Insights into the Mechanism of Bipolar Electrodeposition of Au Films and Its Application in Visual Detection of Prostate Specific Antigens
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Bipolar Deposition
2.4. Fabricating of Specific Sensing Interface for PSA Assay
2.5. ECL Imaging
2.6. Electrochemical Imaging
3. Results and Discussion
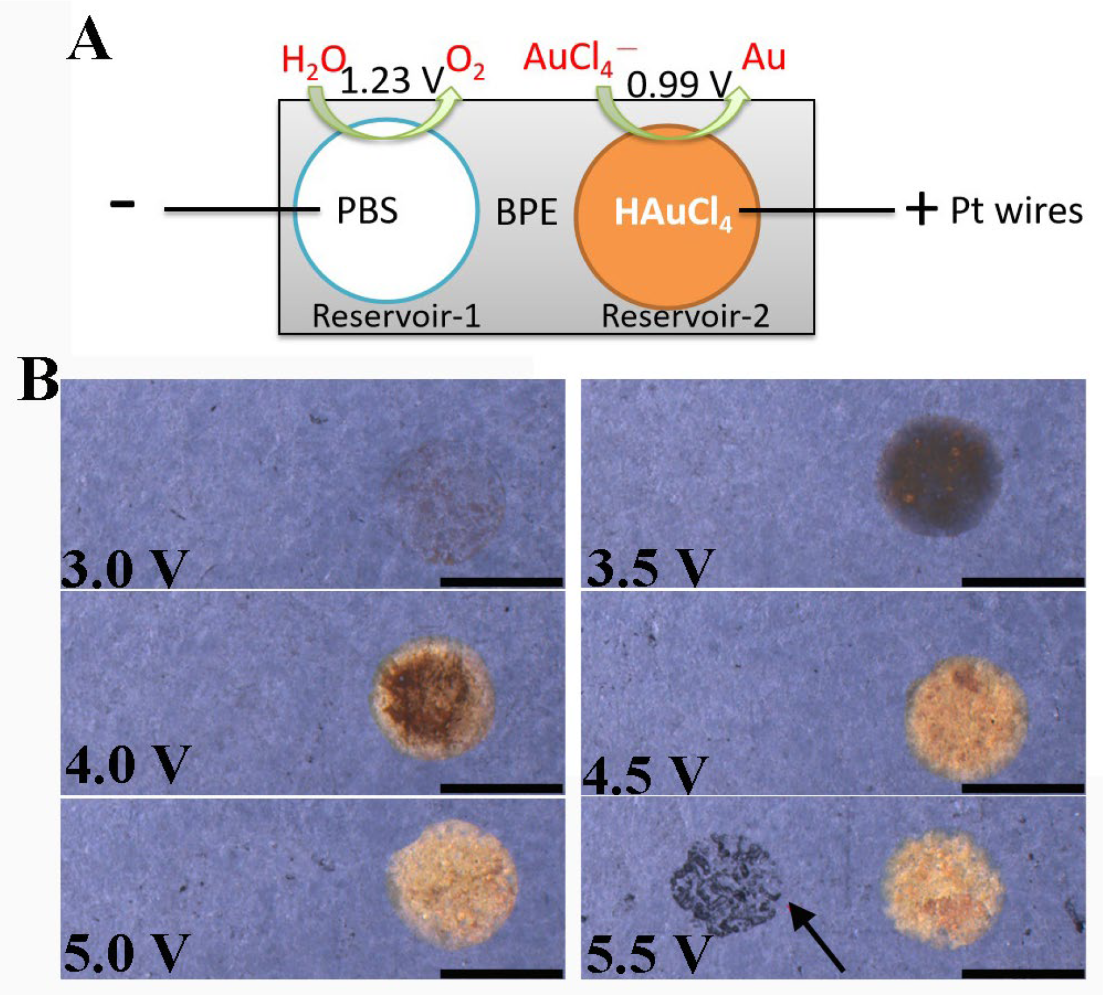
3.1. Bipolar Deposition of Au on Two Poles of BPE
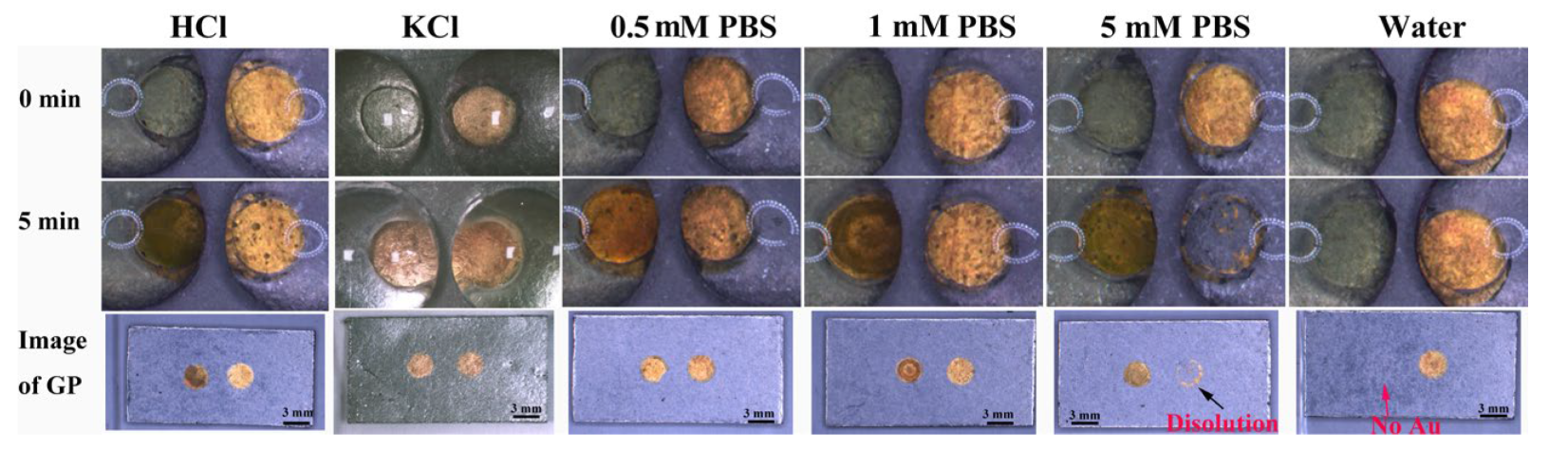
3.2. Mechanism of Au Film Dissolution in BPE Device
3.3. Characterization of Au–Au BPE
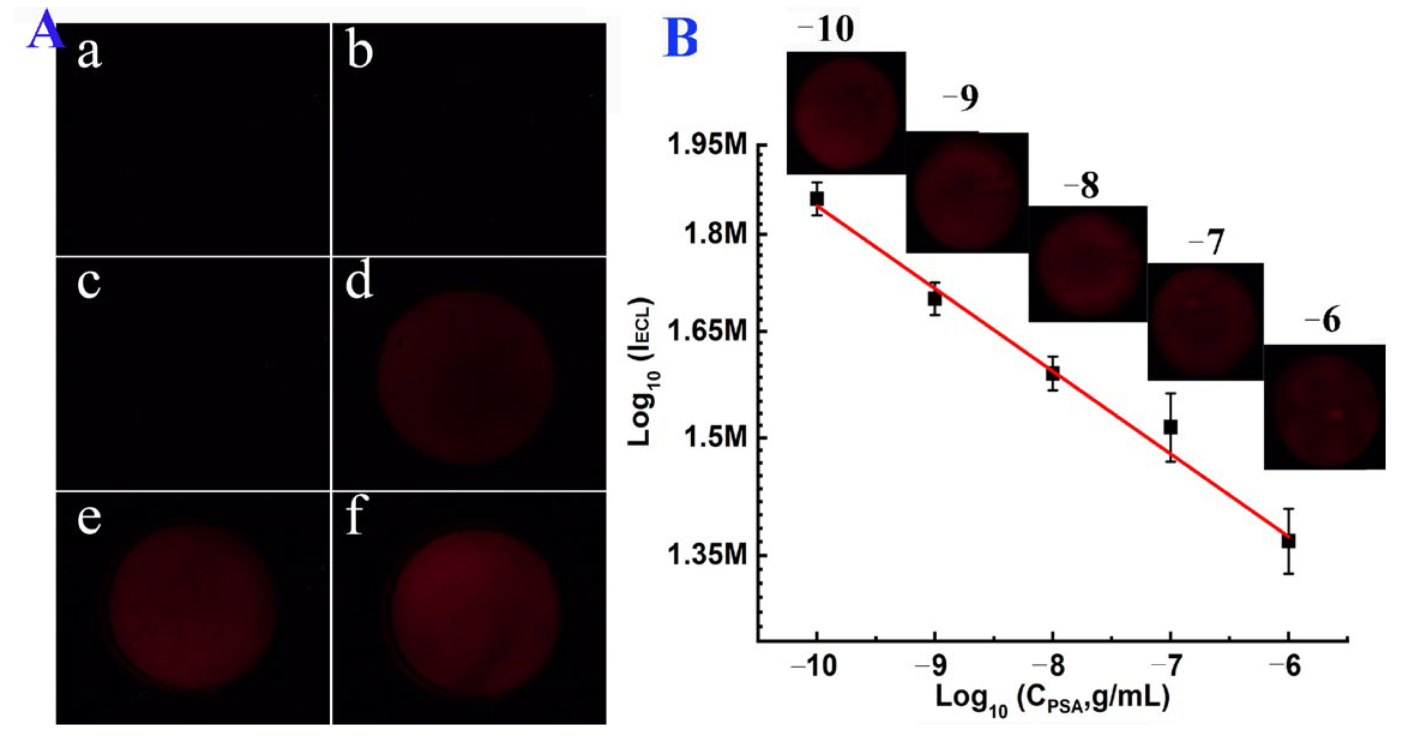
3.4. ECL Imaging of PSA
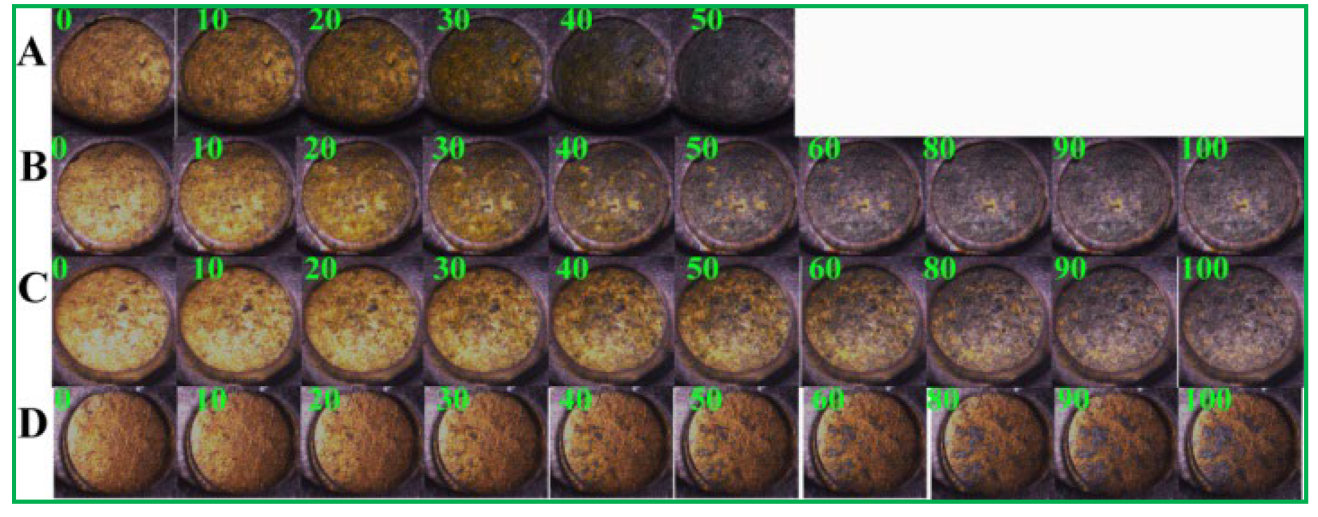
3.5. Electrochemical Imaging of PSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, L.; Qiao, J.; Chen, J.; Xu, N.; Wu, M. Combination of area controllable sensing surface and bipolar electrode-electrochemiluminescence approach for the detection of tetracycline. Talanta 2020, 208, 120404. [Google Scholar] [CrossRef] [PubMed]
- Rafatmah, E.; Hemmateenejad, B. Colorimetric and visual determination of hydrogen peroxide and glucose by applying paper-based closed bipolar electrochemistry. Microchim. Acta 2019, 186, 684. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Mi, L.; Wu, Y.; Shao, F.; Zou, M.; Zhou, Z.; Liu, S. Visual Electrofluorochromic Detection of Cancer Cell Surface Glycoprotein on a Closed Bipolar Electrode Chip. Anal. Chem. 2019, 91, 7902–7910. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, X.; Tian, Z.; Wang, K.; Xia, X.; Wu, Y.; Liu, S. Gold Nanowires Array-Based Closed Bipolar Nanoelectrode System for Electrochemiluminescence Detection of α-Fetoprotein on Cell Surface. Anal. Chem. 2022, 94, 7350–7357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, H.; Xu, C.-H.; Cheng, Y.; Chen, H.-Y.; Xu, J.-J. An Efficient Electrochemiluminescence Enhancement Strategy on Bipolar Electrode for Bioanalysis. Anal. Chem. 2019, 91, 12553–12559. [Google Scholar] [CrossRef]
