Monitoring Changes in Oxygen Muscle during Exercise with High-Flow Nasal Cannula Using Wearable NIRS Biosensors
Abstract
:1. Introduction
2. Materials and Methods
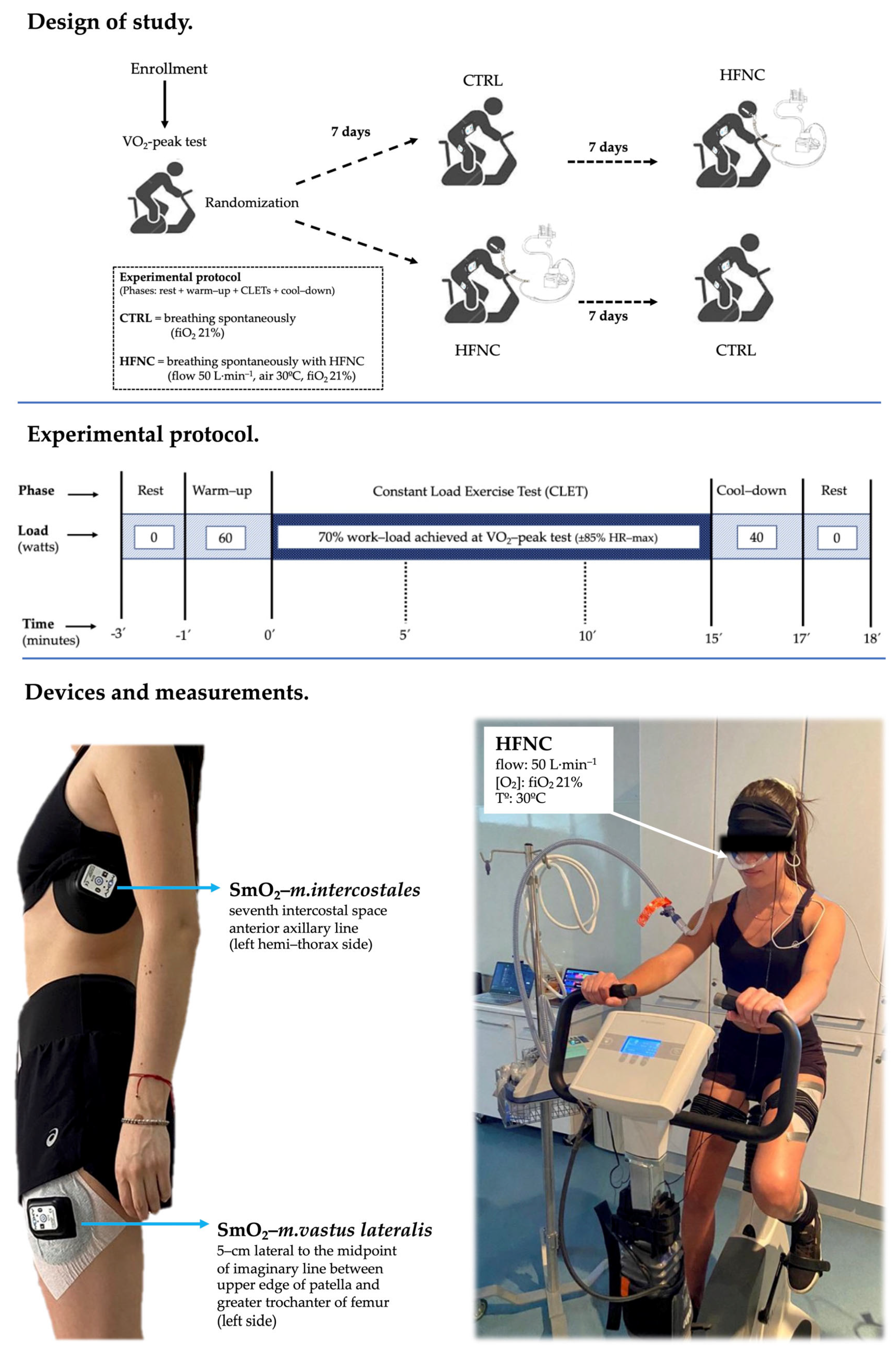
2.1. Study Design and Participants
2.2. Procedures
2.3. Peak Oxygen Consumption (O2-Peak)
2.4. Muscle Oxygen Saturation (SmO2)
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Changes in Physiological Variables during Exercise
3.3. Changes in Symptoms and Effort Level during Exercise
4. Discussion
4.1. Wearable NIRS Devices and Oxygen Muscle Levels during Exercise
4.2. Effect of HFNC on Physiological Variables, Physical Performance, and Symptoms
4.3. Limitations and Directions for Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dempsey, J.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of Exercise-Induced Respiratory Muscle Work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Archiza, B.; Ramsook, A.H.; Mitchell, R.A.; Peters, C.M.; Molgat-Seon, Y.; Henderson, W.R.; Koehle, M.S.; Boushel, R.; Sheel, A.W. Effects of Respiratory Muscle Work on Respiratory and Locomotor Blood Flow during Exercise. Exp. Physiol. 2017, 102, 1535–1547. [Google Scholar] [CrossRef]
- Bell, S.; Saunders, M.; Elborn, J.; Shale, D. Resting Energy Expenditure and Oxygen Cost of Breathing in Patients with Cystic Fibrosis. Thorax 1996, 51, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, C.; Hida, W.; Kikuchi, Y.; Taguchi, O.; Miki, H.; Takishima, T.; Shirato, K. Oxygen Consumption of Respiratory Muscles in Patients with COPD. Chest 1994, 105, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.; Harms, C.; Ainsworth, D. Respiratory Muscle Perfusion and Energetics during Exercise. Med. Sci. Sports Exerc. 1996, 28, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.; McKenzie, D.; Haverkamp, H.; Eldridge, M. Update in the Understanding of Respiratory Limitations to Exercise Performance in Fit, Active Adults. Chest 2008, 134, 613–622. [Google Scholar] [CrossRef]
- Phillips, D.; Stickland, M. Respiratory Limitations to Exercise in Health: A Brief Review. Curr. Opin. Physiol. 2019, 10, 173–179. [Google Scholar] [CrossRef]
- Dipla, K.; Zafeiridis, A.; Koidou, I.; Geladas, N.; Vrabas, I.S. Altered Hemodynamic Regulation and Reflex Control during Exercise and Recovery in Obese Boys. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H2090–H2096. [Google Scholar] [CrossRef] [PubMed]
- Gama, G.; Farinatti, P.; Rangel, M.; Mira, P.A.; Laterza, M.; Crisafulli, A.; Borges, J. Muscle Metaboreflex Adaptations to Exercise Training in Health and Disease. Eur. J. Appl. Physiol. 2021, 121, 2943–2955. [Google Scholar] [CrossRef]
- Crisafulli, A.; Salis, E.; Tocco, F.; Melis, F.; Milia, R.; Pittau, G.; Caria, M.; Solinas, R.; Meloni, L.; Pagliaro, P.; et al. Impaired Central Hemodynamic Response and Exaggerated Vasoconstriction during Muscle Metaboreflex Activation in Heart Failure Patients. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H2988–H2996. [Google Scholar] [CrossRef]
