Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment
Abstract
:1. Introduction
2. Concepts of CNT-Based Electrochemical Biosensor
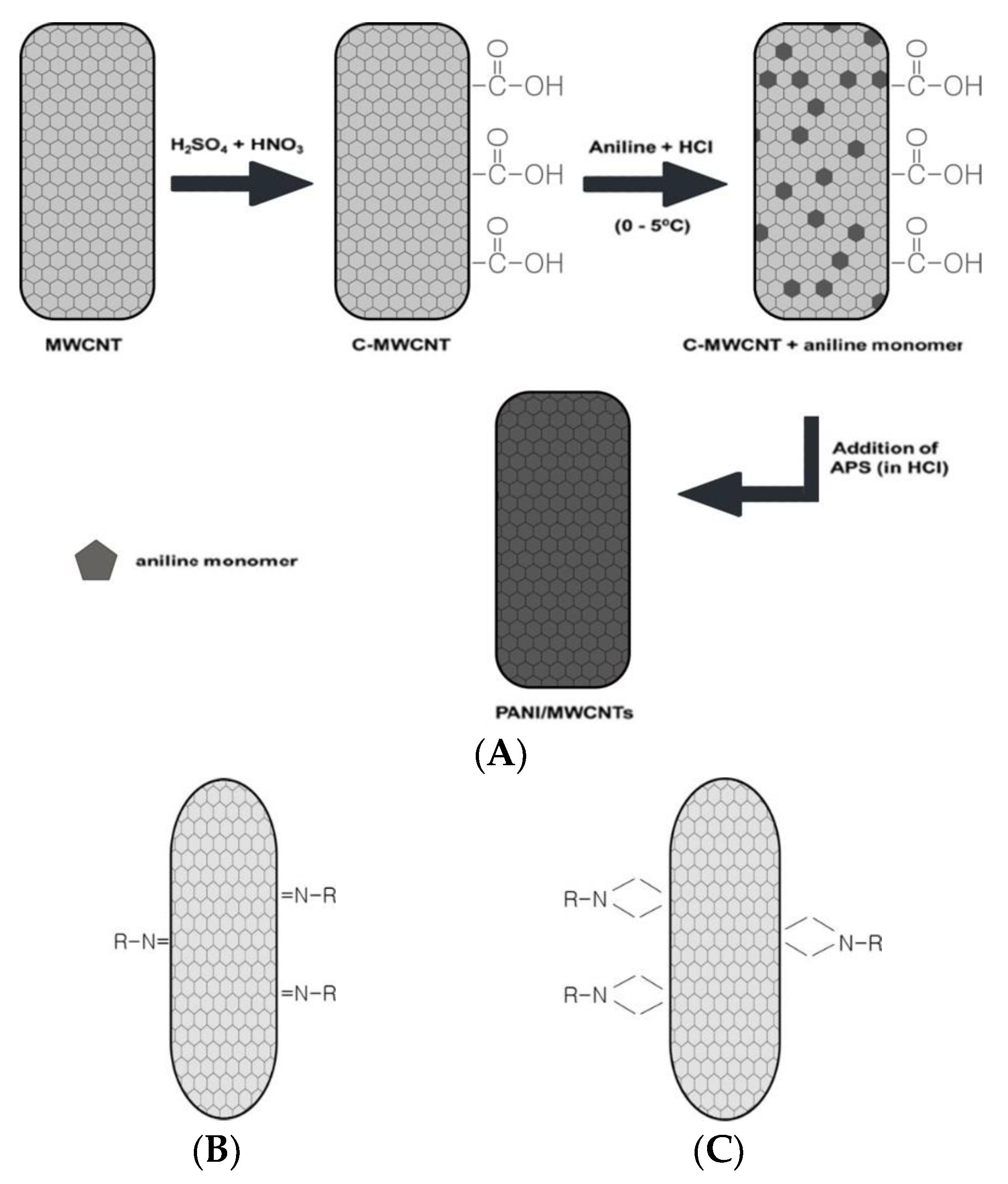
2.1. Covalent Bonding Modification of CNT for Receptor Immobilization
2.2. Non-Covalent Bonding Modification of CNT for Receptor Immobilization
2.3. Sensor Acceptors in CNT-Based Electrochemical Biosensor
| Bio-Acceptors | Analyte | Method | RD | LOD | DR | Ref. |
|---|---|---|---|---|---|---|
| Enzyme | Glucose | AMP | GOx | 3 × 10−4 nM | (1–15) × 10−3 µM | [72] |
| Glucose | AMP | GOx | 5 × 10−5 nM | (0–5) × 10−3 µM | [76] | |
| Glucose | AMP | GOx | 2.99 × 10−6 nM | (3–14) × 10−3 µM | [77] | |
| Alcohol | AMP | ADH | 3.3 × 10−3 nM | (12.5–100) × 10−3 µM | [72] | |
| Ethanol | AMP | ADH | 1 × 10−5 nM | (1–5) × 10−4 µM | [78] | |
| L-malic acid | AMP | MDH | 6 × 10−5 nM | (0–120) × 10−6 µM | [79] | |
| Xanthine | AMP | XOx | 1.2 × 10−7 nM | (2–86) × 10−6 µM | [80] | |
| Choline | AMP | COx | 6 × 10−7 nM | (3–120) × 10−6 µM | [81] | |
| Lead ions | AMP | HRP | 2.5 µg/l | 0.092–0.55 mg/l | [81] | |
| Copper ions | AMP | HRP | 4.2 µg/l | 0.068–2 mg/l | [82] | |
| Antibody | TrT | AMP | Ab | 33 pg/mL | 0.1–10 ng/mL | [83] |
| DVN | AMP | Ab | 35,000 pg/mL | 1000–2500 ng/mL | [84] | |
| ZEA | AMP | Ab | 0.15 pg/mL | 0.001–0.1 ng/mL | [85] | |
| CA19-9 | SWV | Ab | 0.163 pg/mL | 0.001–100 ng/mL | [86] | |
| HER2 | IS | Ab | 7.400 pg/mL | 10–110 ng/mL | [87] | |
| CA19-9 | IS | Ab | 0.35 U/ml | NR | [88] | |
| DNA fragments including aptamer | ALF | DPV | DNA | 3.5 fM | 10−14–10−8 M | [89] |
| HB-DNA | DPV | DNA | 2.5 fM | 10−14–10−10 M | [90] | |
| LNC-RNA | DPV | DNA | 42.8 fM | 10−14–10−7 M | [91] | |
| MicroRNA 21 | DPV | DNA | 0.01 fM | 10−17–10−6 M | [92] | |
| SSE | DPV | DNA | 17 × 10−6 fM | NR | [93] | |
| S-CML | IS | DNA | 1 fM | 10−15–10−6 nM | [94] | |
| Thrombin | DPV | DNA | 0.08 pM | 0.001–4 nM | [95] | |
| Silver ions | SWV | DNA | 1500 pM | 2–100 nM | [96] | |
| HER2 | IS | DNA | 50 fg/ml | 0.1 pg/mL | [97] |
3. CNT-Based Electrochemical Biosensor for Food Safety Assessments
3.1. CNT-Based Biosensor for Food Pathogen Detection
3.2. CNT-Based Biosensor for Food Allergen Detection
3.3. CNT-Based Biosensor for other Food Assessment
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biosensors Market Size, Growth, Trends, Report 2022 to 2030. Available online: https://www.precedenceresearch.com/biosensors-market (accessed on 4 October 2022).
