Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Bacteria Culturing and Sample Preparation
2.3. Surface Functionalization of SS-MWs
2.4. Preparation of CIP-MWs
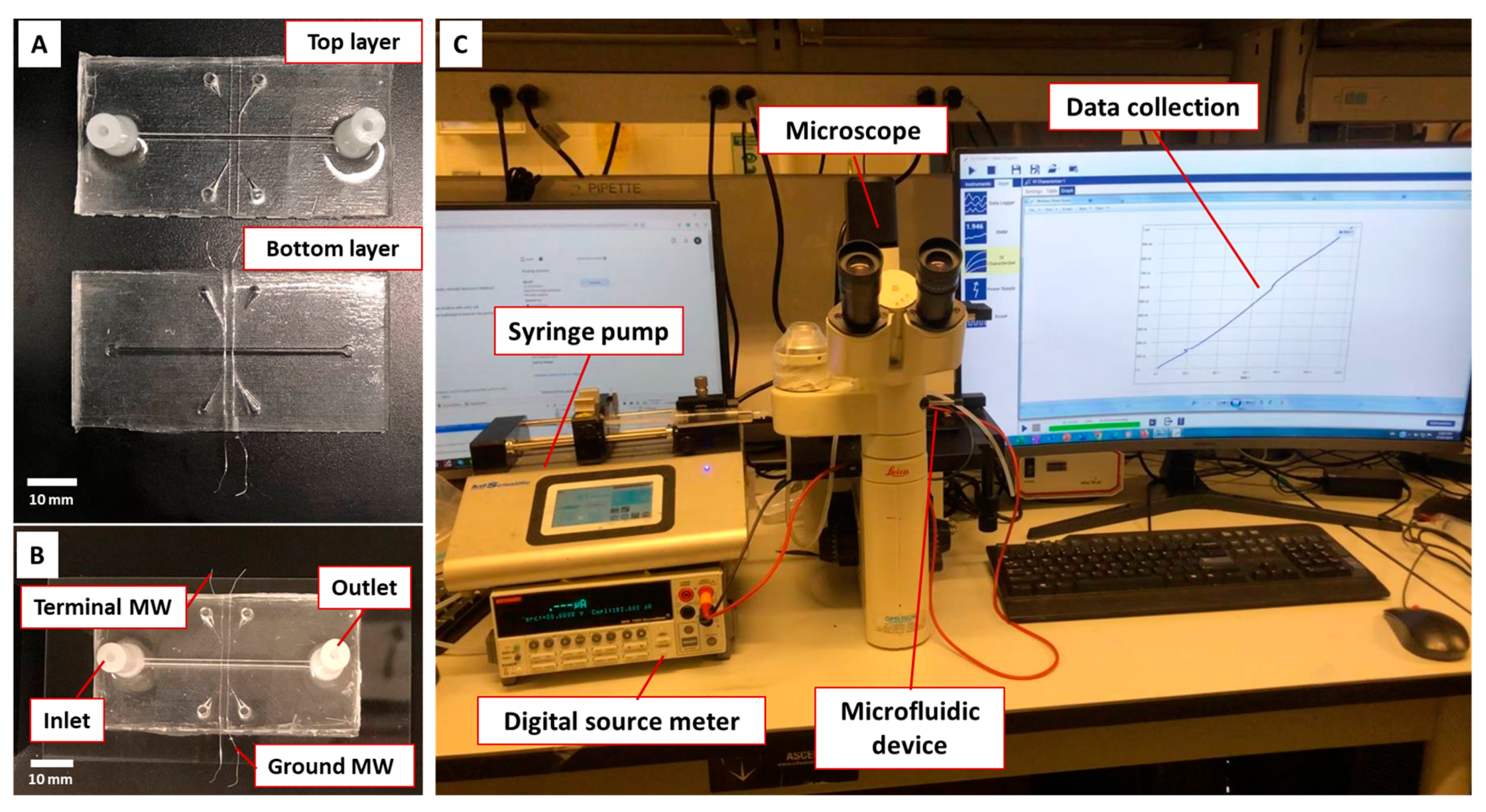
2.5. Microfluidic Device
2.6. Conductometric Measurement and Experimental Setup
2.7. Specificity Experiments
2.8. Data Analysis for Sensor Characterization
3. Results and Discussion
3.1. Bacteria Capturing by Various Microwires inside the Microfluidic Device
3.2. Conductometric Analysis of the Microfluidic Device
3.3. Characterization of the CIP-MW-Based Microfluidic Sensor
3.4. Specificity of the CIP-MW-Based Microfluidic Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A review of methods for the detection of pathogenic microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.D.; Paschoalino, W.J.; Neto, R.C.; Kubota, L.T. Electrochemical point-of-care devices for monitoring waterborne pathogens: Protozoa, bacteria, and viruses—An overview. Case Stud. Chem. Environ. Eng. 2022, 5, 100182. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Mutalib, N.-S.A.; Chan, K.-G.; Lee, L.-H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S.; Adams, C. Monitoring inland water quality using remote sensing: Potential and limitations of spectral indices, bio-optical simulations, machine learning, and cloud computing. Earth Sci. Rev. 2020, 205, 103187. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, J.; Qi, R. Electrochemiluminescence sensing platform for microorganism detection. Biosaf. Health 2022, 4, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhou, T.; Huang, R. Recent Advances in Electrochemiluminescence Sensors for Pathogenic Bacteria Detection. Micromachines 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.-P.; Wang, W.; Shen, Y.; Guo, J.-S. Surface plasmon resonance for water pollutant detection and water process analysis. TrAC Trends Anal. Chem. 2016, 85, 153–165. [Google Scholar] [CrossRef]
- Elcin, E.; Öktem, H.A. Whole-cell fluorescent bacterial bioreporter for arsenic detection in water. Int. J. Environ. Sci. Technol. 2019, 16, 5489–5500. [Google Scholar] [CrossRef]
- Fricke, C.; Harms, H.; Maskow, T. Rapid calorimetric detection of bacterial contamination: Influence of the cultivation technique. Front. Microbiol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Chapter Six—Quartz-Crystal Microbalance (QCM) for Public Health: An Overview of Its Applications. Adv. Protein Chem. Struct. Biol. 2015, 101, 149–211. [Google Scholar] [CrossRef]
- da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de Giarola, J.F.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Razmi, N.; Hasanzadeh, M.; Willander, M.; Nur, O. Recent progress on the electrochemical biosensing of Escherichia coli O157: H7: Material and methods overview. Biosensors 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical methodologies for the detection of pathogens. ACS Sens. 2018, 3, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Khoshroo, A.; Mavaei, M.; Rostami, M.; Valinezhad-Saghezi, B.; Fattahi, A. Recent