- Delaney, E.; Greaney, J.; Edwards, D.; Rose, W.; Fadel, P.; Farquhar, W. Exaggerated Sympathetic and Pressor Responses to Handgrip Exercise in Older Hypertensive Humans: Role of the Muscle Metaboreflex. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H1318–H1327. [Google Scholar] [CrossRef]
- Crisafulli, A. The Impact of Cardiovascular Diseases on Cardiovascular Regulation during Exercise in Humans: Studies on Metaboreflex Activation Elicited by the Post-Exercise Muscle Ischemia Method. Curr. Cardiol. Rev. 2017, 13, 293–300. [Google Scholar] [CrossRef]
- Sheel, A.W.; Boushel, R.; Dempsey, J. Competition for Blood Flow Distribution between Respiratory and Locomotor Muscles: Implications for Muscle Fatigue. J. Appl. Physiol. 2018, 125, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Harada, J.; Nagata, K.; Morimoto, T.; Iwata, K.; Matsunashi, A.; Sato, Y.; Tachikawa, R.; Ishikawa, A.; Tomii, K. Effect of High-Flow Nasal Cannula Oxygen Therapy on Exercise Tolerance in Patients with Idiopathic Pulmonary Fibrosis: A Randomized Crossover Trial. Respirology 2022, 27, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chihara, Y.; Tsuboi, T.; Sumi, K.; Sato, A. Effectiveness of High-Flow Nasal Cannula on Pulmonary Rehabilitation in Subjects with Chronic Respiratory Failure. Respir. Investig. 2022, 60, 658–666. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, C.; Lin, H.; Cheng, S.; Wu, H. Effects of High Flow Nasal Cannula on Exercise Endurance in Patients with Chronic Obstructive Pulmonary Disease. J. Formos. Med. Assoc. 2022, 121, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; De Andrade, A.; Campos, S.; Brandão, D.; Fregonezi, G.; Mourato, I.; Aliverti, A.; De Britto, M. Effects of Noninvasive Ventilation on Treadmill 6-Min Walk Distance and Regional Chest Wall Volumes in Cystic Fibrosis: Randomized Controlled Trial. Respir. Med. 2014, 108, 1460–1468. [Google Scholar] [CrossRef]
- Hui, D.; Mahler, D.; Larsson, L.; Wu, J.; Thomas, S.; Harrison, C.; Hess, K.; Lopez-Mattei, J.; Thompson, K.; Gomez, D.; et al. High-Flow Nasal Cannula Therapy for Exertional Dyspnea in Patients with Cancer: A Pilot Randomized Clinical Trial. Oncologist 2021, 26, e1470–e1479. [Google Scholar] [CrossRef]
- Vitacca, M.; Paneroni, M.; Zampogna, E.; Visca, D.; Carlucci, A.; Cirio, S.; Banfi, P.; Pappacoda, G.; Trianni, L.; Brogneri, A.; et al. High-Flow Oxygen Therapy during Exercise Training in Patients with Chronic Obstructive Pulmonary Disease and Chronic Hypoxemia: A Multicenter Randomized Controlled Trial. Phys. Ther. 2020, 100, 1249–1259. [Google Scholar] [CrossRef]
- Fang, T.; Chen, Y.; Hsiao, H.; Cho, H.; Tsai, Y.; Huang, C.; Hsieh, M.; Wu, H.; Lin, H. Effect of High Flow Nasal Cannula on Peripheral Muscle Oxygenation and Hemodynamic during Paddling Exercise in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Ann. Transl. Med. 2020, 8, 280. [Google Scholar] [CrossRef]