- Sobhan, A.; Oh, J.H.; Park, M.K.; Lee, J. Reusability of a single-walled carbon nanotube-based biosensor for detecting peanut allergens and Y. enterocolitica. Microelectron. Eng. 2020, 225, 111281. [Google Scholar] [CrossRef]
- Sindher, S.B.; Long, A.; Chin, A.R.; Hy, A.; Sampath, V.; Nadeau, K.C.; Chinthrajah, R.S. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022, 77, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Neethirajan, S. A microfluidic biosensor using graphene oxide and aptamer-functionalized quantum dots for peanut allergen detection. Biosens. Bioelectron. 2016, 85, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Newsroom of World Health Organization (WHO). Natural Toxins in Food. Available online: https://www.who.int/news-room/fact-sheets/detail/natural-toxins-in-food (accessed on 3 January 2023).
- Santos, M.J.L.; Merrill, K.A.; Gerdts, J.D.; Ben-Shoshan, M.; Protudjer, J.L.P. Food allergy education and management in schools: A scoping review on current practices and gaps. Nutrients 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.Z.; Geliebter, J.; Tiwari, R.; Li, X.M. Traditional Chinese medicine for food allergy and eczema. Ann. Allergy Asthma Immunol. 2021, 126, 639–654. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticlecoated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Tedner, S.G.; Asarnoj, A.; Thulin, H.; Westman, M.; Konradsen, J.R.; Nilsson, C. Food allergy and hypersensitivity reactions inchildren and adults—A review. J. Intern. Med. 2022, 291, 283–302. [Google Scholar] [CrossRef]
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef]
- Sun, M.; Pei, X.; Xin, T.; Liu, J.; Ma, C.; Cao, M.; Zhou, M.A. Flexible Microfluidic Chip-Based Universal Fully Integrated Nanoelectronic System with Point-of-Care Raw Sweat, Tears, or Saliva Glucose Monitoring for Potential Noninvasive Glucose Management. Anal. Chem. 2022, 94, 1890–1900. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2020, 122, 115701. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, Y.; Yang, R.; Zhang, G. 2D-Materials-Based Wearable Biosensor Systems. Biosensors 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications–An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Puneeth, S.B.; Kulkarni, M.B.; Goel, S. Microfluidic viscometers for biochemical and biomedical applications: A review. Eng. Res. Express 2021, 3, 022003. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goyal, S.; Dhar, A.; Sriram, D.; Goel, S. Miniaturized and IoT enabled Continuous-flow based Microfluidic PCR Device for DNA Amplification. IEEE Trans. Nanobiosci. 2021, 21, 97–104. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Miniaturized DNA amplification platform with soft-lithographically fabricated continuous-flow PCR microfluidic device on a portable temperature controller. Microfluid. Nanofluid. 2021, 25, 69. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Zhu, Y.; Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. In situ growth of TiO2 nanowires on Ti3C2 MXenes nanosheets as highly sensitive luminol electrochemiluminescent nanoplatform for glucose detection in fruits, sweat and serum samples. Biosens. Bioelectron. 2021, 194, 113600. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Applications of a DNA-electrochemical biosensor. TrAC Trends Anal. Chem. 2016, 79, 23–36. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Yashas; Enaganti, P.K.; Amreen, K.; Goel, S. Internet of Things enabled portable thermal management system with microfluidic platform to synthesize MnO2 nanoparticles for electrochemical sensing. Nanotechnology 2020, 31, 425504. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Salve, M.; Goel, S. Miniaturized Thermal Monitoring Module with CO Laser Ablated Microfluidic Device for Electrochemically Validated DNA Amplification. IEEE Trans. Instrum. Meas. 2021, 70, 4006008. [Google Scholar] [CrossRef]
- Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosnensors 2022, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef]
- Henriksson, A.; Neubauer, P.; Birkholz, M. Dielectrophoresis: An Approach to Increase Sensitivity, Reduce Response Time and to Suppress Nonspecific Binding in Biosensors? Biosnensors 2022, 12, 784. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Liu, S.; Yu, S.; Yu, Z.; Low, S.S. Application and Progress of Chemometrics in Voltammetric Biosensing. Biosensors 2022, 12, 494. [Google Scholar] [CrossRef]
- Yoo, M.S.; Shin, M.; Kim, Y.; Jang, M.; Choi, Y.E.; Park, S.J.; Choi, J.; Lee, J.; Park, C. Development of electrochemical biosensor for detection of pathogenic microorganism in Asian dust events. Chemosphere 2017, 175, 269–274. [Google Scholar] [CrossRef]
- Liu, B.; Wu, F.; Gui, H.; Zheng, M.; Zhou, C. Chirality-controlled synthesis and applications of single-wall carbon nanotubes. ACS Nano 2017, 11, 31–53. [Google Scholar] [CrossRef]
- Çevik, S. Xanthine biosensor based on XO/AuNP/PtNP/MWCNT hybrid nanocomposite modified GCPE. Biotechnol. Bioprocess Eng. 2016, 21, 314–320. [Google Scholar] [CrossRef]
- Lee, D.K.; Yoo, J.; Kim, H.; Kang, B.H.; Park, S.H. Electrical and Thermal Properties of Carbon Nanotube Polymer Composites with Various Aspect Ratios. Materials 2022, 15, 1356. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, A.; Badini, C.; Padovano, E.; Pietroluongo, M. Electrical and Thermal Conductivity of Epoxy-Carbon Filler Composites Processed by Calendaring. Materials 2019, 12, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas Sensors Based on Single-Wall Carbon Nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef]
- Cai, B.; Yin, H.; Huo, T.; Ma, J.; Di, Z.; Li, M.; Hu, N.; Yang, Z.; Zhang, Y.; Su, Y. Semiconducting single-walled carbon nanotube/graphene van der Waals junctions for highly sensitive all-carbon hybrid humidity sensors. J. Mater. Chem. C 2020, 8, 3386–3394. [Google Scholar] [CrossRef]
- Cho, H.; Somu, S.; Lee, J.Y.; Jeong, H.; Busnaina, A. High-rate nanoscale offset printing process using directed assembly and transfer of nanomaterials. Adv. Mater. 2015, 27, 1759–1766. [Google Scholar] [CrossRef]
- Dai, R.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. Trends Analyt. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Dong, Y.D.; Zhang, H.; Zhong, G.J.; Yao, G.; Lai, B. Cellulose/carbon composites and their applications in water treatmentea review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon-based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Kumar, V.; Vaid, K.; Bansal, S.A.; Kim, K.H. Nanomaterial-based immunosensors for ultrasensitive detection of pesticides/herbicides: Current status and perspectives. Biosens. Bioelectron. 2020, 165, 112382. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- De Carvalho, A.P.A.; Conte Junior, C.A. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Recent advances in applications of sorted single-walled carbon nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhao, Z.; Hu, Z.; Liu, X.; Yu, G. Flexible sodium-ion based energy storage devices: Recent progress and challenges. Energy Storage Mater. 2020, 26, 83–104. [Google Scholar] [CrossRef]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Mugo, S.M.; Alberkant, J. A biomimetric lactate imprinted smart polymers as capacitive sweat sensors. IEEE Sensor J. 2020, 20, 5741–5749. [Google Scholar] [CrossRef]