advances in electrochemical strategies for bacteria detection. BioImpacts BI 2022, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for molecular imprinting and the evolution of MIP nanoparticles as plastic antibodies—Synthesis and applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Merlier, F.; Avalle, B.; Vieillard, V.; Debré, P.; Haupt, K.; Tse Sum Bui, B. Molecularly imprinted polymer nanoparticles as potential synthetic antibodies for immunoprotection against HIV. ACS Appl. Mater. Interfaces 2019, 11, 9824–9831. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Mattiasson, B. Imprinting of Microorganisms for Biosensor Applications. Sensors 2017, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly imprinted polymers for the selective recognition of microorganisms. Biotechnol. Adv. 2020, 45, 107640. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Bindeus, R.; Haderspöck, C.; Mann, K.-J.; Wirl, B.; Dickert, F.L. Mass-sensitive detection of cells, viruses and enzymes with artificial receptors. Sens. Actuators B Chem. 2003, 91, 316–319. [Google Scholar] [CrossRef]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. Influenza A virus molecularly imprinted polymers and their application in virus sub-type classification. J. Mater. Chem. B 2013, 1, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. A novel approach to identify molecular binding to the influenza virus H5N1: Screening using molecularly imprinted polymers (MIPs). MedChemComm 2014, 5, 617–621. [Google Scholar] [CrossRef]
- Sangma, C.; Lieberzeit, P.A.; Sukjee, W. H5N1 virus plastic antibody based on molecularly imprinted polymers. In Synthetic Antibodies; Springer: Berlin/Heidelberg, Germany, 2017; pp. 381–388. [Google Scholar]
- Sukjee, W.; Thitithanyanont, A.; Wiboon-Ut, S.; Lieberzeit, P.A.; Paul Gleeson, M.; Navakul, K.; Sangma, C. An influenza A virus agglutination test using antibody-like polymers. J. Biomater. Sci. Polym. Ed. 2017, 28, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Klangprapan, S.; Choke-Arpornchai, B.; Lieberzeit, P.A.; Choowongkomon, K. Sensing the classical swine fever virus with molecularly imprinted polymer on quartz crystal microbalance. Heliyon 2020, 6, e04137. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Youssef, K.; Akhtarian, S.; Tabesh, E.; Kraft, G.; Brar, S.K.; Rezai, P. Molecularly imprinted polymer (MIP) based core-shell microspheres for bacteria isolation. Polymer 2022, 251, 124917. [Google Scholar] [CrossRef]
- Akhtarian, S.; Doostmohammadi, A.; Youssef, K.; Kraft, G.; Brar, S.K.; Rezai, P. Metal Microwires Functionalized with Cell-Imprinted Polymer for Capturing Bacteria in Water. ACS Appl. Polym. Mater. 2023, 5, 3235–3246. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.A.; Piletsky, S.A.; Brovko, A.A.; Slinchenko, E.A.; Sergeeva, L.M.; El’Skaya, A. Selective recognition of atrazine by molecularly imprinted polymer membranes. Development of conductometric sensor for herbicides detection. Anal. Chim. Acta 1999, 392, 105–111. [Google Scholar] [CrossRef]