- Cross, T.J.; Gideon, E.A.; Morris, S.J.; Coriell, C.L.; Hubbard, C.D.; Duke, J.W. A Comparison of Methods Used to Quantify the Work of Breathing during Exercise. J. Appl. Physiol. 2021, 131, 1123–1133. [Google Scholar] [CrossRef]
- Contreras-Briceño, F.; Espinosa-Ramirez, M.; Hevia, G.; Llambias, D.; Carrasco, M.; Cerda, F.; López-Fuenzalida, A.; García, P.; Gabrielli, L.; Viscor, G. Reliability of NIRS Portable Device for Measuring Intercostal Muscles Oxygenation during Exercise. J. Sports Sci. 2019, 37, 2653–2659. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, M.; Riquelme, S.; Araya, F.; Rodríguez, G.; Figueroa-Martínez, F.; Gabrielli, L.; Viscor, G.; Reid, W.; Contreras-Briceño, F. Effectiveness of Respiratory Muscles Training by Voluntary Isocapnic Hyperpnea Versus Inspiratory Threshold Loading on Intercostales and Vastus Lateralis Muscles Deoxygenation Induced by Exercise in Physically Active Adults. Biology 2023, 12, 219. [Google Scholar] [CrossRef]
- Scheeren, T.; Schober, P.; Schwarte, L. Monitoring Tissue Oxygenation by near Infrared Spectroscopy (NIRS): Background and Current Applications. J. Clin. Monit. Comput. 2012, 26, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Athanasopoulos, D.; Habazettl, H.; Kuebler, W.; Wagner, H.; Roussos, C.; Wagner, P.; Zakynthinos, S. Intercostal Muscle Blood Flow Limitation in Athletes during Maximal Exercise. J. Physiol. 2009, 587, 3665–3677. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Athanasopoulos, D.; Habazettl, H.; Aliverti, A.; Louvaris, Z.; Cherouveim, E.; Wagner, H.; Roussos, C.; Wagner, P.; Zakynthinos, S. Intercostal Muscle Blood Flow Limitation during Exercise in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 182, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J. Validation of Near-Infrared Spectroscopy in Humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Neary, J. Application of near Infrared Spectroscopy to Exercise Sports Science. Can. J. Appl. Physiol. 2004, 29, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Koirala, B.; Concas, A.; Sun, Y.; Gladden, L.; Lai, N. Relationship between Muscle Venous Blood Oxygenation and Near-Infrared Spectroscopy: Quantitative Analysis of the Hb and Mb Contributions. J. Appl. Physiol. 2023, 134, 1063–1074. [Google Scholar] [CrossRef]
- Perrey, S.; Ferrari, M. Muscle Oximetry in Sports Science: A Systematic Review. Sports Med. 2018, 48, 597–616. [Google Scholar] [CrossRef]
- Sendra-Pérez, C.; Sanchez-Jimenez, J.L.; Marzano-Felisatti, J.M.; Encarnación-Martínez, A.; Salvador-Palmer, R.; Priego-Quesada, J.I. Reliability of Threshold Determination Using Portable Muscle Oxygenation Monitors during Exercise Testing: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 12649. [Google Scholar] [CrossRef]
