Porphyrin-Based Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Peroxidase-like Activity as a Colorimetric Biosensing Platform
Abstract
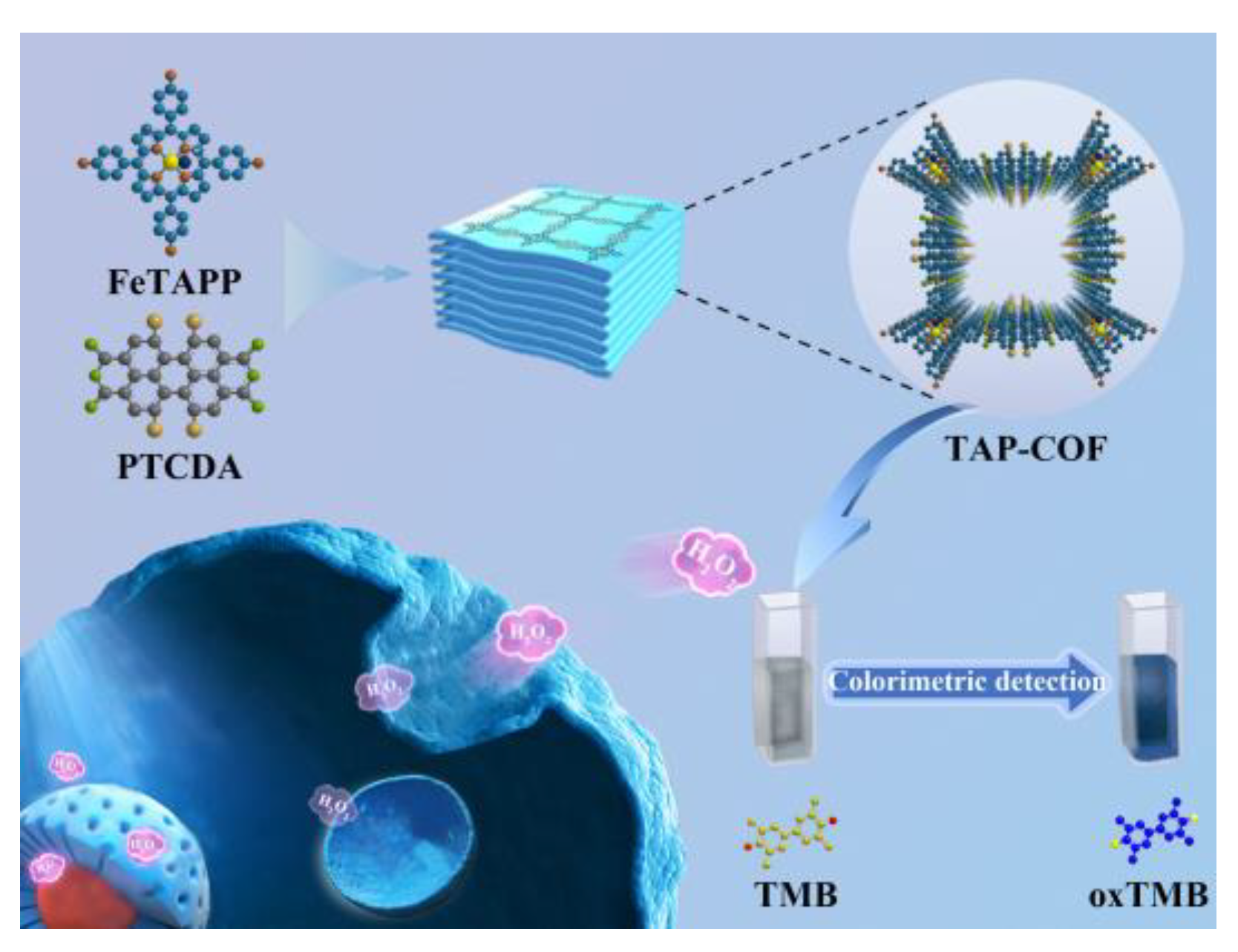
:1. Introduction
2. Materials and Methods
2.1. Peroxidase-like Performance of TAP-COF
2.2. Condition Optimization
2.3. Detection of H2O2 in Cells
3. Results and Discussions
3.1. Structure and Morphology Characterization
3.2. Peroxidase-like Activity of TAP-COF
3.3. Optimization of Experimental Conditions
3.4. Steady-State Kinetic Analysis
3.5. Colorimetric Sensing
3.6. Selectivity for the Detection of Glucose
3.7. Colorimetric Detection Mechanism for H2O2
3.8. Colorimetric Detection of Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef]
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Liu, C.; Du, D.; Lin, Y.; Zhu, C. Fe-N-C Single-Atom Nanozymes for the Intracellular Hydrogen Peroxide Detection. Anal. Chem. 2019, 91, 11994–11999. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of Hydrogen Peroxide (H2O2) Using a Colorimetric Sensor Based on Cellulose Nanowhiskers and Silver Nanoparticles. Carbohydr. Polym 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Fang, L.; Weng, A.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Electrochemiluminescence Sensor for Cancer Cell Detection Based on H2O2-Triggered Stimulus Response System. J. Anal. Test. 2020, 4, 128–135. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, X.; Lu, Z.; Wang, X.; Wang, Z. A Fluorescent Probe for Hydrogen Peroxide in Vivo Based on the Modulation of Intramolecular Charge Transfer. Anal. Chem. 2017, 89, 5278–5284. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Bhagat, S.; Singh, S. Bovine Serum Albumin Decorated Gold Nanoclusters: A Fluorescence-Based Nanoprobe for Detection of Intracellular Hydrogen Peroxide. Sens. Actuators B 2021, 327, 128886. [Google Scholar] [CrossRef]
- Ye, S.; Hu, J.J.; Yang, D. Tandem Payne/Dakin Reaction: A New Strategy for Hydrogen Peroxide Detection and Molecular Imaging. Angew. Chem. Int. Ed. 2018, 57, 10173–10177. [Google Scholar] [CrossRef]
- Kumar, R.; Han, J.; Lim, H.J.; Ren, W.X.; Lim, J.Y.; Kim, J.H.; Kim, J.S. Mitochondrial Induced and Self-Monitored Intrinsic Apoptosis by Antitumor Theranostic Prodrug: In Vivo Imaging and Precise Cancer Treatment. J. Am. Chem. Soc. 2014, 136, 17836–17843. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Dolai, S.; Sun, H.; Jelinek, R. “On/Off/On” Hydrogen-Peroxide Sensor with Hemoglobin-Functionalized Carbon Dots. Sens. Actuators B 2018, 270, 223–230. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Q.; Li, W.; Lin, L.; Qu, F.; Shen, C.; Wei, Y.; Nie, P.; He, Y.; Feng, X. Ultra-Sensitive Detection of Hydrogen Peroxide and Levofloxacin Using a Dual-Functional Fluorescent Probe. J. Hazard. Mater. 2022, 432, 128605. [Google Scholar] [CrossRef]
