigen
2.5. Surface Treatment in Fluorescent Experiments
3. Results and Discussion
3.1. Operational Life of OMBRs
3.2. Specific Detection of HIV-1 p24 Antigen
3.3. Multi-OMBR Sensitivity Equalization and Dynamic Range Enlargement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vollmer, F.; Braun, D.; Libchaber, A.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Dong, C.; He, L.; Xiao, Y.; Gaddam, V.; Özdemir, S.; Han, Z.; Guo, G.; Yang, L. Fabrication of high-Q polydimethylsiloxane optical microspheres for thermal sensing. Appl. Phys. Lett. 2009, 94, 231119. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, X. Optical ring resonators for biochemical and chemical sensing. Anal. Bioanal. Chem. 2011, 399, 205–211. [Google Scholar] [CrossRef]
- Arbabi; Goddard, L. Measurements of the refractive indices and thermo-optic coefficients of Si3N4 and SiOx using microring resonances. Opt. Lett. 2013, 38, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.; Swaim, J.; Vollmer, F. Whispering gallery mode sensors. Adv. Opt. Photonics 2015, 7, 168–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Sun, H. Advances and prospects for whispering gallery mode microcavities. Adv. Opt. Mater. 2015, 3, 1136–1162. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Cheng, W.; Cheng, J.; Zheng, Z. A tunable optical Bragg grating filter based on the droplet sagging effect on a superhydrophobic nanopillar array. Sensors 2019, 19, 3324. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, W.; Zheng, Z.; Cheng, J.; Liu, J. Meridian whispering gallery modes sensing in a sessile microdroplet on micro/nanostructured superhydrophobic chip surfaces. Microfluid. Nanofluid. 2019, 23, 106. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Yin, Q.; Qiu, M.; Su, Y. Ultra-compact parallel label-free biosensors based on concentric micro-ring resonators in silicon-on-insulator. In Proceedings of the Asia Optical Fiber and Optoelectronic 2008 Exposition and Conference, Shanghai, China, 30 October–2 November 2008. [Google Scholar]
- Zhu, H.; Dale, P.S.; Fan, X. Optofluidic ring resonator sensor for sensitive label-free detection of breast cancer antigen CA15-3 in human serum. Proc. SPIE 2009, 7322, 732204. [Google Scholar]
- Hunt, H.; Dahmen, J.; Soteropulos, C. Interfacing whispering gallery mode microresonators for environmental biosensing. Proc. SPIE 2014, 8960, 89600O. [Google Scholar]
- Freeman, L.; Armani, A. Photobleaching of Cy5 conjugated lipid bilayers determined with optical microresonators. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1160–1165. [Google Scholar] [CrossRef]
- Zhu, J.; Özdemir, Ş.K.; Xiao, Y.-F.; Li, L.; He, L.; Chen, D.-R.; Yang, L. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonator. Nat. Photonics 2010, 4, 46–49. [Google Scholar] [CrossRef]
- Sumetsky, M.; Dulashko, Y.; Windeler, R.S. Optical microbubble resonator. Opt. Lett. 2010, 35, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Kulkarni, R.; Fraser, S.; Flagan, R.; Vahala, K. Label-free, single-molecule detection with optical microcavities. Science 2007, 317, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Berneschi, S.; Farnesi, D.; Cosi, F.; Conti, G.; Pelli, S.; Righini, G.; Soria, S. High Q silica microbubble resonators fabricated by arc discharge. Opt. Lett. 2011, 36, 3521–3523. [Google Scholar] [CrossRef]
- Cosci, A.; Quercioli, F.; Farnesi, D.; Berneschi, S.; Giannetti, A.; Cosi, F.; Barucci, A.; Conti, G.; Righini, G.; Pelli, S. Confocal reflectance microscopy for determination of microbubble resonator thickness. Opt. Express 2015, 23, 16693–16701. [Google Scholar] [CrossRef]
- Farnesi, D.; Barucci, A.; Righini, G.; Conti, G.; Soria, S. Generation of hyper-parametric oscillations insilica microbubbles. Opt. Lett. 2015, 40, 4508–4511. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X. Optical manipulation in optofluidic microbubble resonators. Sci. China Phys. Mech. Astron. 2015, 58, 114206. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Jiang, J.; Liu, K.; Yu, Z.; Chen, W.; Liu, W. Micro-bubble-based wavelength division multiplex optical fluidic sensing. In Advanced Sensor Systems and Applications VI; International Society for Optics and Photonics: Bellingham, WA, USA, 2014. [Google Scholar]