- Pei, Z.; Zhang, Q.; Liu, Y.; Zhao, Y.; Dong, X.; Zhang, Y.; Zhang, W.; Sang, S. A high gauge-factor wearable strain sensor array via 3D printed mold fabrication and size optimization of silver-coated carbon nanotubes. Nanotechnology 2020, 31, 305501. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xie, J.; Han, Y.; Huang, Y.; Zhang, H. Exosomal analysis: Advances in biosensor technology. Clin. Chim. Acta 2021, 518, 142–150. [Google Scholar] [CrossRef]
- Bagchi, R.; Elshazly, M.; N’Diaye, J.; Yu, D.; Howe, J.Y.; Lian, K. Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites. Chemistry 2022, 4, 1561–1575. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sensors Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Hsin, T.Y.; Shevtsov, V.Y.; Hhieh, Y.T. Two-way CO2-responsive dispersions of carbon nanotubes in water. Mater. Adv. 2022, 3, 6549–6557. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Norizan, M.; Moklis, M.; Demon, S.; Halim, N.; Samsuri, A.; Mohamad, I.; Knight, V.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.; Prabha, C. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Baibarac, M.; Arzumanyan, G.; Daescu, M.; Udrescu, A.; Mamatkulov, K. Anisotropic Photoluminescence of Poly(3-hexyl thiophene) and Their Composites with Single-Walled Carbon Nanotubes Highly Separated in Metallic and Semiconducting Tubes. Molecules 2021, 26, 294. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, M.J.; Choi, J.H.; Song, J.; Kim, T.H.; Oh, B.K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticles with reduced graphene oxide-modified gold electrode. Nano Converg. 2020, 7, 39. [Google Scholar] [CrossRef]
- Rezaei, B.; Askarpour, N.; Ghiaci, M.; Niyazian, F.; Ensafi, A. Synthesis of functionalized MWCNTs decorated with copper nanoparticles and its application as a sensitive sensor for amperometric detection of H2O2. Electroanalysis 2015, 27, 1457–1465. [Google Scholar] [CrossRef]
- Feizabadi, M.; Soleymanpour, A.; Faridnouri, H.; Ajloo, D. Improving stability of biosensor based on covalent immobilization of horseradish peroxidase by γ-aminobutyric acid and application in detection of H2O2. Int. J. Biol. Macromol. 2019, 136, 597–606. [Google Scholar] [CrossRef]
- Sireesha, M.; Babu, V.J.; Kiran, A.S.K.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applicationsin medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Rajasekar, M.; Agash, S.G.S.; Rajasekar, K. Review of photoresponsive and glycoside dendrimers in biomaterials and sensors applications. RSC Adv. 2022, 12, 35123–35150. [Google Scholar] [CrossRef] [PubMed]
- Yamacli, S.; Avci, M. Investigation and comparison of graphene nanoribbon and carbon nanotube based SARS-CoV-2 detection sensors: An ab initio study. Physica B 2023, 648, 414438. [Google Scholar] [CrossRef]
- Lee, J. Significant Effect of Sample Pretreatment on Ara h1 Extraction and Improved Sensitive SWCNT-Based Detection through Optimization. Processes 2020, 8, 1420. [Google Scholar] [CrossRef]
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, S.H.; Darmokoesoemo, H.; Balogun, V.A. A systematic review on the detection and monitoring of toxic gases using carbon nanotube-based biosensors. Sens. Bio-Sens. Res. 2021, 34, 100463. [Google Scholar] [CrossRef]
