SMART: A Swing-Assisted Multiplexed Analyzer for Point-of-Care Respiratory Tract Infection Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Devices
2.2. Microfluidic Chip Design and Fabrication
2.3. Analyzer Construction
2.4. Sample Preparation
3. Results and Discussion
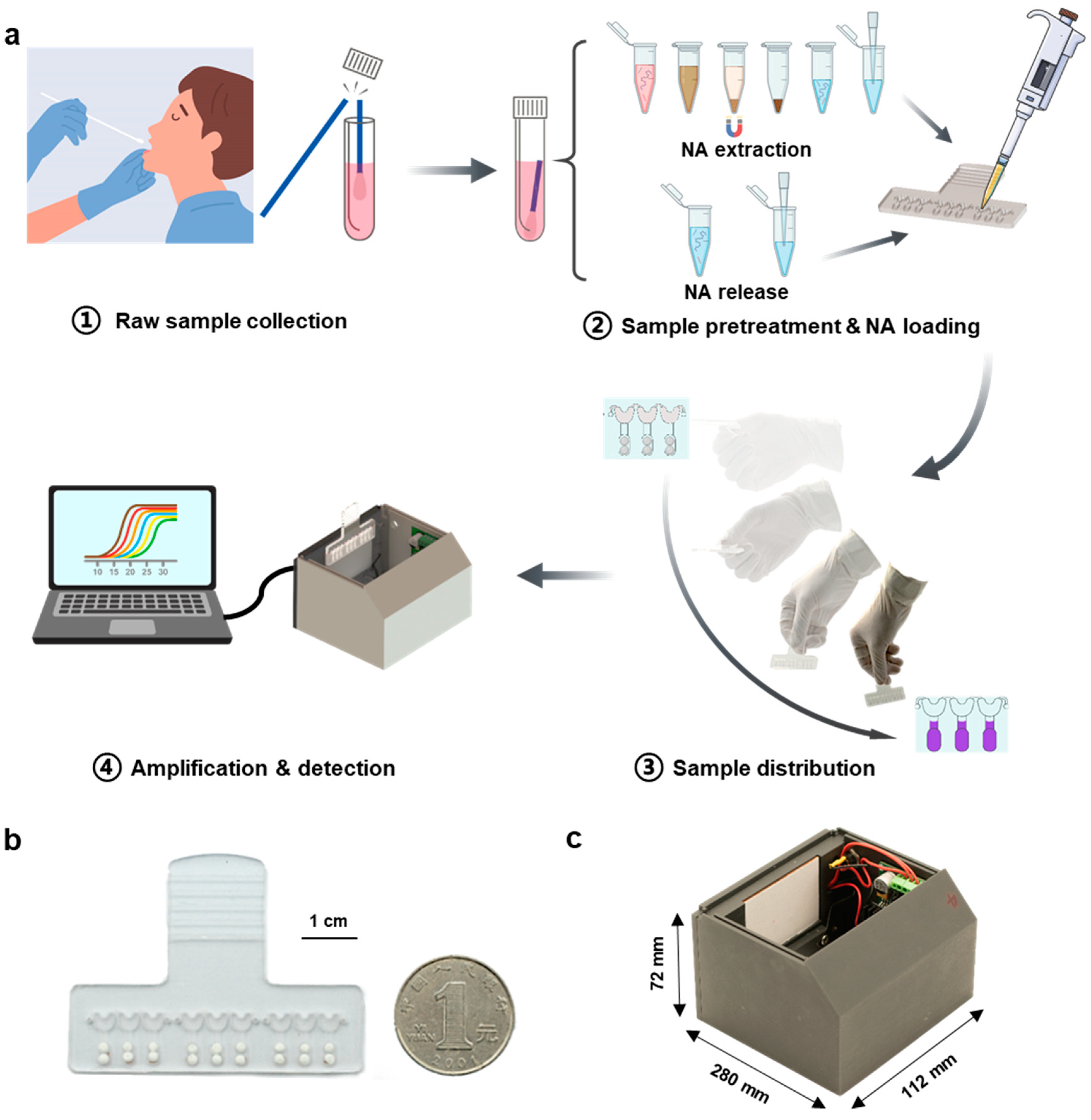
3.1. Workflow of the SMART System
3.2. Chip Structure Optimization
3.3. Cross-Contamination Evaluation
3.4. Sensitivity for SARS-CoV-2 Detection
3.5. Multitarget and Multisample Detection
3.6. Detection of Clinical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 26 December 2022).
- Wilder-Smith, A.; Osman, S. Public health emergencies of international concern: A historic overview. J. Travel Med. 2020, 27, taaa227. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Jankelow, A.; Lim, J.; Kindratenko, V.; Ganguli, A.; White, K.; Kumar, J.; Bashir, R. COVID-19 Point-of-Care Diagnostics: Present and Future. ACS Nano 2021, 15, 7899–7906. [Google Scholar] [CrossRef]
- FDA. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2. (accessed on 26 December 2022).
