In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling
Abstract
:1. Introduction
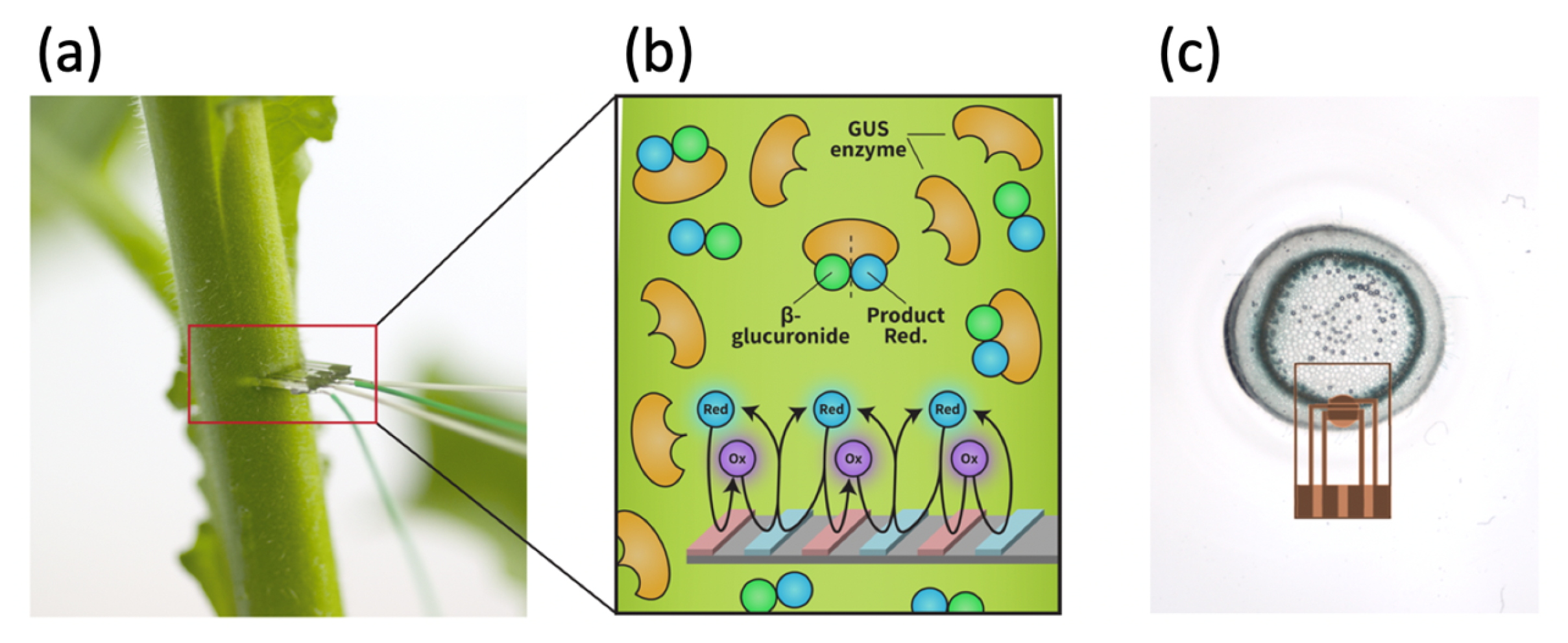
1.1. Plant-Based Biosensing
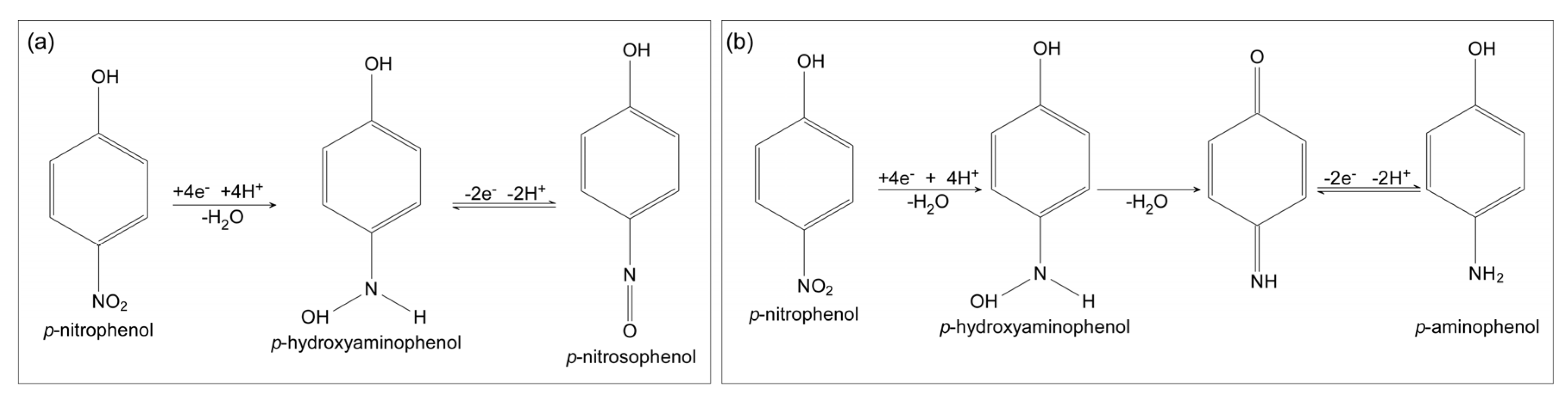
1.2. Amplification Using Redox Cycling
1.3. Electrochemical Characterization
1.4. Heat Shock in Tobacco Plants
2. Materials and Methods
2.1. Generating the Biosensor
2.1.1. Cell Culture
2.1.2. Plant Mode
2.2. Electrode Chip
2.3. Electrochemical Cell
2.4. Plant Model Experimental System
2.5. GUS Staining
2.6. Electrochemical Characterization
2.7. Heat Shock Sensors’ Measurements
3. Results and Discussion
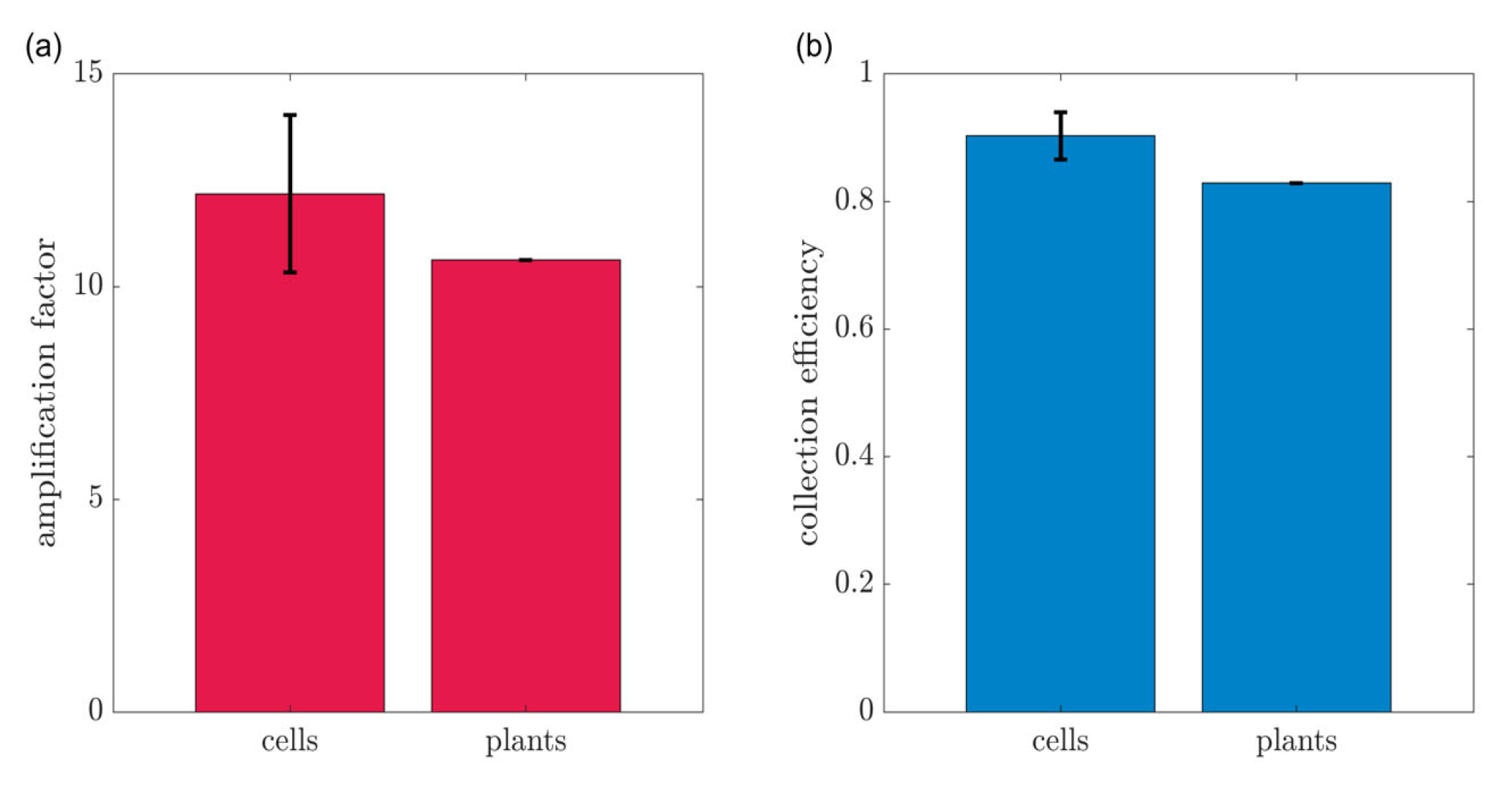
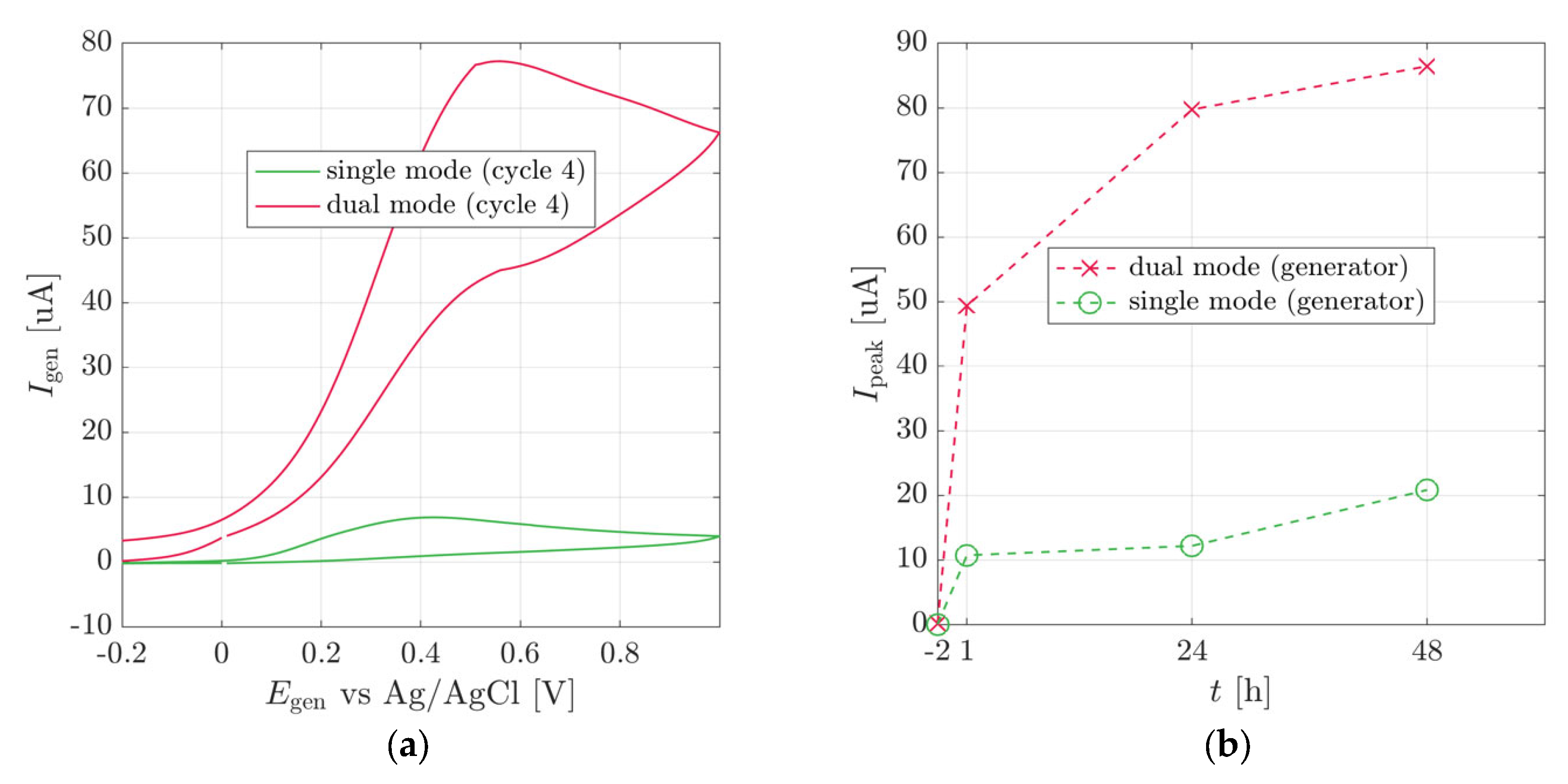
3.1. Cell Culture
3.2. Plant Stem-Mounted Sensor
3.3. Heat Shock Sensor
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, S.; Pandey, A.; Sangwan, R. Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pandey, R.; Teig-Sussholz, O.; Schuster, S.; Avni, A.; Shacham-Diamand, Y. Integrated electrochemical Chip-on-Plant functional sensor for monitoring gene expression under stress. Biosens. Bioelectron. 2018, 117, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Sample, V.; Newman, R.H.; Zhang, J. The structure and function of fluorescent proteins. Chem. Soc. Rev. 2009, 38, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, N.C.A.; Verhees, J.; van Leeuwen, W.; van der Krol, A.R. Evaluation and Comparison of the GUS, LUC and GFP Reporter System for Gene Expression Studies in Plants. Plant Biol. 2003, 5, 103–115. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Burgess, S.M.; Hirsh, D. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc. Natl. Acad. Sci. USA 1986, 83, 8447–8451. [Google Scholar] [CrossRef]