- Sobhan, A.; Lee, J.; Park, M.K.; Oh, J.H. Rapid detection of Yersinia enterocolitica using a single–walled carbon nanotube-based biosensor for Kimchi product. LWT Food Sci. Technol. 2019, 108, 48–54. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.H.; Park, M.K.; Kim, S.W.; Park, C.; Lee, J. Assessment of peanut allergen Ara h1 in processed foods using a SWCNTs-based nanobiosensor. Biosci. Biotechnol. Biochem. 2018, 82, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim-Tirgil, N.; Lee, J.; Cho, H.; Lee, H.; Somu, S.; Busnaina, A.; Gu, A.Z. A SWCNT based aptasensor system for antibiotic oxytetracycline detection in water samples. Anal. Methods 2019, 11, 2692–2699. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Zappi, D.; Caminiti, R.; Ingo, G.M.; Sadun, C.; Tortolini, C.; Antonelli, M.L. Biologically friendly room temperature ionic liquids and nanomaterials for the development of innovative enzymatic biosensors. Talanta 2017, 175, 566–572. [Google Scholar] [CrossRef]
- Xing, X.; Yao, L.; Yan, C.; Xu, Z.; Xu, J.; Liu, G.; Yao, B.; Chen, W. Recent progress of personal glucose meters integrated methods in food safety hazards detection. Crit. Rev. Food. Sci. Nutr. 2022, 62, 7413–7426. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Zhao, Y.W.; Sun, C.H.; Hu, D.G. Deciphering the impact of glucose signaling on fruit quality. Fruit Research 2022, 2, 3. [Google Scholar] [CrossRef]
- Han, J.; Liang, X.; Guo, Y.; Wu, X.; Li, Z.; Hong, T. Agouti-related protein as the glucose signaling sensor in the central melanocortin circuits in regulating fish food intake. Front. Endocrinol. 2022, 13, 1010472. [Google Scholar] [CrossRef]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.-H. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres. Sens. Actuators B 2018, 256, 63–70. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Babu, K.J.; Rayappan, J.B.B. Fabrication of mediator-free hybrid nano-interfaced electrochemical biosensor for monitoring cancer cell proliferation. Biosens. Bioelectron. 2017, 87, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Musameh, M.; Kyratzis, I.L.; Zhang, J.; Bond, A.M.; Hearn, T.W. Enhanced NADH oxidation using polytyramine/carbon nanotube modified electrodes for ethanol biosensing. Electroanalysis 2017, 29, 1985–1993. [Google Scholar] [CrossRef]
- Ruhal, A.; Rana, J.S.; Kumar, S.; Kumar, A. Immobilization of malate dehydrogenase on carbon nanotubes for development of malate biosensor. Cell. Mol. Biol. 2012, 58, 15–20. [Google Scholar]
- Dervisevic, M.; Custiuc, E.; Cevik, E.; Senel, M. Construction of novel xanthine biosensor using polymeric mediator/MWCNT nanocomposite layer for fish freshness detection. Food Chem. 2015, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Ghica, M.E.; Abbas, M.N.; Brett, C.M.A. A novel sensitive amperometric choline biosensor based on multiwalled carbon nanotubes and gold nanoparticles. Talanta 2017, 167, 462–469. [Google Scholar] [CrossRef]
- Moyo, M.; Okonkwo, J.O.; Agyei, N.M. An amperometric biosensor based on horseradish peroxidase immobilized onto maize tassel-multi-walled carbon nanotubes modified glassy carbon electrode for determination of heavy metal ions in aqueous solution. Enzyme Microb. Technol. 2014, 56, 28–34. [Google Scholar] [CrossRef]
- Gomes-Filho, S.L.R.; Dias, A.C.M.S.; Silva, M.M.S.; Silva, B.V.M.; Dutra, R.F. A carbon nanotube-based electrochemical immunosensor for cardiac troponin T. Microchem. J. 2013, 109, 10–15. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Dias, A.C.M.S.; Silva, B.V.M.; Gomes-Filho, S.L.R.; Kubota, L.T.; Goulart, M.O.F.; Dutra, R.F. Electrochemical detection of dengue virus NS1 protein with a poly(allylsamine)/carbon nanotube layered immunoelectrode. J. Chem. Technol. Biotechnol. 2015, 90, 194–2020. [Google Scholar] [CrossRef]