- Zhang, X.; Lazenby, R.A.; Wu, Y.; White, R.J. Electrochromic, Closed-Bipolar Electrodes Employing Aptamer-Based Recognition for Direct Colorimetric Sensing Visualization. Anal. Chem. 2019, 91, 11467–11473. [Google Scholar] [CrossRef]
- Fiorani, A.; Irkham; Valenti, G.; Paolucci, F.; Einaga, Y. Electrogenerated Chemiluminescence with Peroxydisulfate as a Coreactant Using Boron Doped Diamond Electrodes. Anal. Chem. 2018, 90, 12959–12963. [Google Scholar] [CrossRef]
- Chow, K.-F.; Chang, B.-Y.; Zaccheo, B.A.; Mavre, F.; Crooks, R.M. A Sensing Platform Based on Electrodissolution of a Ag Bipolar Electrode. J. Am. Chem. Soc. 2010, 132, 9228–9229. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Pan, J.-B.; Song, J.; Zhao, W.; Chen, H.-Y.; Xu, J.J. Bipolar Electrode Array for Multiplexed Detection of Prostate Cancer Biomarkers. Anal. Chem. 2022, 94, 3005–3012. [Google Scholar] [CrossRef]
- Du, F.; Dong, Z.; Guan, Y.; Zeid, A.M.; Ma, D.; Feng, J.; Yang, D.; Xu, G. Single-Electrode Electrochemical System for the Visual and High-Throughput Electrochemiluminescence Immunoassay. Anal. Chem. 2022, 94, 2189–2194. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.-X.; Zhu, J.-W.; Zong, H.-L.; Hu, Y.-H.; Wang, Y.-Z. Ultrasensitive and Visual Electrochemiluminescence Ratiometry Based on a Constant Resistor-Integrated Bipolar Electrode for MicroRNA Detection. Anal. Chem. 2022, 94, 4303–4310. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Sun, Z.; Liu, F.; Wang, D. Ultrasensitive multicolor electrochromic sensor built on closed bipolar electrode: Application in the visual detection of Pseudomonas aeruginosa. Food Chem. 2023, 403, 134240. [Google Scholar] [CrossRef]
