Fabrication of a Novel and Ultrasensitive Label-Free Electrochemical Aptasensor Based on Gold Nanostructure for Detection of Homocysteine
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Equipment
2.3. Fabrication of CPE
2.4. Modification of CPE Surface
2.5. Construction of Electrochemical Aptasensor
2.6. Homocysteine Determination
3. Results and Discussion
3.1. Structure and Morphology
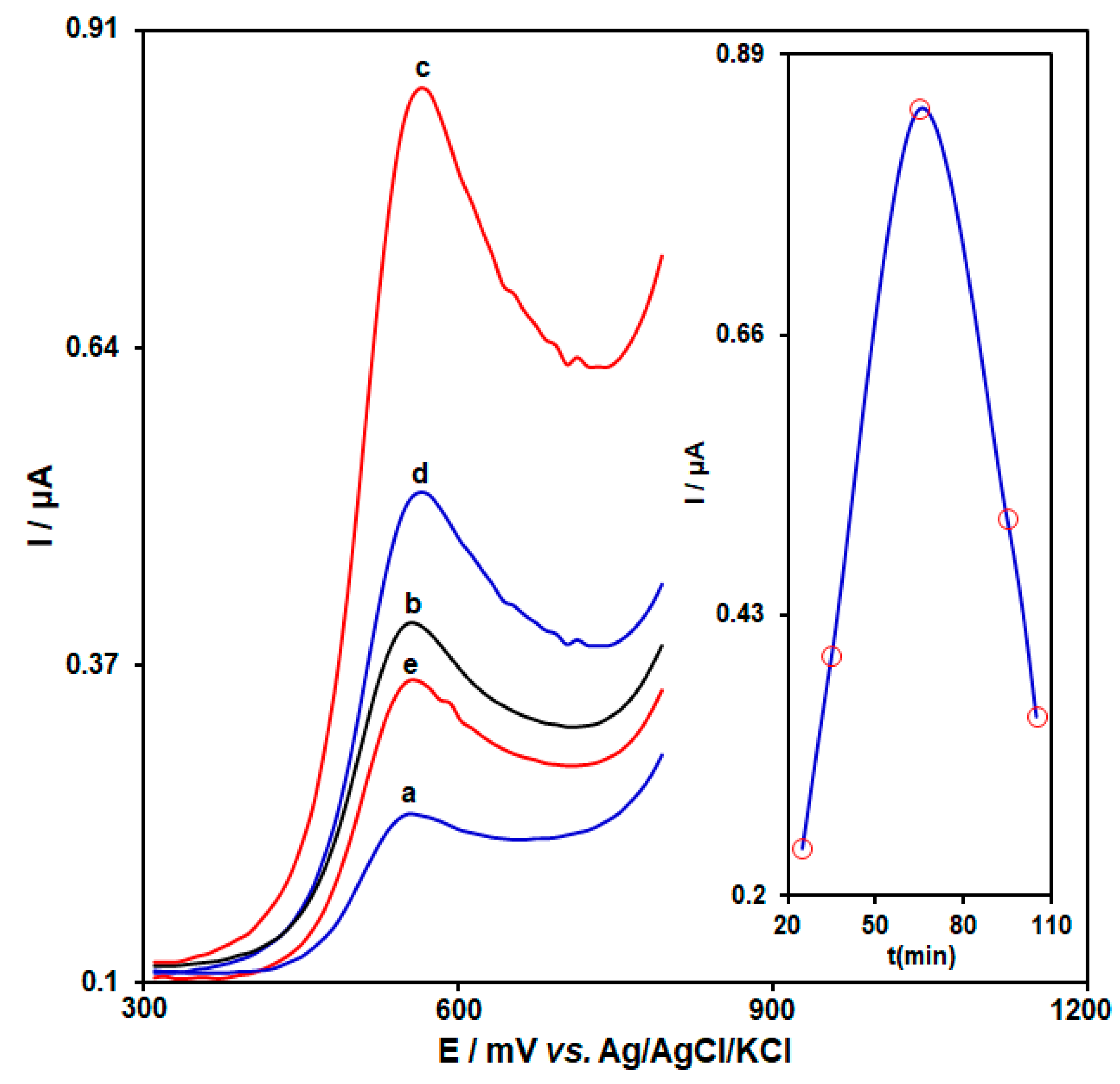
3.2. Experimental Optimization
3.3. Standard Curve and Limit of Detection
3.4. Real Sample Determination
3.5. Comparison of As-Developed Homocysteine Aptasensor with Other Previously Introduced Electrochemical Approaches
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.; Mathiyarasu, J. An electrochemical sensor for homocysteine detection using gold nanoparticle incorporated reduced graphene oxide. Colloids Surf. B 2018, 170, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, P.; Sun, X.; Liu, H. Development of Electrochemical Sensor for Homocysteine Determination as a Cerebrovascular Disease Biomarker. Int. J. Electrochem. Sci. 2017, 12, 8642–8650. [Google Scholar] [CrossRef]
- Refsum, H.; Nurk, E.; Smith, A.D.; Ueland, P.M.; Gjesdal, C.G.; Bjelland, I.; Tverdal, A.; Tell, G.S.; Nygård, O.; Vollset, S.E. The Hordaland Homocysteine Study: A community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 2006, 136, 1731S–1740S. [Google Scholar] [PubMed]
- Concepción-Alvarez, A.; Camayd-Viera, I.; Nuevas-Paz, L. Validation of an HPLC method for total homocysteine quantification in plasma. Rev. del Lab. Clin. 2016, 9, 40–47. [Google Scholar]
- Pasas, S.A.; Lacher, N.A.; Davies, M.I.; Lunte, S.M. Detection of homocysteine by conventional and microchip capillary electrophoresis/electrochemistry. Electrophoresis 2002, 23, 759–766. [Google Scholar] [CrossRef]
- Wrońska, M.; Chwatko, G.; Borowczyk, K.; Piechocka, J.; Kubalczyk, P.; Głowacki, R. Application of GC–MS technique for the determination of homocysteine thiolactone in human urine. J. Chromatogr. B 2018, 1099, 18–24. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Zhu, X.; Zhang, G.; Yang, X.; Li, F.; Zhang, X.; Fang, M.; Yu, J.; Zhou, H. A highly selective two-photon fluorescent chemosensor for tracking homocysteine via situ reaction. Dyes Pigments 2018, 155, 159–163. [Google Scholar] [CrossRef]
- Arora, B.; Narayanasamy, A.; Nirmal, J.; Halder, N.; Patnaik, S.; Ravi, A.K.; Velpandian, T. Development and validation of a LC–MS/MS method for homocysteine thiolactone in plasma and evaluation of its stability in plasma samples. J. Chromatogr. B 2014, 944, 49–54. [Google Scholar]
- Tomaiuolo, M.; Vecchione, G.; Margaglione, M.; Pisanelli, D.; Grandone, E. Stable-isotope dilution LC–ESI-MS/MS techniques for the quantification of total homocysteine in human plasma. J. Chromatogr. B 2009, 877, 3292–3299. [Google Scholar]