- Desagani, D.; Jog, A.; Teig-Sussholz, O.; Avni, A.; Shacham-Diamand, Y. Drought monitoring in tobacco plants by in-vivo electrochemical biosensor. Sens. Actuators B Chem. 2022, 356, 131357. [Google Scholar] [CrossRef]
- Anderson, L.B.; Reilley, C.N. Thin-layer electrochemistry: Steady-state methods of studying rate processes. J. Electroanal. Chem. 1965, 10, 295–305. [Google Scholar] [CrossRef]
- Fan, F.-R.F.; Bard, A.J. Electrochemical Detection of Single Molecules. Science 1995, 267, 871–874. [Google Scholar] [CrossRef]
- Niwa, O.; Morita, M.; Tabei, H. Electrochemical Behavior of Reversible Redox Species at Interdigitated Array Electrodes with Different Geometries: Consideration of Redox Cycling and Collection Efficiency. Anal. Chem. 1990, 62, 447–452. [Google Scholar] [CrossRef]
- Akanda, M.R.; Ju, H. An Integrated Redox Cycling for Electrochemical Enzymatic Signal Enhancement. Anal. Chem. 2017, 89, 13480–13486. [Google Scholar] [CrossRef]
- Yang, H. Enzyme-based ultrasensitive electrochemical biosensors. Curr. Opin. Chem. Biol. 2012, 16, 422–428. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Zhang, Z.; Chen, L. Chemical Redox-Cycling for Improving the Sensitivity of Colorimetric Enzyme-Linked Immunosorbent Assay. Anal. Chem. 2019, 91, 1254–1259. [Google Scholar] [CrossRef]
- Zanetti, C.; Spitz, S.; Berger, E.; Bolognin, S.; Smits, L.M.; Crepaz, P.; Rothbauer, M.; Rosser, J.M.; Marchetti-Deschmann, M.; Schwamborn, J.C.; et al. Monitoring the neurotransmitter release of human midbrain organoids using a redox cycling microsensor as a novel tool for personalized Parkinson’s disease modelling and drug screening. Analyst 2021, 146, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Park, J.-H.; Chang, Y.W.; Cho, S.; Kang, M.-J.; Pyun, J.-C. Chronoamperometry-Based Redox Cycling for Application to Immunoassays. ACS Sens. 2018, 3, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043–2081. [Google Scholar] [CrossRef]