- Contreras-Briceño, F.; Espinosa-Ramírez, M.; Moya-Gallardo, E.; Fuentes-Kloss, R.; Gabrielli, L.; Araneda, O.; Viscor, G. Intercostal Muscles Oxygenation and Breathing Pattern during Exercise in Competitive Marathon Runners. Int. J. Environ. Res. Public Health 2021, 18, 8287. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Briceño, F.; Espinosa-Ramirez, M.; Keim-Bagnara, V.; Carreño-Román, M.; Rodríguez-Villagra, R.; Villegas-Belmar, F.; Viscor, G.; Gabrielli, L.; Andía, M.; Araneda, O.; et al. Determination of the Respiratory Compensation Point by Detecting Changes in Intercostal Muscles Oxygenation by Using Near-Infrared Spectroscopy. Life 2022, 12, 444. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, M.; Moya-Gallardo, E.; Araya-Román, F.; Riquelme-Sánchez, S.; Rodriguez-García, G.; Reid, W.; Viscor, G.; Araneda, O.; Gabrielli, L.; Contreras-Briceño, F. Sex-Differences in the Oxygenation Levels of Intercostal and Vastus Lateralis Muscles during Incremental Exercise. Front. Physiol. 2021, 12, 738063. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Briceño, F.; Valderrama, P.; Moya, E.; Espinosa, M.; Villaseca, Y.; Ira-Ira, C.; Moya, A.; Mieres, J.; Clavería, C.; Contreras-Briceño, F.; et al. Oxigenación de Músculos Respiratorios y Locomotores Durante El Test Cardiopulmonar En Pacientes Con Circulación de Fontan: Serie de Casos. Rev. Chil. Cardiol. 2021, 40, 27–36. [Google Scholar] [CrossRef]
- Da Luz Goulart, C.; Caruso, F.; Garcia de Araújo, A.; Garcia de Moura, S.; Catai, A.; Batista Dos Santos, P.; Kabbach, É.; Arena, R.; Gonçalves Mendes, R.; Borghi-Silva, A. The Effect of Adding Noninvasive Ventilation to High-Intensity Exercise on Peripheral and Respiratory Muscle Oxygenation. Respir. Care 2023, 68, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.; Caruso, F.; de Araújo, A.; Moura, S.; Catai, A.; Agostoni, P.; Mendes, R.; Arena, R.; Borghi-Silva, A. Can Non-Invasive Ventilation Modulate Cerebral, Respiratory, and Peripheral Muscle Oxygenation during High-Intensity Exercise in Patients with COPD-HF? Front. Cardiovasc. Med. 2022, 8, 772650. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Palange, P.; Casaburi, R.; Laveneziana, P.; Maltais, F.; Neder, J.A.; O’Donnell, D.E.; Onorati, P.; Porszasz, J.; Rabinovich, R.; et al. Use of Exercise Testing in the Evaluation of Interventional Efficacy: An Official ERS Statement. Eur. Respir. J. 2016, 47, 429–460. [Google Scholar] [CrossRef]
- Arizono, S.; Taniguchi, H.; Sakamoto, K.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Ogawa, T.; Watanabe, F.; Nishiyama, O.; Nishimura, K.; et al. Endurance Time Is the Most Responsive Exercise Measurement in Idiopathic Pulmonary Fibrosis. Respir. Care 2014, 59, 1108–1115. [Google Scholar] [CrossRef]
- DeCato, T.; Haverkamp, H.; Hegewald, M.; Sockrider, M.; Kaminsky, D. Cardiopulmonary Exercise Testing (CPET). Am. J. Respir. Crit. Care Med. 2020, 201, 1–2. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Day, J.; Rossiter, H.; Coats, E.; Skasick, A.; Whipp, B. The Maximally Attainable VO2 during Exercise in Humans: The Peak vs. Maximum Issue. J. Appl. Physiol. 2003, 95, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-Infrared Spectroscopy-Derived Muscle Oxygen Saturation on a 0% to 100% Scale: Reliability and Validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 115001. [Google Scholar] [CrossRef]