- Yunus, M.H.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ahmad Raston, N.H.; Md Noor, S.S. Simultaneous Amperometric Aptasensor Based on Diazonium Grafted Screen-Printed Carbon Electrode for Detection of CFP10 and MPT64 Biomarkers for Early Tuberculosis Diagnosis. Biosensors 2022, 12, 996. [Google Scholar] [PubMed]
- Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Kirlikovali, K.O.; Le, Q.V.; Jang, H.W.; Varma, R.S.; Farha, O.K.; Shokouhimehr, M. Recent electrochemical applications of metal–organic framework-based materials. Cryst. Growth Des. 2020, 20, 7034–7064. [Google Scholar]
- Mehdizadeh, Z.; Shahidi, S.; Ghorbani-HasanSaraei, A.; Limooei, M.; Bijad, M. ‘Monitoring of Amaranth in Drinking Samples using Voltammetric Amplified Electroanalytical Sensor’. Chem. Methodol. 2022, 6, 246–252. [Google Scholar]
- Chang, Y.; Wang, Y.; Zhang, J.; Xing, Y.; Li, G.; Deng, D.; Liu, L. Overview on the Design of Magnetically Assisted Electrochemical Biosensors. Biosensors 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Piri, F.; Merajoddin, M.; Piri, S.; Mokarian, Z. Synthesis WO3 nanoparticle via the electrochemical method and study its super-hydrophobicity properties. Asian J. Nanosci. Mater. 2021, 4, 240–245. [Google Scholar]
- Mazloum-Ardakani, M.; Taleat, Z.; Khoshroo, A.; Beitollahi, H.; Dehghani, H. Electrocatalytic oxidation and voltammetric determination of levodopa in the presence of carbidopa at the surface of a nanostructure based electrochemical sensor. Biosens. Bioelectron. 2012, 35, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2020, 10, 11699. [Google Scholar]
- Raoof, J.B.; Ojani, R.; Beitollahi, H.; Hosseinzadeh, R. Electrocatalytic Oxidation and Highly Selective Voltammetric Determination of L-Cysteine at the Surface of a 1-[4-(Ferrocenyl ethynyl)phenyl]-1-ethanone Modified Carbon Paste Electrode. Ana. Sci. 2006, 22, 1213–1220. [Google Scholar]
- Naik, T.S.K.; Mwaurah, M.M.; Swamy, B.K. Fabrication of poly (sudan III) modified carbon paste electrode sensor for dopamine: A voltammetric study. J. Electroanal. Chem. 2019, 834, 71–78. [Google Scholar] [CrossRef]
- Liu, L.; Yu, H.; Zhao, Q. The Characterization of Binding between Aptamer and Bisphenol A and Developing Electrochemical Aptasensors for Bisphenol A with Rationally Engineered Aptamers. Biosensors 2022, 12, 913. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Garkani Nejad, F. Simple Preparation and Characterization of Hierarchical Flower-like NiCo2O4 Nanoplates: Applications for Sunset Yellow Electrochemical Analysis. Biosensors 2022, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Ahour, F. Electrochemical sensing of thioridazine in human serum samples using modified glassy carbon electrode. Adv. J. Chem. A 2021, 4, 22–31. [Google Scholar]
- Beitollahi, H.; Dourandish, Z.; Tajik, S.; Sharifi, F.; Mohammadzadeh Jahani, P. Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen. Biosensors 2022, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Alizadeh, M.; Karaman, O.; Vasseghian, Y.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar]