- Noh, H.; Lee, J.; Lee, C.J.; Jung, J.; Kang, J.; Choi, M.; Baek, M.C.; Shim, J.H.; Park, H. Precise evaluation of liquid conductivity using a multi-channel microfluidic chip and direct-current resistance measurements. Sens. Actuators B Chem. 2019, 297, 126810. [Google Scholar] [CrossRef]
- Park, J.K.; Ryu, J.C.; Kim, W.K.; Kang, K.H. Effect of electric field on electrical conductivity of dielectric liquids mixed with polar additives: DC conductivity. J. Phys. Chem. B 2009, 113, 12271–12276. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.J.F.; Tabatabaei, N.; Rezai, P. Low-Cost Resistive Microfluidic Salinity Sensor for High-Precision Detection of Drinking Water Salt Levels. ACS Omega 2022, 7, 15529–15539. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.; Moon, J.; Kim, S.; Kim, S.-D.; Cho, J. Bio-particle separation using microfluidic porous plug for environmental monitoring. Desalination 2007, 202, 215–223. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Yu, X.; Peng, Z.; Zhang, G.; Li, Y. Wearable molecularly imprinted electrochemical sensor with integrated nanofiber-based microfluidic chip for in situ monitoring of cortisol in sweat. Sens. Actuators B Chem. 2023, 381, 133451. [Google Scholar] [CrossRef]
- Karasu, T.; Özgür, E.; Uzun, L. MIP-on-a-chip: Artificial receptors on microfluidic platforms for biomedical applications. J. Pharm. Biomed. Anal. 2023, 226, 115257. [Google Scholar] [CrossRef]
- Erkmen, O. Practice 4—Pure culture techniques. In Laboratory Practices in Microbiology; Erkmen, M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 41–50. [Google Scholar] [CrossRef]
- Ogodo, A.C.; Agwaranze, D.I.; Daji, M.; Aso, R.E. Chapter 13—Microbial techniques and methods: Basic techniques and microscopy. In Analytical Techniques in Biosciences from Basics to Applications; Egbuna, C., Patrick-Iwuanyanwu, K.C., Shah, M.A., Ifemeje, J.C., Rasul, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 201–220. [Google Scholar] [CrossRef]
- DeVilbiss, S.E.; Steele, M.K.; Krometis, L.-A.H.; Badgley, B.D. Freshwater salinization increases survival of Escherichia coli and risk of bacterial impairment. Water Res. 2021, 191, 116812. [Google Scholar] [CrossRef]
- Meride, Y.; Ayenew, B. Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia. Environ. Syst. Res. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Mirzajani, R.; Kardani, F. Fabrication of ciprofloxacin molecular imprinted polymer coating on a stainless steel wire as a selective solid-phase microextraction fiber for sensitive determination of fluoroquinolones in biological fluids and tablet formulation using HPLC-UV detection. J. Pharm. Biomed. Anal. 2016, 122, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Shahhoseini, F.; Azizi, A.; Bottaro, C.S. A critical evaluation of molecularly imprinted polymer (MIP) coatings in solid phase microextraction devices. TrAC Trends Anal. Chem. 2022, 156, 116695. [Google Scholar] [CrossRef]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar] [CrossRef]