- Luo, Y.; Lv, F.; Wang, M.; Lu, L.; Liu, Y.; Xiong, X. A multicolor electrochemiluminescence device based on closed bipolar electrode for rapid visual screening of Salmonella typhimurium. Sens. Actuators B Chem. 2021, 349, 130761. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Wang, Y.-Z.; Wang, L.-L.; Zhu, J.W.; Zhao, J.; Zong, H.-L.; Chen, C.X. Bipolar electrode ratiometric electrochemiluminescence biosensing analysis based on boron nitride quantum dots and biological release system. Biosens. Bioelectron. 2021, 191, 113393. [Google Scholar] [CrossRef]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.-K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef]
- Lu, H.-J.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Visual electrochemiluminescence ratiometry on bipolar electrode for bioanalysis. Biosens. Bioelectron. 2018, 102, 624–630. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, S.-N. Graphite paper-based bipolar electrode electrochemiluminescence sensing platform. Biosens. Bioelectron. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Xiong, X.; Li, Y.; Yuan, W.; Lu, Y.; Xiong, X.; Li, Y.; Chen, X.; Liu, Y. Screen printed bipolar electrode for sensitive electrochemiluminescence detection of aflatoxin B1 in agricultural products. Biosens. Bioelectron. 2020, 150, 111873. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Su, Y.; Shang, Q.; Zhang, C. Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles. Biosens. Bioelectron. 2019, 130, 55–64. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Li, J.; Zhang, L.; Wang, E. New Insight into a Microfluidic-Based Bipolar System for an Electrochemiluminescence Sensing Platform. Anal. Chem. 2013, 85, 5335–5339. [Google Scholar] [CrossRef]