- Rajendran, S.; Zichri, S.B.; Usha Vipinachandran, V.; Jelinek, R.; Bhunia, S.K. Triphenylphosphonium-Derived Bright Green Fluorescent Carbon Dots for Mitochondrial Targeting and Rapid Selective Detection of Tetracycline. ChemNanoMat 2021, 7, 545–552. [Google Scholar] [CrossRef]
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless Steel Electrode for Sensitive Luminol Electrochemiluminescent Detection of H2O2, Glucose, and Glucose Oxidase Activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, W.; Zhang, X.; Chai, H.; Huang, Y. FeNPs@Co3O4 Hollow Nanocages Hybrids as Effective Peroxidase Mimics for Glucose Biosensing. Sens. Actuators B 2018, 263, 575–584. [Google Scholar] [CrossRef]
- UshaVipinachandran, V.; Rajendran, S.; Ali, H.; Ashokan, I.; Bhunia, S.K. Citrate Capped Silver Nanoparticles as an Instantaneous Colorimetric Selective Sensor for Neomycin and Thiamine in Wastewater. New J. Chem. 2022, 46, 14081–14090. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Yang, B.; Liu, Z.; Liu, Q.; Zhang, X. Colorimetric and Ultrasensitive Detection of H2O2 Based on Au/Co3O4-CeOx Nanocomposites with Enhanced Peroxidase-Like Performance. Sens. Actuators B 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Lin, A.; Liu, Q.; Zhang, Y.; Wang, Q.; Li, S.; Zhu, B.; Miao, L.; Du, Y.; Zhao, S.; Wei, H. A Dopamine-Enabled Universal Assay for Catalase and Catalase-Like Nanozymes. Anal. Chem. 2022, 94, 10636–10642. [Google Scholar] [CrossRef]
- Fan, X.; Tian, R.; Wang, T.; Liu, S.; Wang, L.; Xu, J.; Liu, J.; Ma, M.; Wu, Z. An Ultrathin Iron-Porphyrin Based Nanocapsule with High Peroxidase-Like Activity for Highly Sensitive Glucose Detection. Nanoscale 2018, 10, 22155–22160. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Wang, K.; He, D.; Wang, Y.; Mao, Y.; Shi, H.; Wen, L. A Highly Sensitive Electrochemiluminescence Assay for Protein Kinase Based on Double-Quenching of Graphene Quantum Dots by G-quadruplex-Hemin and Gold Nanoparticles. Biosens. Bioelectron. 2015, 70, 54–60. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Gao, W.; Guo, X.; Zhang, X. Porphyrin-Based Porous Organic Polymer as Peroxidase Mimics for Sulfide-Ion Colorimetric Sensing. ACS Sustain. Chem. Eng. 2020, 8, 10870–10880. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, P.; Xu, Z.; Chen, M.; Ding, Y.; Yue, K.; Xu, J. A Facile Strategy to Prepare Porphyrin Functionalized ZnS Nanoparticles and Their Peroxidase-Like Catalytic Activity for Colorimetric Sensor of Hydrogen Peroxide and Glucose. Sens. Actuators B 2017, 251, 339–348. [Google Scholar] [CrossRef]
- Li, D.; Fang, Y.; Zhang, X. Bacterial Detection and Elimination Using a Dual-Functional Porphyrin-Based Porous Organic Polymer with Peroxidase-Like and High Near-Infrared-Light-Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 8989–8999. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Y.; Cao, D.; Huang, L.; Chen, J.F.; Dai, L. Highly Efficient Electrocatalysts for Oxygen Reduction Based on 2D Covalent Organic Polymers Complexed with Non-Precious Metals. Angew. Chem. Int. Ed. 2014, 53, 2433–2437. [Google Scholar] [CrossRef]