- Pourmadadi, M.; Yazdian, F.; Ghorbanian, S.; Shamsabadipour, A.; Khandel, E.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. Construction of Aptamer-Based Nanobiosensor for Breast Cancer Biomarkers Detection Utilizing g-C3N4/Magnetic Nano-Structure. Biosensors 2022, 12, 921. [Google Scholar]
- da Silva, W.; Ghica, M.E.; Ajayi, R.F.; Iwuoha, E.I.; Brett, C.M. Impedimetric sensor for tyramine based on gold nanoparticle doped-poly (8-anilino-1-naphthalene sulphonic acid) modified gold electrodes. Talanta 2019, 195, 604–612. [Google Scholar]
- Sengar, M.S.; Saxena, S.; Satsangee, S.P.; Jain, R. Silver nanoparticles decorated functionalized multiwalled carbon nanotubes modified screen printed sensor for voltammetric determination of butorphanol. J. Appl. Organomet. Chem. 2021, 1, 95–108. [Google Scholar]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with multi-wall carbon nano-tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Joshi, P.; Mehtab, S.; Zaidi, M.G.H.; Tyagi, T.; Bisht, A. Development of polyindole/tungsten carbide nanocomposite-modified electrodes for electrochemical quantification of chlorpyrifos. J. Nanostruct. Chem. 2020, 10, 33–45. [Google Scholar]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar]
- Poo-arporn, Y.; Pakapongpan, S.; Chanlek, N.; Poo-arporn, R.P. The development of disposable electrochemical sensor based on Fe3O4-doped reduced graphene oxide modified magnetic screen-printed electrode for ractopamine determination in pork sample. Sens. Actuators B Chem. 2019, 284, 164–171. [Google Scholar] [CrossRef]
- Peyman, H.; Roshanfekr, H.; Babakhanian, A.; Jafari, H. PVC membrane electrode modified by lawson as synthetic derivative ionophore for determination of cadmium in alloy and wastewater. Chem. Methodol. 2021, 5, 446–453. [Google Scholar]
- Ismaeel, S.A.; Al-Bayati, Y.K. Determination of trace metformin in pharmaceutical preparation using molecularly imprinted polymer based pvc-membrane. Eurasian Chem. Commun. 2021, 3, 812–830. [Google Scholar]
- Khand, N.H.; Palabiyik, I.M.; Buledi, J.A.; Ameen, S.; Memon, A.F.; Ghumro, T.; Solangi, A.R. Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J. Nanostruct. Chem. 2021, 11, 455–468. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shojaei, A.F.; Tabatabaeian, K.; Karimi, F.; Shakeri, S.; Moradi, R. Simultaneous determination of 6-mercaptopruine, 6-thioguanine and dasatinib as three important anticancer drugs using nanostructure voltammetric sensor employing Pt/MWCNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate. Biosens. Bioelectron. 2016, 86, 879–884. [Google Scholar]
- Balasubramanian, P.; Balamurugan, T.S.T.; Chen, S.M.; Chen, T.W.; Lin, P.H. A novel, efficient electrochemical sensor for the detection of isoniazid based on the B/N doped mesoporous carbon modified electrode. Sens. Actuators B Chem. 2019, 283, 613–620. [Google Scholar]