- Chang, B.-Y.; Chow, K.-F.; Crooks, J.A.; Mavré, F.; Crooks, R.M. Two-channel microelectrochemical bipolar electrode sensor array. Analyst 2012, 137, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-W.; Zhao, W.; Liu, Z.; Liu, X.-C.; Wu, M.-S.; Xu, J.-J.; Chen, H.Y. Joint enhancement strategy applied in ECL biosensor based on closed bipolar electrodes for the detection of PSA. Talanta 2016, 154, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, C.; Huang, C.; Li, N.; Wang, Y.; Ge, S.; Yu, J. Paper-based closed Au-Bipolar electrode electrochemiluminescence sensing platform for the detection of miRNA-155. Biosens. Bioelectron. 2019, 150, 111917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, N.; Luo, X.; Ding, S.-N. Patchy gold coated Fe3O4 nanospheres with enhanced catalytic activity applied for paper-based bipolar electrode-electrochemiluminescence aptasensors. Biosens. Bioelectron. 2018, 114, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Koizumi, Y.; Nishiyama, H.; Tomita, I.; Inagi, S. Buoyant force-induced continuous floating and sinking of Janus micromotors. RSC Adv. 2018, 8, 33331–33337. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. FAD-based glucose dehydrogenase immobilized on thionine/AuNPs frameworks grafted on amino-CNTs: Development of high power glucose biofuel cell and biosensor. J. Electroanal. Chem. 2018, 815, 105–113. [Google Scholar] [CrossRef]
- Cao, J.-T.; Wang, Y.-L.; Zhang, J.-J.; Dong, Y.-X.; Liu, F.-R.; Ren, S.-W.; Liu, Y.-M. Immuno-Electrochemiluminescent Imaging of a Single Cell Based on Functional Nanoprobes of Heterogeneous Ru(bpy)32+@SiO2/Au Nanoparticles. Anal. Chem. 2018, 90, 10334–10339. [Google Scholar] [CrossRef]