- Zhu, P.; Li, S.; Zhou, S.; Ren, N.; Ge, S.; Zhang, Y.; Wang, Y.; Yu, J. In Situ Grown COFs on 3D Strutted Graphene Aerogel for Electrochemical Detection of NO Released from Living Cells. Chem. Eng. J. 2021, 420, 127559. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, M.; Wang, L.; Liang, H.; Wang, L.; Song, Y. Iron-Porphyrin-Based Covalent-Organic Frameworks for Electrochemical Sensing H2O2 and pH. Mater. Sci. Eng. C 2020, 112, 110864. [Google Scholar] [CrossRef]
- Li, G.; Tian, W.; Zhong, C.; Yang, Y.; Lin, Z. Construction of Donor-Acceptor Heteroporous Covalent Organic Frameworks as Photoregulated Oxidase-Like Nanozymes for Sensing Signal Amplification. ACS Appl. Mater. Interfaces 2022, 14, 21750–21757. [Google Scholar] [CrossRef]
- Liu, K.; Qi, H.; Dong, R.; Shivhare, R.; Addicoat, M.; Zhang, T.; Sahabudeen, H.; Heine, T.; Mannsfeld, S.; Kaiser, U.; et al. On-Water Surface Synthesis of Crystalline, Few-Layer Two-Dimensional Polymers Assisted by Surfactant Monolayers. Nat. Chem. 2019, 11, 994–1000. [Google Scholar] [CrossRef]
- Han, B.; Ding, X.; Yu, B.; Wu, H.; Zhou, W.; Liu, W.; Wei, C.; Chen, B.; Qi, D.; Wang, H.; et al. Two-Dimensional Covalent Organic Frameworks with Cobalt(II)-Phthalocyanine Sites for Efficient Electrocatalytic Carbon Dioxide Reduction. J. Am. Chem. Soc. 2021, 143, 7104–7113. [Google Scholar] [CrossRef]
- Ma, P.; Kan, J.; Zhang, Y.; Hang, C.; Bian, Y.; Chen, Y.; Kobayshi, N.; Jiang, J. The First Solution-Processable n-Type Phthalocyaninato Copper Semiconductor: Tuning the Semiconducting Nature Via Peripheral Electron-Withdrawing Octyloxycarbonyl Substituents. J. Mater. Chem. 2011, 21, 18552–18559. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Dey, K.; Addicoat, M.; Heine, T.; Banerjee, R. Interplaying Intrinsic and Extrinsic Proton Conductivities in Covalent Organic Frameworks. Chem. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.; Wang, Q.; Zhang, L.; Zhu, P.; Yu, J.; Zhang, Y. Porphyrin-Based Covalent Organic Framework Thin Films as Cathodic Materials for “On–Off–On” Photoelectrochemical Sensing of Lead Ions. ACS Appl. Mater. Interfaces 2021, 13, 20397–20404. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Wu, Z.; Yuan, L.; Fang, W.; Zou, P.; Ma, L.; Wang, X. High-Activity Daisy-Like Zeolitic Imidazolate Framework-67/Reduced Grapheme Oxide-Based Colorimetric Biosensor for Sensitive Detection of Hydrogen Peroxide. J. Colloid Interface Sci. 2022, 608, 3069–3078. [Google Scholar] [CrossRef]