- Liu, D.; Shen, H.; Zhang, Y.; Shen, D.; Zhu, M.; Song, Y.; Zhu, Z.; Yang, C. A microfluidic-integrated lateral flow recombinase polymerase amplification (MI-IF-RPA) assay for rapid COVID-19 detection. Lab Chip 2021, 21, 2019–2026. [Google Scholar] [CrossRef]
- Cao, G.; Huo, D.; Chen, X.; Wang, X.; Zhou, S.; Zhao, S.; Luo, X.; Hou, C. Automated, portable, and high-throughput fluorescence analyzer (APHF-analyzer) and lateral flow strip based on CRISPR/Cas13a for sensitive and visual detection of SARS-CoV-2. Talanta 2022, 248, 123594. [Google Scholar] [CrossRef]
- Yin, K.; Ding, X.; Li, Z.; Sfeir, M.M.; Ballesteros, E.; Liu, C. Autonomous lab-on-paper for multiplexed, CRISPR-based diagnostics of SARS-CoV-2. Lab Chip 2021, 21, 2730–2737. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zhang, L.; Lu, Y.; Wang, X.; Shen, M.; Li, N.; Feng, L.; Jing, J.; Cao, B.; et al. Sensitive and Rapid Diagnosis of Respiratory Virus Coinfection Using a Microfluidic Chip-Powered CRISPR/Cas12a System. Small 2022, 18, e2200854. [Google Scholar] [CrossRef]
- Chaouch, M. Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2. Rev. Med. Virol. 2021, 31, e2215. [Google Scholar] [CrossRef]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef]
- Xun, G.; Lane, S.T.; Petrov, V.A.; Pepa, B.E.; Zhao, H. A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat. Commun. 2021, 12, 2905. [Google Scholar] [CrossRef]
- Bokelmann, L.; Nickel, O.; Maricic, T.; Paabo, S.; Meyer, M.; Borte, S.; Riesenberg, S. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat. Commun. 2021, 12, 1467. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; et al. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef] [PubMed]
- Panpradist, N.; Kline, E.C.; Atkinson, R.G.; Roller, M.; Wang, Q.; Hull, I.T.; Kotnik, J.H.; Oreskovic, A.K.; Bennett, C.; Leon, D.; et al. Harmony COVID-19: A ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection. Sci. Adv. 2021, 7, eabj1281. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.; Liu, F.; Teng, X.; Cui, C.; Wu, F.; Liu, W.; Liu, Y.; Chen, X.; Xu, J.; Ma, B. A Palm Germ-Radar (PaGeR) for rapid and simple COVID-19 detection by reverse transcription loop-mediated isothermal amplification (RT-LAMP). Biosens Bioelectron 2022, 200, 113925. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Nowak, C.; Liu, Y.; Li, Y.; Zhang, T.; Bleris, L.; Qin, Z. Plasmonic LAMP: Improving the Detection Specificity and Sensitivity for SARS-CoV-2 by Plasmonic Sensing of Isothermally Amplified Nucleic Acids. Small 2022, 18, e2107832. [Google Scholar] [CrossRef]
- Xiong, H.; Ye, X.; Li, Y.; Wang, L.; Zhang, J.; Fang, X.; Kong, J. Rapid Differential Diagnosis of Seven Human Respiratory Coronaviruses Based on Centrifugal Microfluidic Nucleic Acid Assay. Anal. Chem. 2020, 92, 14297–14302. [Google Scholar] [CrossRef]
- Tian, F.; Liu, C.; Deng, J.; Han, Z.; Zhang, L.; Chen, Q.; Sun, J. A fully automated centrifugal microfluidic system for sample-to-answer viral nucleic acid testing. Sci. China Chem. 2020, 63, 1498–1506. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Wang, H.; Li, S.; Lin, Y.; Chen, L.; Zhao, Y.; Chao, S.; Huang, X.; Ge, S.; et al. A High-Throughput, Multi-Index Isothermal Amplification Platform for Rapid Detection of 19 Types of Common Respiratory Viruses Including SARS-CoV-2. Engineering 2020, 6, 1130–1140. [Google Scholar] [CrossRef]
- Xing, W.; Wang, J.; Zhao, C.; Wang, H.; Bai, L.; Pan, L.; Li, H.; Wang, H.; Zhang, Z.; Lu, Y.; et al. A Highly Automated Mobile Laboratory for On-site Molecular Diagnostics in the COVID-19 Pandemic. Clin. Chem. 2021, 67, 672–683. [Google Scholar] [CrossRef]