- Ben-Yoav, H.; Winkler, T.E.; Kim, E.; Chocron, S.E.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Redox cycling-based amplifying electrochemical sensor for in situ clozapine antipsychotic treatment monitoring. Electrochim. Acta 2014, 130, 497–503. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Park, J.-H.; Chang, Y.W.; Cho, S.; Kang, M.-J.; Pyun, J.-C. Redox cycling-based immunoassay for detection of carcinogenic embryonic antigen. Anal. Chim. Acta 2017, 971, 33–39. [Google Scholar] [CrossRef]
- Kamath, R.R.; Madou, M.J. Three-Dimensional Carbon Interdigitated Electrode Arrays for Redox-Amplification. Anal. Chem. 2014, 86, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Wagh, M.D.; Sahoo, S.K.; Goel, S. Laser-induced graphene ablated polymeric microfluidic device with interdigital electrodes for taste sensing application. Sens. Actuators A Phys. 2021, 333, 113301. [Google Scholar] [CrossRef]
- Rishi, M.; Amreen, K.; Mohan, J.M.; Javed, A.; Dubey, S.K.; Goel, S. Rapid, Sensitive and Specific Electrochemical Detection of E. coli using Graphitized Mesoporous Carbon Modified Electrodes. Sens. Actuators A Phys. 2022, 338, 113483. [Google Scholar] [CrossRef]
- Singh, P.S.; Kätelhön, E.; Mathwig, K.; Wolfrum, B.; Lemay, S.G. Stochasticity in single-molecule nanoelectrochemistry: Origins, consequences, and solutions. ACS Nano 2012, 6, 9662–9671. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Morita, M. Electrochemical measurements with interdigitated array microelectrodes. Curr. Sep. 2000, 14, 2–8. [Google Scholar]
- Pontié, M.; Thouand, G.; DeNardi, F.; Tapsoba, I.; Lherbette, S. Antipassivating electrochemical process of glassy carbon electrode (gce) dedicated to the oxidation of nitrophenol compounds. Electroanalysis 2011, 23, 1579–1584. [Google Scholar] [CrossRef]