- Riberi, W.I.; Tarditto, L.V.; Zon, M.A.; Arevalo, F.J.; Fernandez, H. Development of an electrochemical immunosensor to determine zearalenone in maize using carbon screen printed electrodes modified with multi-walled carbon nanotubes/polyethyleneimine dispersions. Sens. Actuators B 2018, 254, 1271–1277. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Jana, S.K. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef]
- Arkan, E.; Saber, R.; Karimi, Z.; Shamsipur, M. A novel antibody-antigen based impedimetric immunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode. Anal. Chim. Acta 2015, 874, 66–74. [Google Scholar] [CrossRef]
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Fregnani, J.H.T.G.; Carvalho, A.L.; Oliveira, O.N. Carbon nanotube matrix for highly sensitive biosensors to detect pancreatic cancer biomarker CA19-9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Yang, M.; Fa, H. An ultrasensitive electrochemical DNA biosensor based on a copper oxide nanowires/single-walled carbon nanotube nanocomposite. Appl. Surf. Sci. 2016, 364, 703–709. [Google Scholar] [CrossRef]
- Liu, X.; Shuai, H.-L.; Liu, Y.-J.; Huang, K.-J. An electrochemical biosensor for DNA detection based on tungsten disulfide/multiwalled carbon nanotube composites and hybridization chain reaction amplification. Sens. Actuators B 2016, 235, 603–613. [Google Scholar] [CrossRef]
- Chen, M.; Wu, D.; Tu, S.; Yang, C.; Chen, D.; Xu, Y. A novel biosensor for the ultrasensitive detection of the lncRNA biomarker MALAT1 in non-small cell lung cancer. Sci. Rep. 2021, 11, 3666. [Google Scholar] [CrossRef]
- Sabahi, A.; Salahandish, R.; Ghaffarinejad, A.; Omidinia, E. Electrochemical nano-genosensor for highly sensitive detection of miR-21 biomarker based on SWCNT-grafted dendritic Au nanostructure for early detection of prostate cancer. Talanta 2020, 209, 120595. [Google Scholar] [CrossRef]
- Ozkan-Ariksoysal, D.; Kayran, Y.U.; Yilmaz, F.F.; Ciucu, A.A.; David, I.G.; David, V.; Hosgor-Limoncu, M.; Ozsoz, M. DNA wrapped multi-walled carbon nanotube modified electrochemical biosensor for the detection of Escherichia coli from real samples. Talanta 2017, 166, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ghrera, A.S.; Pandey, C.M.; Malhotra, B.D. Multiwalled carbon nanotube modified microfluidic-based biosensor chip for nucleic acid detection. Sens. Actuators B 2018, 266, 329–336. [Google Scholar] [CrossRef]
- Su, Z.; Xu, X.; Xu, H.; Zhang, Y.; Li, C.; Ma, Y.; Song, D.; Xie, Q. Amperometric thrombin aptasensor using a glassy carbon electrode modified with polyaniline and multiwalled carbon nanotubes tethered with a thiolated aptamer. Microchim. Acta 2017, 184, 1677–1682. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J. A signal-on electrochemical biosensor for sensitive detection of sliver ion based on alkanethiol-carbon nanotube-oligonucleotide modified electrodes. Sens. Actuators B 2014, 202, 1058–1064. [Google Scholar] [CrossRef]
- Rostamabadi, P.F.; Heydari-Bafrooei, E. Impedimetric aptasensing of the breast cancer biomarker HER2 using a glassy carbon electrode modified with gold nanoparticles in a composite consisting of electrochemically reduced graphene oxide and single walled carbon nanotubes. Microchim. Acta 2019, 186, 495. [Google Scholar] [CrossRef]