- Shayesteh, O.H.; Ghavami, R. A novel label-free colorimetric aptasensor for sensitive determination of PSA biomarker using gold nanoparticles and a cationic polymer in human serum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117644. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, Y.; Dong, W.; Sun, H.; Lv, J.; Li, H. Self-powered electrochromic sensing for visual determination of PSA in serum using PB as an indicator. J. Electroanal. Chem. 2019, 839, 108–115. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, K.; Tang, D. A new visual immunoassay for prostate-specific antigen using near-infrared excited CuxS nanocrystals and imaging on a smartphone. Analyst 2019, 144, 3716–3720. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Liu, Y.; Jiang, H.; Yang, H.; Yu, H.; Qiao, J.; Li, Z.; Jin, B.; Wu, M. Insights into the Mechanism of Bipolar Electrodeposition of Au Films and Its Application in Visual Detection of Prostate Specific Antigens. Biosensors 2023, 13, 158. https://doi.org/10.3390/bios13020158
Zhao D, Liu Y, Jiang H, Yang H, Yu H, Qiao J, Li Z, Jin B, Wu M. Insights into the Mechanism of Bipolar Electrodeposition of Au Films and Its Application in Visual Detection of Prostate Specific Antigens. Biosensors. 2023; 13(2):158. https://doi.org/10.3390/bios13020158
Chicago/Turabian StyleZhao, Daoyuan, Yujing Liu, Hong Jiang, Haijian Yang, Huihui Yu, Jingtang Qiao, Zhiwen Li, Bing Jin, and Meisheng Wu. 2023. "Insights into the Mechanism of Bipolar Electrodeposition of Au Films and Its Application in Visual Detection of Prostate Specific Antigens" Biosensors 13, no. 2: 158. https://doi.org/10.3390/bios13020158
APA StyleZhao, D., Liu, Y., Jiang, H., Yang, H., Yu, H., Qiao, J., Li, Z., Jin, B., & Wu, M. (2023). Insights into the Mechanism of Bipolar Electrodeposition of Au Films and Its Application in Visual Detection of Prostate Specific Antigens. Biosensors, 13(2), 158. https://doi.org/10.3390/bios13020158