- Tang, T.; Wu, X.; Liu, L.; Xu, L. Packaged optofluidic microbubble resonators for optical sensing. Appl. Opt. 2016, 55, 395–399. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Xu, L. Ultralow sensing limit in optofluidic micro-bottle resonator biosensor by selfreferenced differential-mode detection scheme. Appl. Phys. Lett. 2014, 104, 033703. [Google Scholar] [CrossRef]
- Pablo, B. Optical Microbottle resonators for sensing. Sensors 2016, 16, 1841. [Google Scholar]
- Li, Z.; Zhu, C.; Guo, Z.; Wang, B.; Wu, X.; Fei, Y. Highly sensitive label-free detection of small molecules with an optofluidic microbubble resonator. Micromachines 2018, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Madugani, R.; Yang, Y.; Le, V.H.; Ward, J.M.; Chormaic, S.N. Linear laser tuning using a pressuresensitive microbubble resonator. IEEE Photonics Technol. Lett. 2016, 28, 1134–1137. [Google Scholar] [CrossRef]
- Zhi, Y.; Yu, X.-C.; Gong, Q.; Yang, L.; Xiao, Y.-F. Single nanoparticle detection using optical microcavities. Adv. Mater. 2017, 29, 1604920. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Liu, L.; Fan, X.; Xu, L. Self-referencing optofluidic ring resonator sensor for highly sensitive biomolecular detection. Anal. Chem. 2013, 85, 9328–9332. [Google Scholar] [CrossRef]
- Wilson, K.A.; Finch, C.A.; Anderson, P.; Vollmer, F.; Hickman, J.J. Whispering gallery mode biosensor quantification of fibronectin adsorption kinetics onto alkylsilane monolayers and interpretation of resultant cellular response. Biomaterials 2012, 33, 225–236. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, Q.; Zhu, C.; Wang, B.; Zhou, Y.; Wu, X. Ultra-sensitive biomolecular detection by external referencing optofluidic microbubble resonators. Opt. Express 2019, 27, 12424–12435. [Google Scholar] [CrossRef]
- Hu, H.; White, I.M.; Suter, J.D.; Dale, P.S.; Fan, X. Analysis of biomolecule detection with optofluidic ring resonator sensors. Opt. Express 2007, 15, 9139–9146. [Google Scholar]
- Zhou, W.; Li, K.; Wei, Y.; Hao, P.; Chi, M.; Liu, Y.; Wu, Y. Ultrasensitive label-free optical microfiber coupler biosensor for detection of cardiac troponin I based on interference turning point effect. Biosens. Bioelectron. 2018, 106, 99–104. [Google Scholar] [CrossRef]
- Srisa-Art, M.; Dyson, E.C.; deMello, A.J.; Edel, J.B. Monitoring of real-time streptavidin−biotin binding kinetics using droplet microfluidics. Anal. Chem. 2008, 80, 7063–7067. [Google Scholar] [CrossRef]
- Jung, L.S.; Nelson, K.E.; Stayton, P.S.; Campbell, C.T. Binding and dissociation kinetics of wild-type and mutant streptavidins on mixed biotin-containing alkylthiolate monolayers. Langmuir 2000, 16, 9421–9432. [Google Scholar] [CrossRef]







| OMBR | Sbulk | Wavelength Shift | Equalized Signal |
|---|---|---|---|
| 1 | 21 nm/RIU | 5.52 pm | 5.52 |
| 2 | 16 nm/RIU | 4.17 pm | 5.47 |
| 3 | 12 nm/RIU | 3.10 pm | 5.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, X.; Liu, L.; Wu, X.; Xu, L. Sensitivity Equalization and Dynamic Range Expansion with Multiple Optofluidic Microbubble Resonator Sensors. Biosensors 2023, 13, 911. https://doi.org/10.3390/bios13100911
Wang Y, Zhao X, Liu L, Wu X, Xu L. Sensitivity Equalization and Dynamic Range Expansion with Multiple Optofluidic Microbubble Resonator Sensors. Biosensors. 2023; 13(10):911. https://doi.org/10.3390/bios13100911
Chicago/Turabian StyleWang, Ye, Xuyang Zhao, Liying Liu, Xiang Wu, and Lei Xu. 2023. "Sensitivity Equalization and Dynamic Range Expansion with Multiple Optofluidic Microbubble Resonator Sensors" Biosensors 13, no. 10: 911. https://doi.org/10.3390/bios13100911
APA StyleWang, Y., Zhao, X., Liu, L., Wu, X., & Xu, L. (2023). Sensitivity Equalization and Dynamic Range Expansion with Multiple Optofluidic Microbubble Resonator Sensors. Biosensors, 13(10), 911. https://doi.org/10.3390/bios13100911