- Hwang, J.H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. The selective electrochemical detection of homocysteine in the presence of glutathione, cysteine, and ascorbic acid using carbon electrodes. Analyst 2014, 139, 3755–3762. [Google Scholar] [CrossRef]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. The use of screen-printed electrodes in a proof of concept electrochemical estimation of homocysteine and glutathione in the presence of cysteine using catechol. Sensors 2014, 14, 10395–10411. [Google Scholar]
- Wang, K.; Liao, J.; Yang, X.; Zhao, M.; Chen, M.; Yao, W.; Tan, W.; Lan, X. A label-free aptasensor for highly sensitive detection of ATP and thrombin based on metal-enhanced PicoGreen fluorescence. Biosens. Bioelectron. 2015, 63, 172–177. [Google Scholar] [PubMed]
- Wang, S.; Zhang, L.; Wan, S.; Cansiz, S.; Cui, C.; Liu, Y.; Cai, R.; Hong, C.; Teng, I.T.; Shi, M.; et al. Aptasensor with expanded nucleotide using DNA nanotetrahedra for electrochemical detection of cancerous exosomes. ACS Nano 2017, 11, 3943–3949. [Google Scholar]
- Huo, Y.; Qi, L.; Lv, X.J.; Lai, T.; Zhang, J.; Zhang, Z.Q. A sensitive aptasensor for colorimetric detection of adenosine triphosphate based on the protective effect of ATP-aptamer complexes on unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 315–320. [Google Scholar] [PubMed]
- Yu, Z.G.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of ampicillin in complex samples. Talanta 2018, 176, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Somerson, J.; Plaxco, K.W. Electrochemical Aptamer-Based Sensors for Rapid Point-of-Use Monitoring of the Mycotoxin Ochratoxin A Directly in a Food Stream. Molecules 2018, 23, 912. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.P.; Zhao, R.N.; Wang, L.J.; Ma, R.N.; Zhang, W.; Shang, L.; Wang, H.S. Aptamer based electrochemical assay for protein kinase activity by coupling hybridization chain reaction. Biosens. Bioelectron. 2018, 117, 690–695. [Google Scholar] [PubMed]
- McKeague, M.; Foster, A.; Miguel, Y.; Giamberardino, A.; Verdin, C.; Chan, J.Y.; DeRosa, M.C. Development of a DNA aptamer for direct and selective homocysteine detection in human serum. RSC Adv. 2013, 3, 24415–24422. [Google Scholar]
- Peng, H.; Hui, Y.; Pu, M.; Yang, D.; Zhao, A.; Wang, W.; Wu, S.; Wang, B. Electrochemical aptasensor based on PEI-Fe-MOF/Au@ Ag NPs nanocomposites for streptomycin detection in dairy products. J. Food Compos. Anal. 2022, 116, 105091. [Google Scholar] [CrossRef]
- Rahmani, H.R.; Adabi, M.; Pooshang Bagheri, K.; Karim, G. Effect of processing variables on the performance of electrochemical aptasensor for determination of aflatoxin M1. Nanomed. Res. J. 2020, 5, 378–382. [Google Scholar]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Electrochemical aptasensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1237–1250. [Google Scholar]