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 1–26. [Google Scholar] [CrossRef]
- Pontié, M.; Sikpo, L.; Thouand, G.; Rahan, R.; Tapsoba, I.; Mallet, R.; Feng, T. Direct Electroanalysis of p-Nitrophenol (PNP) in Estuarine and Surface Waters by a High Sensitive Type C/p-NiTSPc Coating Carbon Fiber Microelectrode (CFME). Electroanalysis 2011, 23, 433–441. [Google Scholar] [CrossRef]
- Cañizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical treatment of 2,4-dinitrophenol aqueous wastes using boron-doped diamond anodes. Electrochim. Acta 2004, 49, 4641–4650. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. Voltammetric behavior of p-nitrophenol and its trace determination in human urine by liquid chromatography with a dual reductive mode electrochemical detection system. Electroanalysis 2007, 19, 2176–2184. [Google Scholar] [CrossRef]
- Hu, S.; Xu, C.; Wang, G.; Cui, D. Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 2001, 54, 115–123. [Google Scholar] [CrossRef]
- Liu, Z.; Du, J.; Qiu, C.; Huang, L.; Ma, H.; Shen, D.; Ding, Y. Electrochemical sensor for detection of p-nitrophenol based on nanoporous gold. Electrochem. Commun. 2009, 11, 1365–1368. [Google Scholar] [CrossRef]
- El Mhammedi, M.A.; Achak, M.; Bakasse, M.; Chtaini, A. Electrochemical determination of para-nitrophenol at apatite-modified carbon paste electrode: Application in river water samples. J. Hazard. Mater. 2009, 163, 323–328. [Google Scholar] [CrossRef]
- Barbero, C.; Silber, J.J.; Sereno, L. Formation of a novel electroactive film by electropolymerization of ortho-aminophenol. J. Electroanal. Chem. Interfacial Electrochem. 1989, 263, 333–352. [Google Scholar] [CrossRef]
- Vierling, E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Key, J.L.; Lin, C.Y.; Chen, Y.M. Heat shock proteins of higher plants. Proc. Natl. Acad. Sci. USA 1981, 78, 3526–3530. [Google Scholar] [CrossRef]