- Deng, X.; Fang, Y.; Lin, S.; Cheng, Q.; Liu, Q.; Zhang, X. Porphyrin-Based Porous Organic Frameworks as a Biomimetic Catalyst for Highly Efficient Colorimetric Immunoassay. ACS Appl. Mater. Interfaces 2017, 9, 3514–3523. [Google Scholar] [CrossRef]
- Xie, J.; Cao, H.; Jiang, H.; Chen, Y.; Shi, W.; Zheng, H.; Huang, Y. Co3O4-Reduced Graphene Oxide Nanocomposite as an Effective Peroxidase Mimetic and Its Application in Visual Biosensing of Glucose. Anal. Chim. Acta 2013, 796, 92–100. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A Nanosized Metal-Organic Framework of Fe-MIL-88NH2 as a Novel Peroxidase Mimic Used for Colorimetric Detection of Glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon Nanodots as Peroxidase Mimetics and Their Applications to Glucose Detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.J.; Yang, Y.S.; Zhu, H.L. Recent Progress In the Development of Small-Molecule Fluorescent Probes for the Detection of Hydrogen Peroxide. TrAC Trends Anal. Chem. 2019, 118, 625–651. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, S.; Zhu, L.; Lv, X.; Zhang, J.; Zhang, L.; Zhu, P.; Yu, J. DNAzyme-Triggered Visual and Ratiometric Electrochemiluminescence Dual-Readout Assay for Pb(II) Based on an Assembled Paper Device. Anal. Chem. 2020, 92, 3874–3881. [Google Scholar] [CrossRef]
- Hu, F.X.; Miao, J.; Guo, C.; Yang, H.B.; Liu, B. Real-Time Photoelectrochemical Quantification of Hydrogen Peroxide Produced by Living Cells. Chem. Eng. J. 2021, 407, 127203. [Google Scholar] [CrossRef]
- Dong, Y.L.; Zhang, H.G.; Rahman, Z.U.; Su, L.; Chen, X.J.; Hu, J.; Chen, X.G. Graphene Oxide-Fe3O4 Magnetic Nanocomposites with Peroxidase-Like Activity for Colorimetric Detection of Glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef]
- Zhu, D.; Kong, H.; Yang, G.; He, P.; Luan, X.; Guo, L.; Wei, G. Peptide Nanosheet-Inspired Biomimetic Synthesis of CuS Nanoparticles on Ti3C2 Nanosheets for Electrochemical Biosensing of Hydrogen Peroxide. Biosensors 2022, 13, 14. [Google Scholar] [CrossRef]
- Lu, W.; Arumugam, S.; Senapati, D.; Khan, S.A.; Yu, H.; Ray, P. Multifunction Oval-Shaped Gold-Nanoparticle-Based Selective Detection of Breast Cancer Cells Using Simple Colorimetric and Highly Sensitive Two-Photon Scattering Assay. ACS Nano 2010, 4, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Gao, Y.; Hu, N.; Zhang, Y.; Guo, C.; Gao, G.; Ma, Z.; Ivan Ivanovich, K.; Qiu, Y. Electronic Coupling Between Molybdenum Disulfide and Gold Nanoparticles to Enhance the Peroxidase Activity for the Colorimetric Immunoassays of Hydrogen Peroxide and Cancer Cells. J. Colloid Interface Sci. 2020, 578, 366–378. [Google Scholar] [CrossRef]
- Li, M.; Lao, Y.H.; Mintz, R.L.; Chen, Z.; Shao, D.; Hu, H.; Wang, H.X.; Tao, Y.; Leong, K.W. A Multifunctional Mesoporous Silica-Gold Nanocluster Hybrid Platform for Selective Breast Cancer Cell Detection Using a Catalytic Amplification-Based Colorimetric Assay. Nanoscale 2019, 11, 2631–2636. [Google Scholar] [CrossRef]