- Crum, E.M.; O’Connor, W.J.; Van Loo, L.; Valckx, M.; Stannard, S.R. Validity and Reliability of the Moxy Oxygen Monitor during Incremental Cycling Exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Iannetta, D.; Qahtani, A.; Mattioni-Maturana, F.; Murias, J. The Near-Infrared Spectroscopy Derived Deoxygenated Haemoglobin Breaking-Point Is a Repeatable Measure That Demarcates Exercise Intensity Domains. J. Sci. Med. Sport 2017, 20, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Vasquez Bonilla, A.; González-Custodio, A.; Timón, R.; Cardenosa, A.; Camacho-Cardenosa, M.; Olcina, G. Training Zones through Muscle Oxygen Saturation during a Graded Exercise Test in Cyclists and Triathletes. Biol. Sport 2023, 40, 439–448. [Google Scholar] [CrossRef]
- Thiel, C.; Vogt, L.; Himmelreich, H.; Hübscher, M.; Banzer, W. Reproducibility of Muscle Oxygen Saturation. Int. J. Sports Med. 2011, 32, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.; Arêas, G.; Caruso, F.; Araújo, A.; de Moura, S.; Catai, A.; Beltrame, T.; Junior, L.; dos Santos, P.; Roscani, M.; et al. Effect of High-Intensity Exercise on Cerebral, Respiratory and Peripheral Muscle Oxygenation of HF and COPD-HF Patients. Heart Lung 2021, 50, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Steppan, J.; Hogue, C.W. Cerebral and Tissue Oximetry. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 429–439. [Google Scholar] [CrossRef]
- Ekkekakis, P. Illuminating the Black Box: Investigating Prefrontal Cortical Hemodynamics during Exercise with near-Infrared Spectroscopy. J. Sport Exerc. Psychol. 2009, 31, 505–553. [Google Scholar] [CrossRef]
- Watzman, H.M.; Kurth, C.D.; Montenegro, L.M.; Rome, J.; Steven, J.M.; Nicolson, S.C. Arterial and Venous Contributions to Near-Infrared Cerebral Oximetry. Anesthesiology 2000, 93, 947–953. [Google Scholar] [CrossRef]
- Thavasothy, M.; Broadhead, M.; Elwell, C.; Peters, M.; Smith, M. A Comparison of Cerebral Oxygenation as Measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia 2002, 57, 999–1006. [Google Scholar] [CrossRef]
- Chatila, W.; Nugent, T.; Vance, G.; Gaughan, J.; Criner, G. The Effects of High-Flow vs Low-Flow Oxygen on Exercise in Advanced Obstructive Airways Disease. Chest 2004, 126, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Spoletini, G.; Watson, R.; Lim, W.; Pollard, K.; Etherington, C.; Clifton, I.; Peckham, D. Nasal High-Flow Therapy as an Adjunct to Exercise in Patients with Cystic Fibrosis: A Pilot Feasibility Trial. J. Cyst. Fibros. 2021, 20, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ando, M.; Kimura, T.; Kataoka, K.; Yokoyama, T.; Shiroshita, E.; Kondoh, Y. The Impact of High-Flow Nasal Cannula Oxygen Therapy on Exercise Capacity in Fibrotic Interstitial Lung Disease: A Proof-of-Concept Randomized Controlled Crossover Trial. BMC Pulm. Med. 2020, 20, 51. [Google Scholar] [CrossRef]