- Ostovan, A.; Arabi, M.; Wang, Y.; Li, J.; Li, B.; Wang, X.; Chen, L. Greenificated molecularly imprinted materials for advanced applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.A.; Matthew, N.O. Fundamentals of Electric Circuits; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Xu, L.; Ivanov, P.C.; Hu, K.; Chen, Z.; Carbone, A.; Stanley, H.E. Quantifying signals with power-law correlations: A comparative study of detrended fluctuation analysis and detrended moving average techniques. Phys. Rev. E 2005, 71, 51101. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, S.; Nakadoi, Y.; Takahashi, M.; Ikemizu, M.; Kadoma, T.; Saimatsu, K.; Dung, L.Q.; Shiigi, H.; Nagaoka, T. Label-free and selective bacteria detection using a film with transferred bacterial configuration. Anal. Chem. 2013, 85, 4925–4929. [Google Scholar] [CrossRef] [PubMed]
- El Ichi, S.; Leon, F.; Vossier, L.; Marchandin, H.; Errachid, A.; Coste, J.; Jaffrezic-Renault, N.; Fournier-Wirth, C. Microconductometric immunosensor for label-free and sensitive detection of Gram-negative bacteria. Biosens. Bioelectron. 2014, 54, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Yang, Q.; Jiang, X.; Li, Y.; Zhao, J.; Qu, K. Conductometric sensor for viable Escherichia coli and Staphylococcus aureus based on magnetic analyte separation via aptamer. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wan, Y.; Zhang, D. Impedimetric biosensor based on cell-mediated bioimprinted films for bacterial detection. Biosens. Bioelectron. 2013, 39, 282–288. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, Y.; Wang, X.; Li, Y.; Ma, P.; Gu, B.; Li, H. Recent advances in rapid pathogen detection method based on biosensors. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1021–1037. [Google Scholar] [CrossRef]
- Yilmaz, E.; Majidi, D.; Ozgur, E.; Denizli, A. Whole cell imprinting based Escherichia coli sensors: A study for SPR and QCM. Sens. Actuators B Chem. 2015, 209, 714–721. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- Canale-Parola, E. Sarcina. Bergey’s Man. Syst. Archaea Bact. 2015, 1–9. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtarian, S.; Doostmohammadi, A.; Archonta, D.-E.; Kraft, G.; Brar, S.K.; Rezai, P. Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water. Biosensors 2023, 13, 943. https://doi.org/10.3390/bios13100943
Akhtarian S, Doostmohammadi A, Archonta D-E, Kraft G, Brar SK, Rezai P. Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water. Biosensors. 2023; 13(10):943. https://doi.org/10.3390/bios13100943
Chicago/Turabian StyleAkhtarian, Shiva, Ali Doostmohammadi, Daphne-Eleni Archonta, Garrett Kraft, Satinder Kaur Brar, and Pouya Rezai. 2023. "Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water" Biosensors 13, no. 10: 943. https://doi.org/10.3390/bios13100943
APA StyleAkhtarian, S., Doostmohammadi, A., Archonta, D.-E., Kraft, G., Brar, S. K., & Rezai, P. (2023). Microfluidic Sensor Based on Cell-Imprinted Polymer-Coated Microwires for Conductometric Detection of Bacteria in Water. Biosensors, 13(10), 943. https://doi.org/10.3390/bios13100943