- Li, N.; Shen, M.; Liu, J.; Zhang, L.; Wang, H.; Xu, Y.; Cheng, J. Multiplexed detection of respiratory pathogens with a portable analyzer in a "raw-sample-in and answer-out" manner. Microsyst. Nanoeng. 2021, 7, 94. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Akhtar, A.S.; Pinto, I.F.; Lapins, N.; Barrett, D.; Sandh, G.; Yin, X.; Pelechano, V.; Russom, A. Sample-to-answer COVID-19 nucleic acid testing using a low-cost centrifugal microfluidic platform with bead-based signal enhancement and smartphone read-out. Lab Chip 2021, 21, 2932–2944. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Bui, H.K.; Phan, V.M.; Seo, T.S. An internet of things-based point-of-care device for direct reverse-transcription-loop mediated isothermal amplification to identify SARS-CoV-2. Biosens. Bioelectron. 2022, 195, 113655. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Woo, S.; Kim, J.; Lee, H.; Yoo, Y.E.; Hong, S. Rapid and simple single-chamber nucleic acid detection system prepared through nature-inspired surface engineering. Theranostics 2021, 11, 6735–6745. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, H.; Zhang, Y.; Jiang, K.; Lu, Y.; Ren, Y.; Wang, H.; Wang, S.; Xing, W. A fully sealed plastic chip for multiplex PCR and its application in bacteria identification. Lab Chip 2015, 15, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Liu, J.; Zhao, H.; Fan, X.P.; Xu, Y.C.; Li, C.X. Construction of a rapid microfluidic-based SNP genotyping (MSG) chip for ancestry inference. Forensic Sci. Int. Genet. 2019, 41, 145–151. [Google Scholar] [CrossRef]
- Panpradist, N.; Wang, Q.; Ruth, P.S.; Kotnik, J.H.; Oreskovic, A.K.; Miller, A.; Stewart, S.W.A.; Vrana, J.; Han, P.D.; Beck, I.A.; et al. Simpler and faster COVID-19 testing: Strategies to streamline SARS-CoV-2 molecular assays. EBioMedicine 2021, 64, 103236. [Google Scholar] [CrossRef]
- Garcia-Venzor, A.; Rueda-Zarazua, B.; Marquez-Garcia, E.; Maldonado, V.; Moncada-Morales, A.; Olivera, H.; Lopez, I.; Zuniga, J.; Melendez-Zajgla, J. SARS-CoV-2 Direct Detection Without RNA Isolation With Loop-Mediated Isothermal Amplification (LAMP) and CRISPR-Cas12. Front. Med. 2021, 8, 627679. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Yan, H.; Zhu, Y.; Wang, L.; Zhang, Y.; Lu, Y.; Xing, W. Sensitive and rapid detection of pathogenic bacteria from urine samples using multiplex recombinase polymerase amplification. Lab Chip 2018, 18, 2441–2452. [Google Scholar] [CrossRef]
- Zong, N.; Gao, Y.; Chen, Y.; Luo, X.; Jiang, X. Automated Centrifugal Microfluidic Chip Integrating Pretreatment and Molecular Diagnosis for Hepatitis B Virus Genotyping from Whole Blood. Anal. Chem. 2022, 94, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ching, J.; Xi, L.; Hughes, S.J.; Chang, R.; Wong, W.; McMillan, W.; Gooding, W.E.; McCarty, K.S., Jr.; Chestney, M.; et al. Technology for automated, rapid, and quantitative PCR or reverse transcription-PCR clinical testing. Clin. Chem. 2005, 51, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Cepheid. GeneXpert® Infinity Systems. Available online: https://www.cepheid.com/en_US/systems/GeneXpert-Family-of-Systems/GeneXpert-Infinity. (accessed on 26 December 2022).
- Senok, A.; Alfaresi, M.; Khansaheb, H.; Nassar, R.; Hachim, M.; Al Suwaidi, H.; Almansoori, M.; Alqaydi, F.; Afaneh, Z.; Mohamed, A.; et al. Coinfections in Patients Hospitalized with COVID-19: A Descriptive Study from the United Arab Emirates. Infect. Drug Resist. 2021, 14, 2289–2296. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, X.; Liu, D.; Wu, Y.; Feng, L.; Han, C.; Liu, J.; Lu, Y.; Sotnikov, D.V.; Xu, Y.; et al. SMART: A Swing-Assisted Multiplexed Analyzer for Point-of-Care Respiratory Tract Infection Testing. Biosensors 2023, 13, 228. https://doi.org/10.3390/bios13020228
Zhang L, Wang X, Liu D, Wu Y, Feng L, Han C, Liu J, Lu Y, Sotnikov DV, Xu Y, et al. SMART: A Swing-Assisted Multiplexed Analyzer for Point-of-Care Respiratory Tract Infection Testing. Biosensors. 2023; 13(2):228. https://doi.org/10.3390/bios13020228
Chicago/Turabian StyleZhang, Li, Xu Wang, Dongchen Liu, Yu Wu, Li Feng, Chunyan Han, Jiajia Liu, Ying Lu, Dmitriy V. Sotnikov, Youchun Xu, and et al. 2023. "SMART: A Swing-Assisted Multiplexed Analyzer for Point-of-Care Respiratory Tract Infection Testing" Biosensors 13, no. 2: 228. https://doi.org/10.3390/bios13020228