- Hui, D.; Hernandez, F.; Urbauer, D.; Thomas, S.; Lu, Z.; Elsayem, A.; Bruera, E. High-Flow Oxygen and High-Flow Air for Dyspnea in Hospitalized Patients with Cancer: A Pilot Crossover Randomized Clinical Trial. Oncologist 2021, 26, e883–e892. [Google Scholar] [CrossRef]
- Richardson, R.; Poole, D.; Knight, D.; Kurdak, S.; Hogan, M.; Grassi, B.; Johnson, E.; Kendrick, K.; Erickson, B.; Wagner, P. High Muscle Blood Flow in Man: Is Maximal O2 Extraction Compromised? J. Appl. Physiol. 1993, 75, 1911–1916. [Google Scholar] [CrossRef]
- Ramsook, A.; Peters, C.; Leahy, M.; Archiza, B.; Mitchell, R.; Jasinovic, T.; Koehle, M.; Guenette, J.; Sheel, A.W. Near-Infrared Spectroscopy Measures of Sternocleidomastoid Blood Flow during Exercise and Hyperpnoea. Exp. Physiol. 2020, 105, 2226–2237. [Google Scholar] [CrossRef] [PubMed]




| Variables | Mean ± Standard Deviation |
|---|---|
| Sex (n) | female = 9; male = 9 |
| Age (years) | 22 ± 2 |
| Height (cm) | 173.0 ± 5.8 |
| Weight (kg) | 65.1 ± 11.2 |
| BMI | 21.6 ± 2.8 |
| FEV1 (L) | 4.32 ± 0.56 |
| FVC (L) | 5.13 ± 0.78 |
| FEV1 · FVC−1 (%) | 84.2 ± 3.4 |
| Load max (watts) | 226 ± 62 |
| O2-peak (mL·kg−1·min−1) | 48.3 ± 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Briceño, F.; Espinosa-Ramírez, M.; Rivera-Greene, A.; Guerra-Venegas, C.; Lungenstrass-Poulsen, A.; Villagra-Reyes, V.; Caulier-Cisterna, R.; Araneda, O.F.; Viscor, G. Monitoring Changes in Oxygen Muscle during Exercise with High-Flow Nasal Cannula Using Wearable NIRS Biosensors. Biosensors 2023, 13, 985. https://doi.org/10.3390/bios13110985
Contreras-Briceño F, Espinosa-Ramírez M, Rivera-Greene A, Guerra-Venegas C, Lungenstrass-Poulsen A, Villagra-Reyes V, Caulier-Cisterna R, Araneda OF, Viscor G. Monitoring Changes in Oxygen Muscle during Exercise with High-Flow Nasal Cannula Using Wearable NIRS Biosensors. Biosensors. 2023; 13(11):985. https://doi.org/10.3390/bios13110985
Chicago/Turabian StyleContreras-Briceño, Felipe, Maximiliano Espinosa-Ramírez, Augusta Rivera-Greene, Camila Guerra-Venegas, Antonia Lungenstrass-Poulsen, Victoria Villagra-Reyes, Raúl Caulier-Cisterna, Oscar F. Araneda, and Ginés Viscor. 2023. "Monitoring Changes in Oxygen Muscle during Exercise with High-Flow Nasal Cannula Using Wearable NIRS Biosensors" Biosensors 13, no. 11: 985. https://doi.org/10.3390/bios13110985
APA StyleContreras-Briceño, F., Espinosa-Ramírez, M., Rivera-Greene, A., Guerra-Venegas, C., Lungenstrass-Poulsen, A., Villagra-Reyes, V., Caulier-Cisterna, R., Araneda, O. F., & Viscor, G. (2023). Monitoring Changes in Oxygen Muscle during Exercise with High-Flow Nasal Cannula Using Wearable NIRS Biosensors. Biosensors, 13(11), 985. https://doi.org/10.3390/bios13110985