- Gholami-Orimi, F.; Taleshi, F.; Biparva, P.; Karimi-Maleh, H.; Beitollahi, H.; Ebrahimi, H.R.; Shamshiri, M.; Bagheri, H.; Fouladgar, M.; Taherkhani, A. Voltammetric determination of homocysteine using multiwall carbon nanotube paste electrode in the presence of chlorpromazine as a mediator. J. Anal. Methods Chem. 2012, 2012, 902184. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Mokhtari, B.; Nematollahi, D. Selective electrochemical determination of homocysteine in the presence of cysteine and glutathione. Electrochim. Acta 2014, 123, 353–361. [Google Scholar] [CrossRef]
- Saeed, J.; Mirzaei, M.; Torkzadeh-Mahani, M. A selective and regenerable voltammetric aptasensor for determination of homocysteine. Microchim. Acta 2016, 183, 2205–2210. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Khoshroo, A.; Adib, K.; Rahimi-Nasrabadi, M.; Ahmadi, F. Determination of homocysteine using a dopamine-functionalized graphene composite. Microchem. J. 2021, 165, 106124. [Google Scholar]
- Wen, X.H.; Zhao, X.F.; Peng, B.F.; Yuan, K.P.; Li, X.X.; Zhu, L.Y.; Lu, H.L. Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]





| Sample | Spiked | Found | Recovery (%) | R.S.D. (%) |
|---|---|---|---|---|
| Urine | 0 | 0.7 (±0.02) | - | 3.2 |
| 6.0 | 6.6 (±0.1) | 98.5 | 2.2 | |
| 8.0 | 8.8 (±0.1) | 101.1 | 1.8 | |
| Human serum | 0 | 4.1 (±0.1) | - | 2.6 |
| 4.0 | 8.4 (±0.1) | 103.7 | 1.9 | |
| 5.0 | 9.0 (±0.3) | 98.9 | 3.2 |
| Method | Limit of Detection (LOD) | linear Dynamic Range (LDR) | Ref. |
|---|---|---|---|
| Voltammetry/Square wave voltammetry | 0.08 μM | 0.1–210.0 μM | [51] |
| Voltammetry/Linear sweep voltammograms | 3.3 μM | 5.0–800.0 μM | [52] |
| Voltammetry/Differential pulse voltammetry | 0.89 μM | 2.5–1000.0 μM | [53] |
| Voltammetry/Differential pulse voltammetry | 0.15 μM | 0.5–900.0 μM | [54] |
| Voltammetry/Differential pulse voltammetry | 1.0 μM | 1.0–100.0 μM | [55] |
| Voltammetry/Differential pulse voltammetry | 0.03 μM | 0.1–30.0 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaimbashi, R.; Tajik, S.; Beitollahi, H.; Torkzadeh-Mahani, M. Fabrication of a Novel and Ultrasensitive Label-Free Electrochemical Aptasensor Based on Gold Nanostructure for Detection of Homocysteine. Biosensors 2023, 13, 244. https://doi.org/10.3390/bios13020244
Zaimbashi R, Tajik S, Beitollahi H, Torkzadeh-Mahani M. Fabrication of a Novel and Ultrasensitive Label-Free Electrochemical Aptasensor Based on Gold Nanostructure for Detection of Homocysteine. Biosensors. 2023; 13(2):244. https://doi.org/10.3390/bios13020244
Chicago/Turabian StyleZaimbashi, Reza, Somayeh Tajik, Hadi Beitollahi, and Masoud Torkzadeh-Mahani. 2023. "Fabrication of a Novel and Ultrasensitive Label-Free Electrochemical Aptasensor Based on Gold Nanostructure for Detection of Homocysteine" Biosensors 13, no. 2: 244. https://doi.org/10.3390/bios13020244
APA StyleZaimbashi, R., Tajik, S., Beitollahi, H., & Torkzadeh-Mahani, M. (2023). Fabrication of a Novel and Ultrasensitive Label-Free Electrochemical Aptasensor Based on Gold Nanostructure for Detection of Homocysteine. Biosensors, 13(2), 244. https://doi.org/10.3390/bios13020244