- Abdinejad, M.; Dao, C.; Zhang, X.A.; Kraatz, H.B. Enhanced Electrocatalytic Activity of Iron Amino Porphyrins Using a Flow Cell for Reduction of CO2 to CO. J. Energy Chem. 2021, 58, 162–169. [Google Scholar] [CrossRef]
- Pegis, M.L.; Martin, D.J.; Wise, C.F.; Brezny, A.C.; Johnson, S.I.; Johnson, L.E.; Kumar, N.; Raugei, S.; Mayer, J.M. Mechanism of Catalytic O2 Reduction by Iron Tetraphenylporphyrin. J. Am. Chem. Soc. 2019, 141, 8315–8326. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, P.E.; Mauck, C.M.; Harris, M.A.; Young, R.M.; Wu, Y.L.; Marks, T.J.; Wasielewski, M.R. Influence of Anion Delocalization on Electron Transfer in a Covalent Porphyrin Donor-Perylenediimide Dimer Acceptor System. J. Am. Chem. Soc. 2017, 139, 749–756. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yan, Y.; Xia, F.; Huang, A.; Xian, Y. Highly Fluorescent Polyimide Covalent Organic Nanosheets as Sensing Probes for the Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 13415–13421. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Q.; Liu, Q.; Deng, X.; Liu, T.; Li, D.; Zhang, X. Porphyrin-Based Porous Organic Framework: An Efficient and Stable Peroxidase-Mimicking Nanozyme for Detection of H2O2 and Evaluation of Antioxidant. Sens. Actuators, B 2018, 277, 86–94. [Google Scholar] [CrossRef]
- Wang, N.; Sun, J.; Chen, L.; Fan, H.; Ai, S. A Cu2(OH)3Cl-CeO2 Nanocomposite with Peroxidase-Like Activity, and Its Application to the Determination of Hydrogen Peroxide, Glucose and Cholesterol. Microchim. Acta 2015, 182, 1733–1738. [Google Scholar] [CrossRef]
- Socaci, C.; Pogacean, F.; Biris, A.R.; Coros, M.; Rosu, M.C.; Magerusan, L.; Katona, G.; Pruneanu, S. Graphene Oxide vs. Reduced Graphene Oxide as Carbon Support in Porphyrin Peroxidase Biomimetic Nanomaterials. Talanta 2016, 148, 511–517. [Google Scholar] [CrossRef]
- Yang, H.; Zha, J.; Zhang, P.; Xiong, Y.; Su, L.; Ye, F. Sphere-Like CoS with Nanostructures as Peroxidase Mimics for Colorimetric Determination of H2O2 and Mercury Ions. RSC Adv. 2016, 6, 66963–66970. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. One-Step Synthesis of Uniform Nanoparticles of Porphyrin Functionalized Ceria with Promising Peroxidase Mimetics for H2O2 and Glucose Colorimetric Detection. Sens. Actuators, B 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Q.; Deng, G.; Chen, X.; Yang, Y.; Lan, W.; Hu, Y.; Zhang, L.; Xu, L.; Li, C.; et al. Rapid and Highly Sensitive Colorimetric Biosensor For the Detection of Glucose and Hydrogen Peroxide Based on Nanoporphyrin Combined with Bromine as a Peroxidase-Like Catalyst. Sens. Actuators, B 2021, 343, 130104. [Google Scholar] [CrossRef]