- Kharrati-Koopaee, H.; Rezaei, V.; Esmailizadeh, A.; Sabahi, F. DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A mini-review. J. Cell Dev. Biol. 2020, 3, 28–35. [Google Scholar]
- Choi, H.K.; Lee, J.; Park, M.K.; Oh, J.H. Development of Single-Walled Carbon Nanotube-Based Biosensor for the Detection of Staphylococcus aureus. J. Food Qual. 2017, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Kim, C.-T.; Kim, J.-H.; Chung, J.-H.; Lee, H.G.; Jun, S. Single Walled Carbon Nanotube-Based Junction Biosensor for Detection of Escherichia coli. PLoS ONE 2014, 9, e105767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.R.; Pulingam, T.; Appaturi, J.N.; Zifruddin, A.N.; Teh, S.J.; Lim, T.W.; Ibrahim, F.; Leo, B.F.; Thong, K.L. Carbon nanotube-based aptasensor for sensitive electrochemical detection of whole-cell Salmonella. Anal. Biochem. 2018, 554, 34–43. [Google Scholar] [CrossRef]
- Ghanbari, K.; Roushani, M. A nanohybrid probe based on double recognition of an aptamer MIP grafted onto a MWCNTs-Chit nanocomposite for sensing hepatitis C virus core antigen. Sens. Actuators B 2018, 258, 1066–1071. [Google Scholar] [CrossRef]
- Li, T.; Zhu, F.; Guo, W.; Gu, H.; Zhao, J.; Yan, M.; Liu, S. Selective capture and rapid identification of E. coli O157:H7 by carbon nanotube multilayer biosensors and microfluidic chip-based LAMP. RSV Adv. 2017, 7, 30446–30452. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Jia, M.; Ji, J.; Guan, L.; Zhang, Y.; Tang, L.; Li, Z. Enzymatic amplification detection of peanut allergen Ara h1 using a stem-loop DNA biosensor modified with a chitosan-mutiwalled carbon nanotube nanocomposite and spongy gold film. Talanta 2015, 131, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ye, Y.; He, S.; Wu, Z.; Yue, J.; Sun, H.; Cao, X. A novel oriented antibody immobilization based voltammetric immunosensor for allergenic activity detection of lectin in kidney bean by using AuNPs-PEI-MWCNTs modified electrode. Biosens. Bioelectron. 2019, 143, 111607. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Alec GMaddaus, A.G.; Song, E. A Low-Cost Inkjet-Printed Aptamer-Based Electrochemical Biosensor for the Selective Detection of Lysozyme. Biosensors 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, B.; Jamei, H.R.; Ensafi, A.A. An ultrasensitive and selective electrochemical aptasensor based on rGO/MWCNTs/Chitosan/carbon quantum dot for the detection of lysozyme. Biosens. Bioelectron. 2018, 115, 37–44. [Google Scholar] [CrossRef]
- Jiang, D.; Sheng, K.; Jiang, H.; Wang, L. A biomimetic “intestinal microvillus” cell sensor based on 3D bioprinting for the detection of wheat allergen gliadin. Bioelectrochemistry 2021, 142, 107919. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.H.; Park, M.K.; Kim, S.W.; Park, C.; Lee, J. Single walled carbon nanotube based biosensor for detection of peanut allergy-inducing protein ara h1. Korean J. Chem. Eng. 2018, 35, 172–178. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.H.; Park, M.K.; Lee, J. Detection of Peanut Allergen Ara h 6 in Commercially Processed Foods using a Single-Walled Carbon Nanotube–Based Biosensor. J. AOAC Int. 2018, 101, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Dizajdizi, B.Z. Development of nonenzymatic hydrogen peroxide sensor based on catalytic properties of copper nanoparticles/Rutin/MWCNTs/IL/Chit. Catal. Commun. 2015, 69, 133–137. [Google Scholar] [CrossRef]
- Zhao, K.; Veksha, A.; Ge, L.; Lisak, G. Near real-time analysis of para-cresol in wastewater with a laccase-carbon nanotube-based biosensor. Chemosphere 2020, 269, 128699. [Google Scholar] [CrossRef]