- Neumann, D.; Nover, L.; Parthier, B.; Rieger, R.; Scharf, K.D.; Wollgiehn, R.; Nieden, U.Z. Heat shock and other stress response systems of plants. Results Probl. Cell Differ. 1989, 16, 1–155. [Google Scholar] [PubMed]
- Altschuler, M.; Mascarenhas, J.P. Heat shock proteins and effects of heat shock in plants. Plant Mol. Biol. 1982, 1, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Herrera-Estrella, L.; Inze, D.; Van Haute, E.; Van Montagu, M.; Schell, J.; Zambryski, P. Introduction of genetic material into plant cells. In Biotechnology & Biological Frontiers; Routledge: New York, NY, USA, 2019; pp. 480–493. [Google Scholar]
- Narasimhulu, S.B.; Deng, X.-B.; Sarria, R.; Gelvin, S.B. Early Transcription of Agrobacterium T-DNA Genes in Tobacco and Maize. Plant Cell 1996, 8, 873. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Grosskopf, D.G.; Regenass, M.; Boller, T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc. Natl. Acad. Sci. USA 1991, 88, 8831–8834. [Google Scholar] [CrossRef]
- Jefferson, R.A. The GUS reporter gene system. Nature 1989, 342, 837–838. [Google Scholar] [CrossRef]
- Takahashi, T.; Komeda, Y. Characterization of two genes encoding small heat-shock proteins in Arabidopsis thaliana. MGG Mol. Gen. Genet. 1989, 219, 365–372. [Google Scholar] [CrossRef]
- Van Engelen, F.A.; Molthoff, J.W.; Conner, A.; Nap, J.-P.; Pereira, A.; Stiekema, W.J. pBINPLUS: An improved plant transformation vector based on pBIN19. Transgenic Res. 1995, 4, 288–290. [Google Scholar] [CrossRef]
- Popovtzer, R.; Neufeld, T.; Ron, E.z.; Rishpon, J.; Shacham-Diamand, Y. Electrochemical detection of biological reactions using a novel nano-bio-chip array. Sens. Actuators B Chem. 2006, 119, 664–672. [Google Scholar] [CrossRef]