- Jin, L.; Shang, L.; Guo, S.; Fang, Y.; Wen, D.; Wang, L.; Yin, J.; Dong, S. Biomolecule-Stabilized Au Nanoclusters as a Fluorescence Probe for Sensitive Detection of Glucose. Biosens. Bioelectron. 2011, 26, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Wen, J.; Liu, L.; He, D.; Kuang, R.Y.; Shi, T. A Mediator-Free Glucose Biosensor Based on Glucose Oxidase/Chitosan/Alpha-Zirconium Phosphate Ternary Biocomposite. Anal. Biochem. 2014, 445, 24–29. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Lu, W.; Shi, L.; Wang, R.; Pang, R.; Cao, Y.; Wang, F.; Xu, X. One-Step Electrodeposition of AuNi Nanodendrite Arrays as Photoelectrochemical Biosensors for Glucose and Hydrogen Peroxide Detection. Biosens. Bioelectron. 2019, 142, 111577. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, W.; Zheng, J.; Niu, Z.; Li, Z. Nonenzymatic Amperometric Response of Glucose on A Nanoporous Gold Film Electrode Fabricated by A Rapid and Simple Electrochemical Method. Biosens. Bioelectron. 2011, 26, 3555–3561. [Google Scholar] [CrossRef]
- Aksorn, J.; Teepoo, S. Development of The Simultaneous Colorimetric Enzymatic Detection of Sucrose, Fructose and Glucose Using a Microfluidic Paper-based Analytical Device. Talanta 2020, 207, 120302. [Google Scholar] [CrossRef]
- Wang, K.; Li, N.; Zhang, J.; Zhang, Z.; Dang, F. Size-Selective QD@MOF Core-Shell Nanocomposites for the Highly Sensitive Monitoring of Oxidase Activities. Biosens. Bioelectron. 2017, 87, 339–344. [Google Scholar] [CrossRef]





| Catalyst | Km/mM | Vmax/10−8 M s−1 | Reference | ||
|---|---|---|---|---|---|
| H2O2 | TMB | H2O2 | TMB | ||
| FePor-TFPA-COP | 2.77 | 0.027 | 83.3 | 1.76 | [32] |
| H2TCPP-Co3O4 | 3.70 | 0.43 | 8.71 | 10.0 | [33] |
| Fe-MIL-88NH2 | 6.10 | 0.028 | 0.710 | 0.670 | [34] |
| C-Dots | 39.1 | 0.011 | 1.40 | 4.80 | [35] |
| TAP-COF | 0.780 | 0.18 | 2.94 | 4.78 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Lv, L.; Chi, W.; Bai, Y.; Gao, W.; Zhu, P.; Yu, J. Porphyrin-Based Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Peroxidase-like Activity as a Colorimetric Biosensing Platform. Biosensors 2023, 13, 188. https://doi.org/10.3390/bios13020188
Wang Q, Lv L, Chi W, Bai Y, Gao W, Zhu P, Yu J. Porphyrin-Based Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Peroxidase-like Activity as a Colorimetric Biosensing Platform. Biosensors. 2023; 13(2):188. https://doi.org/10.3390/bios13020188
Chicago/Turabian StyleWang, Qian, Liang Lv, Wenhao Chi, Yujiao Bai, Wenqing Gao, Peihua Zhu, and Jinghua Yu. 2023. "Porphyrin-Based Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Peroxidase-like Activity as a Colorimetric Biosensing Platform" Biosensors 13, no. 2: 188. https://doi.org/10.3390/bios13020188
APA StyleWang, Q., Lv, L., Chi, W., Bai, Y., Gao, W., Zhu, P., & Yu, J. (2023). Porphyrin-Based Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Peroxidase-like Activity as a Colorimetric Biosensing Platform. Biosensors, 13(2), 188. https://doi.org/10.3390/bios13020188