- Cakiroglu, B.; Ozacar, M. A self-powered photoelectrochemical glucose biosensor based on supercapacitor Co3O4–CNT hybrid on TiO2. Biosens. Bioelectron. 2018, 119, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, B.; Ye, J.; Ye, J. A sensitive and selective amperometric hydrazine sensor based on palladium nanoparticles loaded on cobalt-wrapped nitrogen-doped carbon nanotubes. J. Eelctroanal. Chem. 2017, 801, 215–223. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Zhang, J.; Wang, X.; Yin, F.; Li, Z.; Zhang, M. Universal DNA detection realized by peptide based carbon nanotube biosensors. Nanoscale Adv. 2020, 2, 717–723. [Google Scholar] [CrossRef] [PubMed]
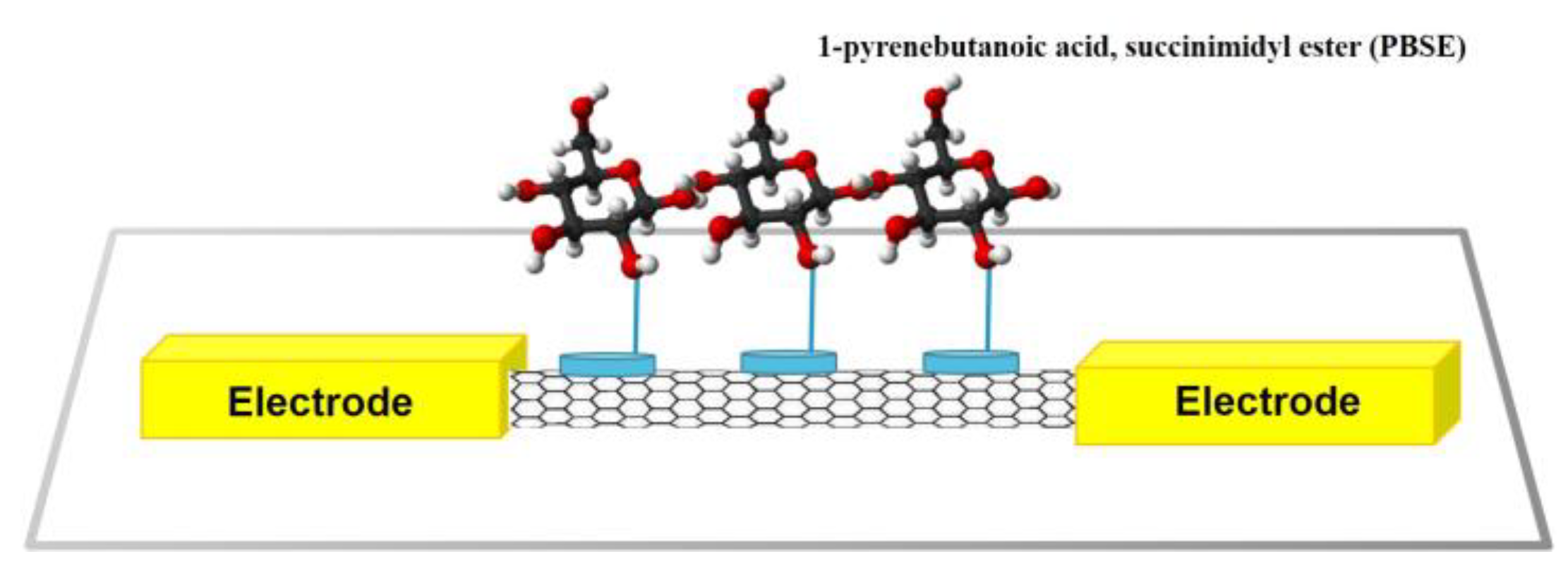
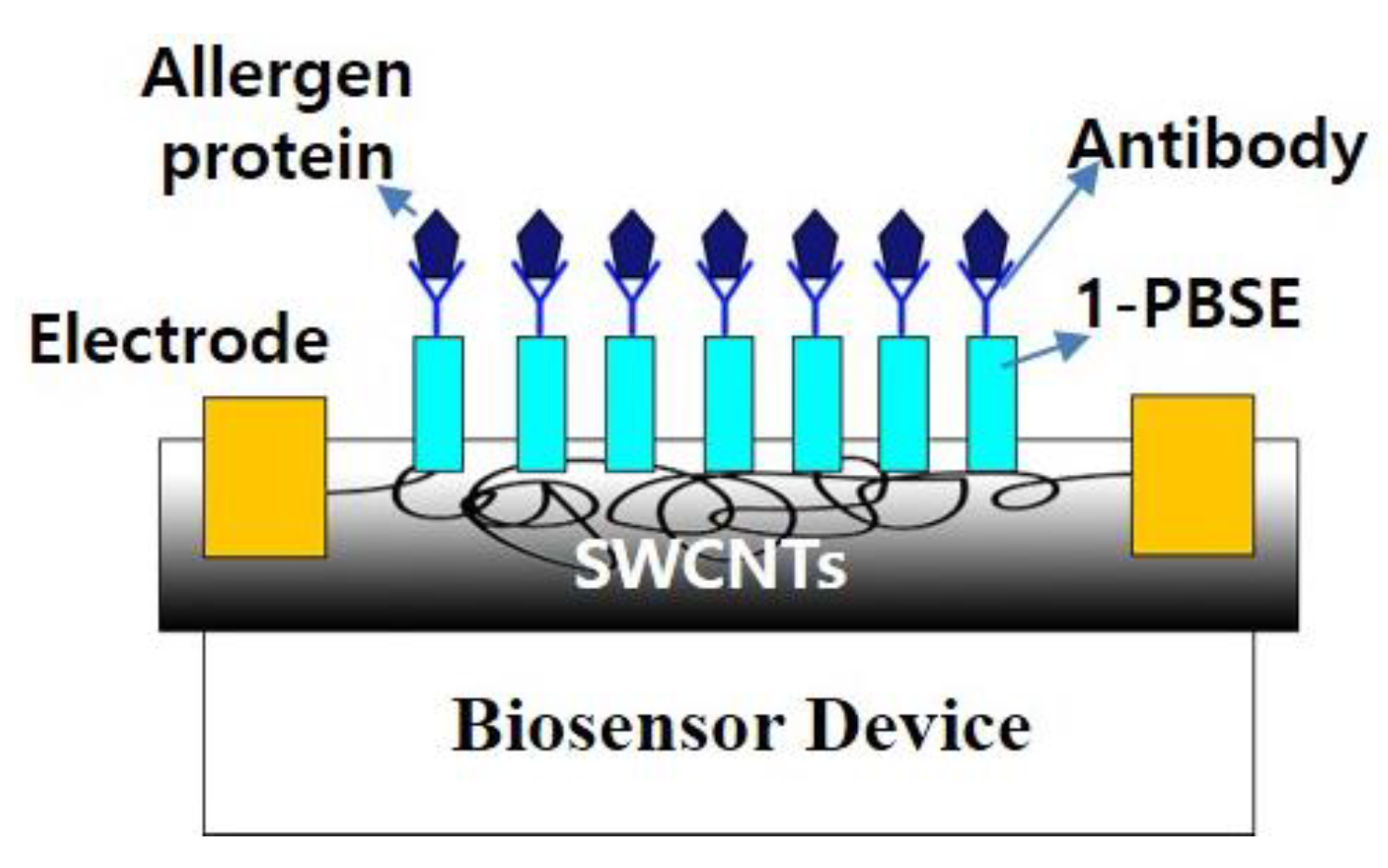
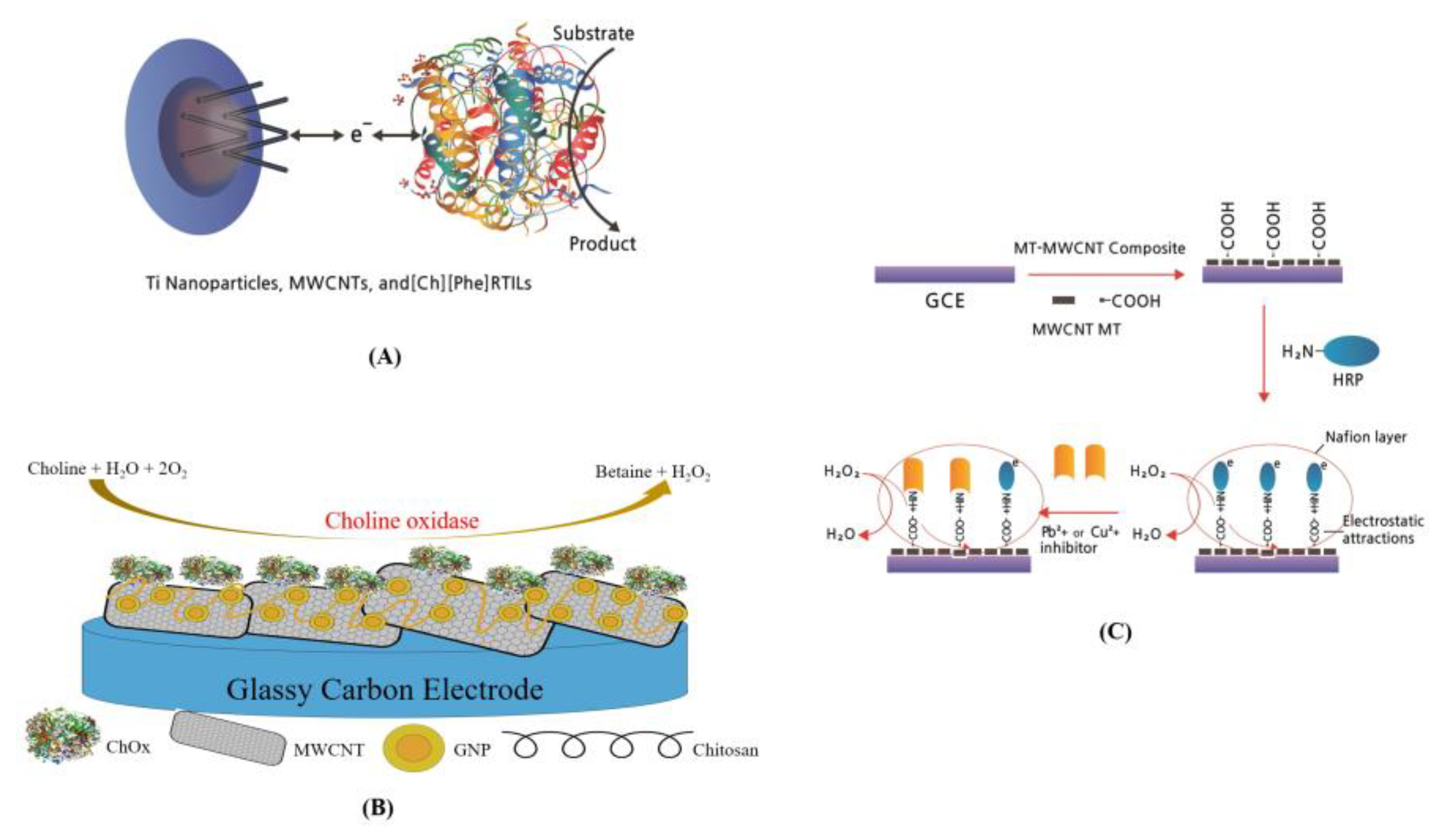

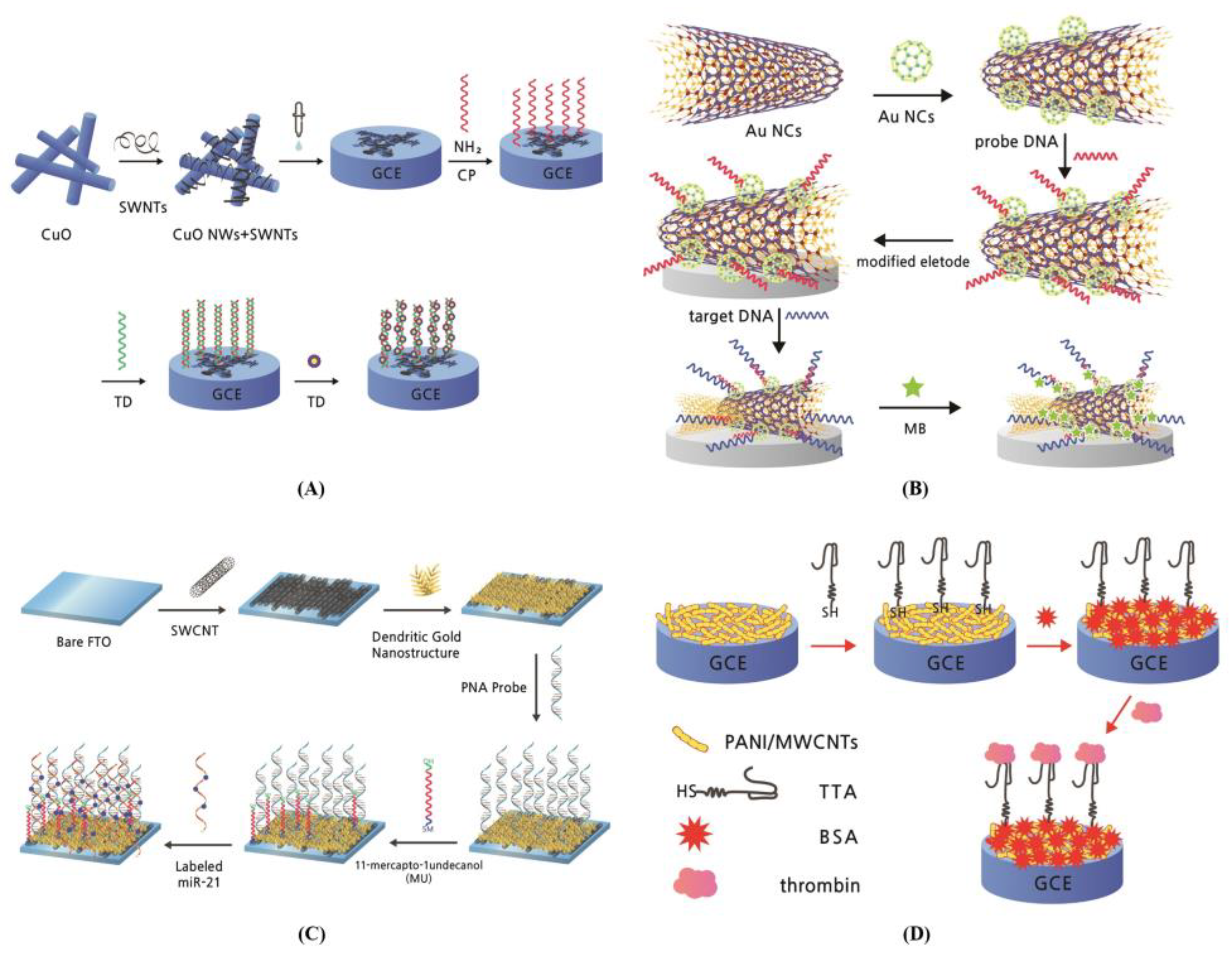



| Target Types | Modified Electrode | Electrical Method | Analyte | LOD | DR | Ref. | |
|---|---|---|---|---|---|---|---|
| Pathogens | SWCNTs/1-PBSE–Ab- Staphylococcus aureus | AMP | S. aureus in Kimchi samples | 104 CFU/mL | 104–107 CFU/mL | [99] | |
| Gold tungsten wires/PEI/SWCNTs –Ab-E. coli | AMP | E. coli O157:H7 | 102 CFU/mL | 102–105 CFU/mL | [100] | ||
| Amino-modified Apt (ssDNA)/COOH-rich MWCNTs/ITO | CV/IMP | S. Typhiurium S. Enteritidis | 6.7 × 10 Typhiurium 5.5 × 10 Enteritidis (CFU/mL) | 6.7 × 10–6.7 × 105, Typhiurium 5.5 × 10–5.5 × 106 Enteritidis (CFU/mL) | [101] | ||
| Apt/MWCNTs -Chit/GCE | EIS | HCV in human serum | 1.67 fg/mL | 5.0 fg/mL–1.0 pg/mL | [102] | ||
| ITO/MWCNTs-PSS/PEI -Ab- E. coli O157:H7 | EIS | E. coli O157:H7 | 1.0 CFU/mL | 1.0 –104 CFU/ml | [103] | ||
| Allergen | Au film/CS –MWCNT/GCE | DPV | Ara h1 | 1.3×10−17 mol/L | (3.91–125) × 10−17 mol/L | [104] | |
| Ab-SPA/ AuNPs/PEI_MWCNTs nanocomposite | DPV | KBL in kidney bean milk samples | 0.023 µg/mL | 0.05–100 µg/mL | [105] | ||
| Printable ink including a CNT–aptamer complex and [Fe(CN)6]−4/−3 as the redox probe | EIS | Lys | 90 ng/mL | 0–1.0 µg/mL | [106] | ||
| rGO/MWCNTs/CQDs /CS nanocomposite | DPV/EIS | Lys | 3.7 fmol/L (DPV) 1.9 fmol/L (EIS) | 20 fmol/L–10 nmol/L (DPV) 10 fmol/L–100 nmol/L (EIS) | [107] | ||
| FCONPs/MWCNTs -CDH/ GelMA | EIS | Gliadin | 0.036 ng/mL | 0.1–0.8 ng/mL | [108] | ||
| SWCNTs/1-PBSE –Ab-Ara h1 nanocomposite | AMP | Ara h1 | 1.0 ng/mL | 1.0–1000 ng/mL | [109] | ||
| SWCNTs/1-PBSE –Ab-Ara h6 nanocomposite | AMP | Ara h6 | 10 pg/ml | 1.0–107 pg/L | [110] | ||
| Other molecules | Cu NPs/Rutin/MWCNTs/ IL/ChI/GCE | CV | H2O2 | 0.11 µm | 0.35–2500 µM | [111] | |
| LAC-CNTs-SPCE | EC | Paracresol | 0.05 ppm | 0.2–25 ppm | [112] | ||
| Co3O4-SWCNTs/TiO2 | PLC | Glucose | 0.16 µM | 0–4 mM | [113] | ||
| Pd/Co-NCNT | CV/EIS | Hydrazine | 0.007 µm | 0.05–406.045 µm | [114] | ||
| SWCNTs-TFT | TFT | DNA | 0.88 µg/L | 1.6 × 10−4–5 µmol/L | [115] | ||
| SWCNTs/1-PBSE/Apt | EC | OTC | 1.125 mg/L | 10–75 mg/L | [69] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J. Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment. Biosensors 2023, 13, 183. https://doi.org/10.3390/bios13020183
Lee J. Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment. Biosensors. 2023; 13(2):183. https://doi.org/10.3390/bios13020183
Chicago/Turabian StyleLee, Jinyoung. 2023. "Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment" Biosensors 13, no. 2: 183. https://doi.org/10.3390/bios13020183
APA StyleLee, J. (2023). Carbon Nanotube-Based Biosensors Using Fusion Technologies with Biologicals & Chemicals for Food Assessment. Biosensors, 13(2), 183. https://doi.org/10.3390/bios13020183