- Aoki, K.; Morita, M.; Niwa, O.; Tabei, H. Quantitative analysis of reversible diffusion-controlled currents of redox soluble species at interdigitated array electrodes under steady-state conditions. J. Electroanal. Chem. Interfacial Electrochem. 1988, 256, 269–282. [Google Scholar] [CrossRef]
- Groß, A.; Heintz, A. Diffusion coefficients of aromatics in nonporous PEBA membranes. J. Memb. Sci. 2000, 168, 233–242. [Google Scholar] [CrossRef]
- Groß, A.; Heintz, A. Diffusion Coefficients of Aromatics in Aqueous Solution. J. Chem. Eng. Data 2000, 45, 1121–1124. [Google Scholar]
- Aloni, R. Vascular Differentiation and Plant Hormones; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Saito, K.; Yamazaki, M.; Kaneko, H.; Murakoshi, I.; Fukuda, Y.; Van Montagu, M. Tissue-specific and stress-enhancing expression of the TR promoter for mannopine synthase in transgenic medicinal plants. Planta 1991, 184, 40–46. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotan, T.; Jog, A.; Kadan-Jamal, K.; Avni, A.; Shacham-Diamand, Y. In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling. Biosensors 2023, 13, 219. https://doi.org/10.3390/bios13020219
Dotan T, Jog A, Kadan-Jamal K, Avni A, Shacham-Diamand Y. In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling. Biosensors. 2023; 13(2):219. https://doi.org/10.3390/bios13020219
Chicago/Turabian StyleDotan, Tali, Aakash Jog, Kian Kadan-Jamal, Adi Avni, and Yosi Shacham-Diamand. 2023. "In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling" Biosensors 13, no. 2: 219. https://doi.org/10.3390/bios13020219
APA StyleDotan, T., Jog, A., Kadan-Jamal, K., Avni, A., & Shacham-Diamand, Y. (2023). In Vivo Plant Bio-Electrochemical Sensor Using Redox Cycling. Biosensors, 13(2), 219. https://doi.org/10.3390/bios13020219






